Introduction
High-altitude Mountain ecosystems located above 2 800 m (Challenger and Soberón, 2008) contain numerous transition zones between plant communities (knows as ecotones), which are highly dynamic (Kark, 2017). Treeline, which represents the transition zone between forested areas and other types of vegetation, marks the uppermost extent of forested patches on slopes with the same aspect (Richardson and Friedland, 2009; Kark, 2017). There is not clarity about the global mechanisms that control tree growth beyond treeline. However, there is no doubt about the influence of temperature over the ecological and physiological processes of treeline in the high mountains, which gives them a high dynamic useful in the early detection of shifts induced by global warming (Holtmeier, 2009; Treml and Chuman, 2015; Kark, 2017). Nevertheless, both vegetation structure and plant composition of treeline are key for understanding the response of mountain ecosystems to changes in environmental conditions (Richardson and Friedland, 2009; Harsch and Bader, 2011). Moreover, they can promote plant growth and resilience in the face of extreme conditions, potentially extending the longevity of plant life (Wielgolaski et al., 2017).
As part of an evolutionary adaptation process, many plant species in high mountain ecosystems have developed notable structural, phenotypical, and physiological modifications to mitigate the abiotic stress faced at high altitudes (Parmesan, 2006; Alberto et al., 2013). These adaptations have generated a large variety of plant life forms and endemism in high mountain ecosystems (Cavieres and Badano, 2009; Körner, 2017). The diversity of life forms in these ecosystems is a crucial factor in the establishment and survival of plants across their distribution areas (Callaway et al., 2002), at new sites (Cavieres and Badano, 2009; Badano et al., 2015) and influences the latitudinal or altitudinal plant migrations in response to changing environmental conditions. As a result, many alpine plants develop stress-tolerance strategies such as small size or slow growth rate, buffering effects on the microenvironment, will not change much with time (Anthelme et al., 2014). In addition, the altitudinal migration of a forest could be promoted or limited as a function of the plant composition in the ecotone (Alberto et al., 2013; Badano et al., 2015).
The upper altitudinal limit of Mexican pine (Pinus hartwegii Lindl.) is the highest in the world (Calderón de Rzedowski and Rzedowski, 2005). This species has adapted to extremely adverse aboveground (extremely low temperatures, freezing and abrasive winds, and high solar radiation) (Körner et al., 2021) and belowground environmental conditions (shallow and unconsolidated soils, low nutrient availability and freezing soils) (Kozlowski and Pallardy, 2002; Körner et al., 2021). These adaptations, however, make Pinus hartwegii extremely sensitive to environmental changes.
Several studies have analyzed changes in its upper altitudinal limit at the Nevado de Toluca and Iztaccíhuatl-Popocatépetl (both volcanoes in central Mexico) stemming from an average local temperature increase of 1.5 °C in the past 50 years (Pérez-Suárez et al., 2022). This phenomenon is especially relevant considering that the temperature of central Mexico is expected to increase 2 °C by 2080 (Arriaga and Gómez, 2004; Alfaro-Ramírez et al., 2020). Furthermore, little knowledge has been complied on the composition, structure and functioning of the species that compose the treeline ecotone of Pinus hartwegii or on the presence of facilitative and competitive relationships between different life forms in this ecotone, especially in the case of annual life forms, which respond more quickly to environmental changes compared to longer living life forms such as trees.
In this context, this study aimed to know what is the composition of the plant community found along the altitudinal gradient of the treeline ecotone of Pinus hartwegii? What proportion of life forms accompany Pinus hartwegii? As well as if, is the proportion of existing life forms across the altitudinal gradient related to a reduction in Pinus hartwegii trees at increasing altitudes? To answer these questions, the present study determined the species diversity and the proportion of life forms of the plant community along the altitudinal gradient of the Pinus hartwegii treeline ecotone in the Nevado de Toluca, central Mexico. The resulting data could serve as a baseline for future comparisons and form the basis for more in-depth analyses of the competitive and facilitative relationships that either promote or limit the upward migration of the Pinus hartwegii forest. Upon understanding these relationships, more precise models could be created to potentially predict the altitudinal migration of Pinus hartwegii based on plant community composition of its treeline ecotone.
