Introduction
Selenium (Se) deficiency in ruminants is a common problem in various grazing areas worldwide. The content of Se varies with the type of soil. Consequently, forages could have low amounts of this mineral1. Se deficiency causes diseases such as nutritional muscular dystrophy, anemia, mastitis, placental retention and infertility2-4. Nutritional muscular dystrophy has been reported as one of the leading causes of death in kids from 28 to 90 d of age in the Mexican plateau5. Se supplementation prevents deficiency disorders in animals6. Selenium can be administered with inorganic compounds such as selenite (Na2SeO3) and sodium selenate (Na2SeO4) or organic forms such as selenomethionine (C5H11NO2Se) and selenocysteine (C3H7NO2Se), widely available in yeast cultures7,8. The NRC9 recommends the amount of 0.2 mg of Se per kilo of dry matter in small ruminants. However, animal needs can be met through injectable solutions or extended-release forms, such as intraruminal boluses10-12. Intraruminal boluses have the advantage of continuously releasing of the required dose of Se for long periods; they are administered orally, remaining in the reticulo-rumen when the size and density are adequate13,14. The adequate dosage of Se is reflected in the body with the increase in Se levels in the blood15.
Selenium deficiencies can cause an increase in free radicals, generating an imbalance known as oxidative stress (OE). The excessive formation of reactive oxygen species (ROS) and the inhibited antioxidant system causes damage to molecules such as phospholipids, proteins and DNA16. Antioxidants prevent ROS formation, intercepting the formation of reactive species, eliminating molecules damaged by apoptosis, capturing reactive metabolites, and converting them to less or non-toxic products17,18. ROS can cause lipoperoxidation, DNA mutations, and protein inactivation, causing disorders in cell metabolism and contributing to the appearance of diseases in animals19. Lipoperoxidation damages cell membranes, receptors, and enzymes, increasing permeability and causing cell death. Lipoperoxidation products, such as unsaturated aldehydes, inactivate proteins, leading to DNA adducts and mutagenesis20,21.
There are different forms against ROS, such as low molecular weight oxidant scavengers (ascorbic acid, tocopherols, urates, thiols), enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), catalase (CAT), pyridoxines, reductases of methionine sulfoxide (MetSO), disulfide reductases, sulfiredoxins, proteasomes, lysosomes, DNA repair enzymes, phospholipases and lastly, non-enzymatic defenses like reduced glutathione (GSH)21,22. The selenoproteins are distributed in the body, catalyzing oxidation-reduction reactions23 and protecting against oxidative damage. Oxidative stress in the body can be measured in the blood, through the concentration and activity of antioxidants that circulate in this tissue22,24, or with the measurement of the products formed by the oxidation of molecules, for example, aldehydes like the thiobarbituric acid reactive substances (TBARS)20.
Oxidative stress biomarkers indicate the oxidative balance in the body25. The deficiency or administration of Se to animals modifies the concentration of biomarkers such as GSH and TBARS26. When Se levels are low and oxidative stress levels increase, GSH levels rise to prevent cellular liperoxidation19,27. Finally, old animals increase their erythrocyte levels GSH to control OE as a result of age, unlike young animals28. On the other hand, it has been observed that when there is an increase in aldehyde concentrations, it is due to the decrease in antioxidant activity29. Kohen et al30 observed a positive correlation between the increase in TBARS, lipoperoxidation and cellular damage in the organism. Elsheikh et al31 supplemented Se in goats, measured concentrations of TBARS such as malondialdehyde (MDA) and observed that supplementation reduces the levels of this aldehyde, improving the activity of antioxidants. This review hypothesized that an association may exist between the levels of oxidative stress biomarkers in the blood of goats with and without Se supplement. The objective of this study was to evaluate the effect of Se administered through intraruminal boluses in goats and to correlate it with the levels of the mineral and biomarkers of oxidative stress in blood.
Material and methods
Preparation of intraruminal boluses
The manufacture of the Se and placebo boluses was carried out by granulation by fusion14. Sodium selenite was used as a source of selenium, and sodium sulfamethazine was used as a source for sulfamethazine, a lipid excipient to control the release of the active principles and a densifier excipient to achieve adequate density. Boluses of sodium selenite at 12 g weight, 1.4 cm wide, 5 cm long and 0.95 thickness measurements, boluses Se-SMZ at 20 g weight, 2.1 cm wide, 5.2 cm long and 1.3 thickness measurements and boluses placebo at 12 g weight,1.3 cm wide, 4.5 cm long and 1.2 thickness measurements.