Materials and Methods
Study area
The study area was located in the State of Mexico in central Mexico between Toluca and Tenango valleys within the Nevado de Toluca Flora and Fauna Protection Area (18°51'-19°19' N and 99°38'-100°09' W; Figure 1). This area encompasses a range of altitude from 3 000 to 4 680 m (Vargas, 1984). Semicold, subhumid climate (C[E]wig) and cold climate (E[T]Hwig) are dominant (García, 1990), with average temperatures below 10 °C and low rainfall, as well as snowfall during the winter season (Challenger and Soberón, 2008). Main soil group is andosol, as well as feozem, regosol, cambisol, and litosol soils in lesser proportions (Vargas, 1984). Temperate coniferous forests are abundant and dominated by species of Abies Mill., Pinus L., and Quercus L. genera between 3 000 and 4 100 m, while above 3 950 and up to 4 500 m high mountain grasses are present and dominated by Festuca L. and Calamagrostis Adans. (Calderón de Rzedowski and Rzedowski, 2005).
Sampling design
A stratified systematic sampling design was used to subdivide the study area according to altitudinal level and to subsequently identify any patterns in the spatial variation of plant communities (Matteucci and Colma, 1982).
Five perpendicular transects of 220 m in length along the treeline ecotone were established to cover an altitudinal gradient. Transects that were accessible and that had a similar state of conservation at the treeline and a similar gradient were selected. Concerning the volcanic crater, two transects had a northwesterly orientation, and three had an easterly-southeasterly exposure.
Six 20×20 m plots were established along each transect based on the experimental model of Camarero and Fortin (2006). Three plots were located below the forest line (forested area), which were called from lowest to highest altitudes as Bos1, Bos2 and Bos3, while the other three were located above the forest line (grassy area) being called Pas4, Pas5 and Pas6 idem. Central points of each plot were separated by 40 m. Each plot was further divided into four quadrants of 10×10 m numbered clockwise from 1 to 4. In each plot, two opposite quadrants were alternately selected (1 and 3 or 2 and 4); in these quadrants, two plots of 3×3 m were randomly established to assess the herbaceous strata. Therefore, data were generated per plot and altitudinal level considering plant cover on both sides of the forest line and sampling sites orientation.
Characterization of life forms
The diversity of life forms at treeline ecotone of Pinus hartwegii was determined through collecting and identifying all plant species from August to October 2018. The number of individuals of each species was recorded in each plot to subsequently calculate relative abundance of each species and specific diversity was calculated of each altitudinal level according to the Shannon diversity index (H') (Buckland et al., 2005). The required sampling effort was based on rarefaction and extrapolation curves generated in iNEXT Online version (Chao et al., 2022) using Hill numbers (Chao et al., 2014; Cox et al., 2017). Hill numbers were used since it that incorporates species richness and relative abundance and allows to explicitly choose how sensitive the diversity metric is to rare species (Chao et al., 2014; Cox et al., 2017). We used a Hill number with q=1 (i. e., Hill Shannon diversity, which emphasizes neither rare nor common species to allow characterizing gradients in biodiversity) (Chao et al., 2014).
Sample robustness was measured with sample-size-based diversity accumulation curves, in which the expected Hill Shannon diversity is plotted as a function of sample size using the number of individuals (Chao et al., 2014; Cox et al., 2017). Coverage-based diversity curves were used as well to plot expected diversity as a function of interpolated (observed) and extrapolated (expected) coverage.
All plant species found at upper treeline ecotone of Pinus hartwegii were then categorized as phanerophytes, chamaephytes, hemicryptophytes, cryptophytes or therophytes life forms according to the classification of Raunkiaer (1934). This classification is based on height of plants’ buds during seasons with adverse conditions. Life forms proportion at each altitudinal level, and the percentage cover (species coverage) occupied by each life form were calculated. Collected specimens were identified in the Hortorio ChAPA Herbarium of the Colegio de Postgraduados in Montecillo campus.
Structure of the alpine treeline ecotone of Pinus hartwegii
Each plot was georeferenced (Garmin GPSmap® 64s) to calculate the average altitude (altitudinal level) and to establish the treeline ecotone´s limits. In all plots, tree height, normal diameter (ND) and tree density were determined through the moving split-window method adapted from Camarero and Fortin (2006). This method consists of calculating the difference using Euclidean distances squared, between two halves of a moving window that runs along the transect.
At following, altitudinal distribution model of the upper treeline ecotone of Pinus hartwegii was obtained in the Idrisi Selva software (Eastman, 2012). Altitudinal dimensions and limits of the treeline ecotone of Pinus hartwegii were defined via a supervised classification based on georeferenced points, which were also used to confirm the generated model. Finally, by using the data generated by the elevation distribution model, the form of treeline ecotone was characterized according to Harsch and Bader (2011), who recognized four possible forms: (I) Diffuse: characterized by a gradual decrease in height and tree density along the treeline ecotone; (II) Abrupt: characterized by a continuous forest (>3 m tall) directly bordering low alpine vegetation. Tree height and density thus changes rapidly; although trees may be present above the continuous forest, but their presence is infrequent; (III) Island: characterized by clumped patches or linear strips of Krummholz or trees above the continuous forest limit; or (IV) Krummholz: characterized by severely stunted or deformed multi-stemmed trees. This growth form can occur in clumped patches above the upright forest, in which case we class the treeline as an island treeline, or it can occur as a dispersed or contiguous band above the upright forest.
Statistical analysis
The Shapiro-Wilk test and the homoscedasticity by the Levene test checked the normality of the data given the number of samples (Rahman and Govindarajulu, 1997). A one-way analysis of variance (ANOVA) was carried out with tree density and species diversity (H') as the main factors and with altitudinal level as the classification factor and was followed by Tukey’s multiple comparison test (Zar, 1998). A linear regression was applied for the associations of the altitude-height and altitude-ND variables. Life forms diversity was evaluated through calculating their proportional distribution per transect and per altitude strata. All statistical analyses were performed in JMP8 (SAS, 2008) at a 95 % confidence level.
Results
Life forms diversity and richness
A total of 43 plant species belonging to 30 genera and 18 botanical families were identified Pinus hartwegii treeline ecotone in Nevado de Toluca. Asteraceae (12 species) and Poaceae (8 species) families had the highest diversity and abundance (Table 1). Dominant species were Calamagrostis tolucensis (Kunth) Trin. ex Steud., Agrostis tolucensis Kunth, Festuca tolucensis Kunth, among other (Table 1). Diversity accumulation curves were saturated for the two altitudinal levels (Figure 2a-b). Sample coverage reached an asymptote with the sample effort employed in all altitudinal levels (Figure 2c-d). Sample coverage on Hill Shannon diversity was on average ≥98 % for all altitudinal level (Figure 2e-f).
Table 1 Families and species present in the alpine treeline ecotone of Pinus hartwegii Lindl. at Nevado de Toluca Flora and Fauna Protection Area.
| Family Species |
RA (%) by altitudinal level | LF | |||||
|---|---|---|---|---|---|---|---|
| Bos1 | Bos2 | Bos3 | Pas4 | Pas5 | Pas6 | ||
| Alliaceae Allium L. sp. | 0 | 2.23 | 0.90 | 0 | 0.09 | 0 | Chamaephytes |
| Apiaceae Eryngium monocephalum Cav. | 3.00 | 2.23 | 5.28 | 6.75 | 9.84 | 10.71 | Hemicryptophytes |
| Asteraceae Conyza coronopifolia Kunth | 1.22 | 4.93 | 6.79 | 1.44 | 0.68 | 0.67 | Chamaephytes |
| Conyza schiedeana (Less.) Cronquist | 0.13 | 0.58 | 1.20 | 0.24 | 0 | 0 | Chamaephytes |
| Erigeron galeottii (A. Gray) Greene | 0.13 | 0.23 | 0 | 0.24 | 0 | 0 | Hemicryptophytes |
| Eupatorium prunellifolium Kunth | 0 | 0.23 | 0 | 0.36 | 0.38 | 0 | Hemicryptophytes |
| Gnaphalium lavandulaceum DC. | 0 | 0 | 0.60 | 2.77 | 3.11 | 1.25 | Chamaephytes |
| Gnaphalium liebmannii Sch. Bip. ex Klatt | 0 | 0 | 0 | 0.60 | 1.75 | 1.83 | Hemicryptophytes |
| Gnaphalium oxyphyllum DC. | 0.27 | 0.82 | 3.02 | 1.44 | 1.85 | 2.12 | Hemicryptophytes |
| Gnaphalium sarmentosum Klatt | 0.13 | 1.52 | 1.66 | 2.89 | 1.26 | 2.60 | Hemicryptophytes |
| Hieracium dysonymum S. F. Blake | 2.18 | 1.52 | 2.87 | 2.53 | 4.38 | 3.57 | Hemicryptophytes |
| Hieracium pringlei A. Gray | 0.81 | 0.35 | 1.35 | 0.72 | 1.65 | 2.02 | Hemicryptophytes |
| Senecio mairetianus DC. | 0.68 | 1.29 | 1.05 | 1.32 | 0.38 | 0.48 | Phanerophytes |
| Senecio roseus Sch. Bip. | 0 | 0.35 | 0 | 0 | 3.89 | 0.38 | Hemicryptophytes |
| Asparagaceae Echeandia durangensis (Greenm.) Cruden | 0 | 0.23 | 0 | 0.12 | 0 | 0 | Chamaephytes |
| Brassicaceae Draba L. sp. | 0.95 | 0.58 | 0.45 | 0.12 | 1.85 | 1.15 | Chamaephytes |
| Draba jorullensis Kunth | 0.13 | 1.41 | 1.05 | 1.20 | 3.99 | 1.73 | Chamaephytes |
| Draba tolucensis Kunth | 0 | 0.82 | 0.15 | 0.84 | 2.14 | 0.77 | Chamaephytes |
| Caryophyllaceae Arenaria lanuginosa (Michx.) Rohrb. | 0 | 0.23 | 0.60 | 1.56 | 1.94 | 1.25 | Hemicryptophytes |
| Arenaria bryoides Willd. ex D. F. K. Schltdl. | 0.81 | 2.23 | 2.11 | 6.99 | 3.70 | 6.08 | Chamaephytes |
| Euphorbiaceae Euphorbia L. sp. | 10.38 | 22.56 | 6.04 | 6.27 | 2.14 | 6.56 | Chamaephytes |
| Fabaceae Lupinus aschenbornii S. Schauer | 2.45 | 6.81 | 4.38 | 7.84 | 9.25 | 9.26 | Terophytes |
| Lupinus montanus Kunth | 1.63 | 3.29 | 1.51 | 4.82 | 3.60 | 5.21 | Terophytes |
| Lupinus L. sp. | 0.13 | 0.70 | 0.15 | 0 | 0 | 0 | Terophytes |
| Gentianaceae Halenia plantaginea (Kunth) G. Don | 0 | 0.35 | 0 | 0.96 | 0.19 | 2.60 | Hemicryptophytes |
| Geranium L. sp. | 0 | 0.11 | 0.15 | 0 | 0 | 0 | Chamaephytes |
| Juncaceae Luzula racemosa Desv. | 0 | 0.35 | 0.90 | 3.61 | 2.82 | 3.76 | Chamaephytes |
| Orobanchaceae Castilleja lithospermoides Kunth | 0.13 | 0 | 0.30 | 0.36 | 0 | 0.09 | Chamaephytes |
| Oxalidaceae Oxalis alpina (Rose) Rose ex R. Knuth | 24.31 | 11.75 | 8.30 | 13.51 | 15.20 | 13.80 | Cryptophytes |
| Plantaginaceae Penstemon gentianoides (Kunth) Poir. | 0.54 | 3.52 | 0.45 | 0.24 | 0 | 0 | Phanerophytes |
| Plantago tolucensis Pilg. | 0.54 | 0 | 0.60 | 0 | 0.58 | 0.19 | Chamaephytes |
| Poaceae Agrostis tolucensis Kunth | 2.04 | 1.99 | 6.79 | 1.80 | 2.24 | 1.93 | Chamaephytes |
| Blepharoneuron tricholepis (Torr.) Nash | 1.22 | 0.23 | 1.20 | 0.12 | 0 | 0.57 | Chamaephytes |
| Calamagrostis orizabae (Rupr. ex E. Fourn.) Beal | 0.81 | 0.58 | 1.51 | 0.48 | 0 | 0.19 | Chamaephytes |
| Calamagrostis tolucensis (Kunth) Trin. ex Steud. | 23.08 | 16.45 | 22.20 | 17.73 | 13.45 | 12.93 | Chamaephytes |
| Festuca L. sp. | 4.50 | 0.23 | 4.07 | 1.44 | 0.29 | 0.67 | Chamaephytes |
| Festuca tolucensis Kunth | 7.65 | 5.05 | 3.02 | 4.10 | 5.94 | 5.11 | Chamaephytes |
| Poa annua L. | 0.13 | 0.11 | 0.15 | 0.12 | 0 | 0.09 | Chamaephytes |
| Trisetum spicatum (L.) K. Richt. | 3.00 | 1.29 | 2.56 | 2.53 | 0.87 | 0.96 | Chamaephytes |
| Rosaceae Alchemilla vulcanica Schltdl. & Cham. | 0 | 1.99 | 4.83 | 1.56 | 0.09 | 0.09 | Chamaephytes |
| Potentilla richardii Lehm. | 6.83 | 0.35 | 1.05 | 0 | 0.09 | 0.09 | Chamaephytes |
| Solanaceae Physalis L. sp. | 0 | 0.35 | 0.60 | 0.24 | 0.19 | 0.09 | Chamaephytes |
| Pinaceae Pinus hartwegii Lindl. | 19.90 | 13.40 | 15.80 | 4.20 | 1.30 | 0.50 | Phanerophytes |
Relative abundance (RA, %) by altitudinal level and life form (LF) of registered species. Bold numbers indicate the highest abundance values.
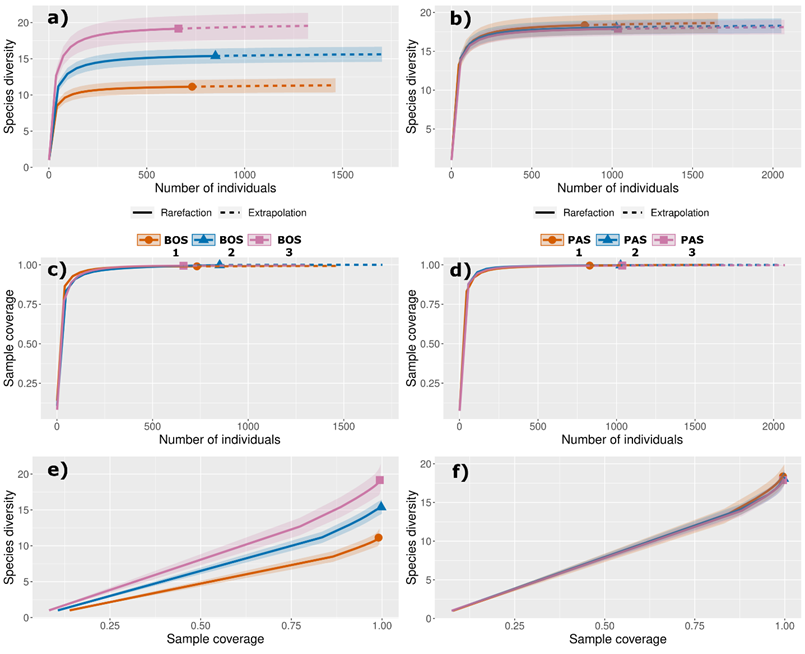
a-b = Hill Shannon diversity as a function of number of individuals (i. e., sample-sized-based); c-d = Sample coverage for rarefied samples as a function of number of individuals; e-f = Hill Shannon diversity as a function of sample coverage (i. e., coverage-based for each altitudinal level). Continuous lines represent interpolation (i. e., observed) and dashed lines represent extrapolation (i. e., expected) of species diversity.
Figure 2 Diversity accumulation curves for each altitudinal level.
Altitudinal gradients and life forms composition
Bunch grasses had highest cover throughout the ecotone, and Calamagrostis tolucensis (20-60 % cover), Festuca tolucensis (10-30 %), Luzula racemosa Desv. (10-35 %), and Agrostis tolucensis (2-15 %) were the most dominant in all plots. Chamaephytes were the dominant life form throughout Pinus hartwegii treeline ecotone (56 % of total recorded species), followed by hemicryptophytes (26 %), phanerophytes (16 %), and cryptophytes (2 %). Cover of life forms changed as increasing altitude, phanerophyte cover decreased by 91 %, while chamaephyte cover increased by 54 % (Figure 3a). However, the proportion of phanerophyte cover reduced by 94 %, and chamaephyte remained stable along the altitudinal gradient (Figure 3b).
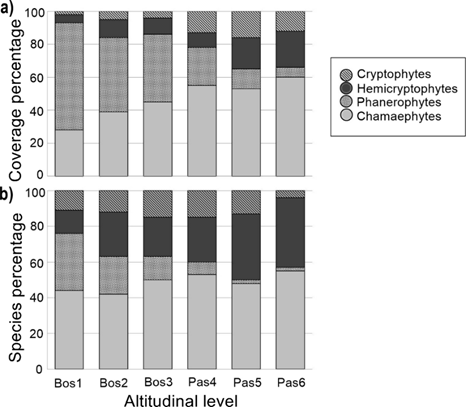
a = Dominant life forms per altitudinal level in forest (Bos1, Bos2, Bos3) and grassland (Pas4, Pas5, Pas6); b = Species belonging to different life forms in Pinus hartwegii Lind. treeline ecotone at Nevado de Toluca.
Figure 3 Relative covers.
Highest diversity (H’=2.13) was recorded at altitudinal level Pas6 (4 096 m), while the lowest diversity (H’=1.5) was recorded at altitudinal level Bos1 (4 043 m; Figure 4). Shannon diversity index was positively related with increasing altitude at plots with a northwesterly aspect (F=15.22, P=0.0022) and at plots with an easterly-southeasterly exposure (F=8.47, P=0.0012; Figure 5). Diversity of sample sites also differed significantly (F=3.67, P=0.017); sites 1, 2, and 3, which had an easterly-southeasterly exposure, had greatest diversity (Figure 5).
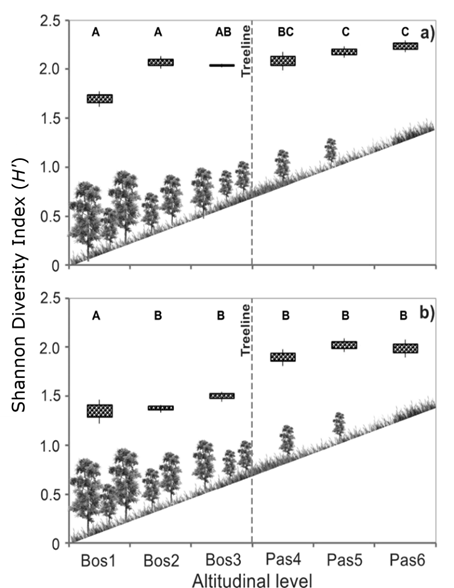
a = Easterly-southeasterly aspect; b = Northwesterly aspect. Means with common letter are not significantly different (P≤0.05).
Figure 4 Shannon’s diversity index (H’) of plant life forms by altitudinal level, in forest (Bos1, Bos2, Bos3) and grassland (Pas4, Pas5, Pas6) covers at sampling sites.
Characterization of ecotone structure
The lower limit of Pinus hartwegii treeline ecotone of Nevado de Toluca was located at 3 980 to 4 090 m and its upper limit at 4 030 to 4 130 m. Tree density decreased significantly along the altitudinal gradient from 70 % at the lower limit to 8 % at the upper limit, indicating that Pinus hartwegii conforms a diffuse ecotone where in the tree density gradually decrease as altitude increases. Forested area of altitudinal level Bos1 (4 043 m) had the highest average tree density (925 trees ha-1), while altitudinal level Pas6 (4 096 m) had the lowest average tree density (25 trees ha-1). Tree height and ND were also significantly and negatively related with increasing altitude (r 2 =0.21, P=0.0001; r 2 =0.12, P=0.0001, respectively). Tree height reduced by 48 % from the lowest to the highest altitudinal range, and the ND also reduced by 20 %. Along the altitudinal gradient of the ecotone, tree height ranged from 0.3 to 22.0 m, and average tree height was 3.88 m. Both shortest and tallest trees were recorded at 4 043 m (Bos1). Overall, ND of trees ranged from 1.0 to 72.0 cm, and average ND was 11.82 cm. Largest trees ranged from 50 to 72 cm in diameter and were recorded at the Bos1 (4 043 m) and Bos2 (4 050 m) altitudinal levels.
Diffuse structure of the Pinus hartwegii ecotone was characterized by an increasing diversity and cover of high-mountain grasses species, such as Eryngium monocephalum Cav., Lupinus aschenbornii S. Schauer, Arenaria bryoides Willd. ex D. F. K. Schltdl., Oxalis alpina (Rose) Rose ex R. Knuth, Draba tolucensis Kunth, and Poa annua L., among others, with increasing altitude (Table 1). These species increased in abundance by up to 80 % over the altitudinal gradient, and their cover also increased by up to 90 %. Species accompanying Pinus hartwegii at lower altitude levels, including Festuca tolucensis and Trisetum spicatum (L.) K. Richt. gradually reduced their cover by 10 to 40 % with increasing altitude. Meanwhile, species such as Calamagrostis tolucensis and Gnaphalium sarmentosum Klatt gradually increased their cover by 5 to 30 % with increasing altitude. Similarly, the abundance of species such as Poa annua and Draba tolucensis above the forest line, which were recorded in plots Pas4, Pas5, and Pas6 (4 072 to 4 096 m), gradually increased by 10 to 35 % as altitude increase. Other species such as Senecio mairetianus DC. and Castilleja lithospermoides Kunth had a relatively stable relative abundance and cover along the altitudinal gradient of the treeline ecotone of Pinus hartwegii.
Discussion
Pinus hartwegii, native to the mountains of Mexico (Calderón de Rzedowski and Rzedowski, 2005), is therefore an ideal model for studying the mechanisms that regulate the survival of tree life forms at high altitudes (Körner and Paulsen, 2004; Pérez-Suárez et al., 2022) and the impacts of climate change on species distribution. At the Nevado de Toluca, Pinus hartwegii forms a diffuse ecotone that is characterized by the convergence of tree life forms, or therophytes, and herbaceous life forms, or chamaephytes, such as Calamagrostis tolucensis, which dominates high-mountain grasses (Calderón de Rzedowski and Rzedowski, 2005). The capacity of this transition zone to respond to changes in environmental conditions is closely related with the composition and proportion of life forms along the altitudinal gradient.
The study of plant life forms makes it possible to identify the interactions between species and environment (Treml and Chuman, 2015; Kark, 2017; Körner, 2017), as well as their morphological and physiological adaptations (Heslop-Harrison, 2017; dos Santos et al., 2022), which allows to observe the selection and adaptation processes that could regulate the survival of plant species at increasing temperatures (Körner and Paulsen, 2004; Brooker et al., 2008). In the present study, therophytic life forms such as Lupinus montanus Kunth and L. aschenbornii were found to survive under unfavorable conditions in seed form; these species then subsequently emerge or germinate when environmental conditions are more favorable (Parmesan, 2006). The effects of increasing temperatures on the survival of distinct species, including those associated with Pinus hartwegii, are important to understand and to assess the potential response of species to climate changes (Ramírez-Contreras and Rodríguez-Trejo, 2009).
Notably, herbaceous life forms have developed adaptations that allow them to germinate, establish, and grow in shallow soils with scarce nutrients and freezing temperatures (Kozlowski and Pallardy, 2002; Körner et al., 2021). These life forms are also able of modifying the microsites where they establish (Badano et al., 2015), thereby directly or indirectly favoring the establishment of other life forms (Brooker et al., 2008; Anthelme et al., 2014). Herbaceous plants may create viable microsites for other species and facilitate the colonization process of more complex life forms such as Pinus hartwegii (Ramírez-Contreras and Rodríguez-Trejo, 2009). Thus, life forms proportion along an ecotone can potentially change the structure of an ecosystem and its processes (Badano et al., 2015; Körner, 2017) and influence altitudinal migration of species (Aitken et al., 2008).
Density and diversity of plant life forms observed in our study within of Pinus hartwegii treeline ecotone at Nevado de Toluca evidence the diffuse form of this ecotone. This ecotone is characterized by a gradual reduction in density of trees and a gradual increase in plants of smaller dimensions such as herbaceous plants and bunch grasses (Harsch and Bader, 2011; Alfaro-Ramírez et al., 2017). Diffuse ecotones are the most common around the world (Harsch and Bader, 2011), and a frequent altitudinal variation have been reported within these ecotones (Bader et al., 2007), which suggest an increase in facilitation relationships between herbaceous and forest species (Callaway et al., 2002; Brooker et al., 2008; Badano et al., 2015), as well as changes in composition and life forms proportion in the altitudinal gradient (Callaway, 2007; Arzac et al., 2011).
Along the treeline ecotone of Nevado de Toluca, chamaephytic life forms are dominant. Chamaephytes can preserve their buds under a layer of dry leaves and can more quickly emerge during favorable climate conditions (Kozlowski and Pallardy, 2002) than plants found in seed form (Parmesan, 2006; Maestre et al., 2009). In the study area, greatest number of recorded species belonged to the Asteraceae family, species that are chamaephytic in life form.
Species diversity was greatest above the forest line in areas with predominant grass cover. This is partially due to the transition between ecosystems and the convergence of species at their distributional limits, which led to high species richness (Cavieres and Badano, 2009; Körner, 2017). However, some species as Muhlenbergia hintonii Swallen, M. vaginata Swallen and Agrostis calderoniae Acosta Cast. associated with Pinus hartwegii in the forest nucleus (Calderón de Rzedowski and Rzedowski, 2005), were not found within the treeline ecotone. In addition, some species abundant at the lower portion of the ecotone, such as Festuca tolucensis and Trisetum spicatum, were also found at higher altitudes, although their abundance and cover decreased with increasing altitude.
In contrast, the abundance of other species, such as Calamagrostis tolucensis and Gnaphalium sarmentosum, decreased at low altitude yet increased at higher altitudes. Consequently, as altitude increases, the coverage of small, cold-resistant species, including several bunch grasses, also increases (Calderón de Rzedowski and Rzedowski, 2005; Arzac et al., 2011). In a similar way, the diversity of cushion plants, such as Arenaria bryoides, increased with increasing altitudes, as well as those that form rosettes, such as Gnaphalium sarmentosum and Hieracium dysonymum S. F. Blake (chamaephytic plants). These plant forms are adapted to environmental conditions such as extreme low temperatures and intense winds, which are frequent at high altitudes (Körner, 2017; Körner et al., 2021).
In the present study, the dominant group of chamaephytes may regulate microsite conditions (Cavieres and Badano, 2009; Heslop-Harrison, 2017; dos Santos et al., 2022) and may possibly facilitate the establishment of other groups, thereby compensating for the negative effects of extreme environmental conditions (Brooker et al., 2008). As climate changes, chamaephytes that have a facilitative relationship with forest species such as Pinus hartwegii could enable these species to establish at increasingly higher altitudes (Maestre et al., 2009; Ramírez-Contreras and Rodríguez-Trejo, 2009). Even so, altitudinal migrations are extremely complex and depend on numerous factors that could also potentially limit the capacity of Pinus hartwegii to colonize new sites at higher altitudes. This relationship is evidence that particular species and life forms that can favor the colonization process of Pinus hartwegii at new sites and at greater altitudes in Nevado de Toluca, especially given the changing climate.
Conclusions
The diversity and distribution of life forms along alpine treeline ecotones influence the capacity of species present in these environments to react to changing environmental conditions. Ecotones with a diffuse structure are the most abundant around the world, such as that of treeline of Pinus hartwegii in Nevado de Toluca. Altitudinal advance and recession of plants has been frequently reported in such diffuse ecotones, favoring the increase in the diversity of life forms found in this transition zone. The present study found a consistent pattern in the life forms accompanying Pinus hartwegii along its altitude gradient. As altitude increases, the abundance of forest species along the altitudinal gradient of Pinus hartwegii reduces, and P. hartwegii is increasingly accompanied by high-mountain grasses indicating a potential relationship between life forms yet not necessarily between species. However, Lupinus montanus, Calamagrostis tolucensis, Festuca tolucensis, and Arenaria bryoides, presented important relationships with the presence of Pinus hartwegii. Considering the changing climate in central Mexico, Pinus hartwegii could be benefitted by certain life forms like chamaephytes that encourage its establishment at increasingly higher altitudes. The results of this work show a close relationship between chamaephytes and terophytes in the Pinus hartwegii treeline ecotone, so it would be valuable to assess further this interaction between life forms.











 texto em
texto em 