Animals and treatments
Fifteen (15) goat kids from 8 to 9 wk old from the goat production module of the Facultad de Estudios Superiores Cuautitlán (FESC) of Alpine breed with an average weight of 13.7 kg were used. The goat kids were isolated for adaptation in the pens of the FESC experimentation unit; after one month of adaptation the kids were randomly divided into three groups: Selenium group (n= 5), a sodium selenite bolus was administered per animal orally, with a content equivalent to 90 mg of Se: group Se-SMZ (n= 5). A bolus with sodium selenite and sodium sulfamethazine was administered per animal, with a content equivalent to 90 mg of Se and 4 g of sulfamethazine: Placebo group (n= 5); placebo bolus was administered per animal orally, without any active ingredient. The experiment was carried out with the approval of the Internal Committee for the Care and Use of Experimental Animals CICUAE FESC C11_02.
Sampling
Blood samples were collected by puncturing the jugular vein using sterile BD Vacutainer® needles 20G- X 38mm and 6 mL vacuum tubes with Heparin Vacutainer® BD; the samples were collected on d-0 (before bolus administration), 1, 3, 5 and 24 h, the days 2, 4, 8, 11, 18, 25 and 32 after bolus dosification. The samples taken were stored at 4 ºC, once in the laboratory, the samples were centrifuged at 2,500 rpm for 15 min. The plasma obtained was separated in microtubes and stored at -20 ºC until its analysis.
Quantification of Se in plasma
For the quantification of Se in plasma, 0.5 g of each sample was weighed into a microwave Teflon beaker, and acid digestion was carried out: 5 mL of Milli Q water, 2.5 mL of nitric acid (55 %) and .01 mL of H2O2 at 30 %, left for 30 min at room temperature and digestion was carried out in a MARS microwave oven-EMC digestion. The digested samples were transferred into 25 mL flasks and were brought to capacity with 7M HCl. The samples were evaluated in a Varian® hydride generator atomic absorption spectrophotometer and compared with the reference curve.
Quantification of TBARS
One hundred (100) µL of plasma were taken, 100 µL of 2.5 % perchloric acid were added and it was left at room temperature for 10 min; they were centrifuged for 15 min at 1,200 rpm at 4 °C, 100 µL of the supernatant were mixed with 100 µL of thiobarbituric acid 0.67 %; they were incubated at 90 °C for 30 min. The samples were read on a UVvisCary100 Varian® spectrophotometer at a wavelength of 532 nm.
Quantification of reduced glutathione GSH in erythrocytes
A methodology was implemented to evaluate the content of GSH using the Ellman reagent32. An aliquot of 400 µL was taken from each sample, 400 µL of 5 % sulfosalicylic acid were added to them, they were left at room temperature for 30 min at 4 ºC, they were centrifuged at 13,500 rpm 4 ºC for 15 min. An aliquot of 200 µL was taken of each of the supernatants and placed in a polystyrene Medium Bindding costar® microplate, 100 µL of the reaction mixture (0.52 mM DTNB and 0.15 mM EDTA) were added and left at room temperature for 5 min. The microplate reader (96 wells) by mrcScientific Instruments® was used to read samples at 405 nm.
Statistical analysis
The data were analyzed as a completely randomized design with a 3 × 12 factorial arrangement, using a repeated measures analysis over time. The independent variables were treatment and sampling time, and the dependent variables were blood selenium concentration, TBARS levels, and GSH levels. The analysis of results was carried out with the statistical software Statgraphics Centurion XV, version 15.2.05. Statistical significance considered for all comparisons of P<0.05. The following model was used:
Where:
Y ijk = variable response,
μ= general mean,
Ti = effect of treatment (Intraruminal boluses treatment),
S j = effect of sampling time,
(T× S) ij = effect of T × S interaction at i, j levels,
ξ ij = random error.
Results
In the study, the 0-hour measurement includes the average of all the animals. After administering boluses of Selenium and Se-SMZ orally to the goat kids, the hematic Se levels increased from 3 h after dosing. This caused a significant difference (P<0.05) between the Selenium group and the Se-SMZ group until 3 and 24 h. Placebo group had a mean of 0.18 µg Se in plasma before 24 h. After this time, Se levels was variable decreased up to 32 d (Figure 1).

Figure 1 Selenium concentrations in plasma during 1 to 24 hours (bars on the left) and 2 to 32 days (bars on the right)
All groups had variability in plasma TBARS levels without showing a constant trend among groups. Mainly, the Placebo group had a lower content of plasma TBARS at 1 h after dosing. However, at 24 h after dosing, the range of plasma TBARS increased vs selenium group and Se-SMZ (P<0.05). Se-SMZ group had the highest TBARS level at 2, 4, 8 and 11d vs Selenium and Placebo groups, then all groups decreased plasma TBARS levels up to 32 d without significant difference (P>0.05) (Figure 2).

Figure 2 Plasma TBARS levels during 1 to 24 hours (bars on the left) 2 to 32 days (bars on the right)
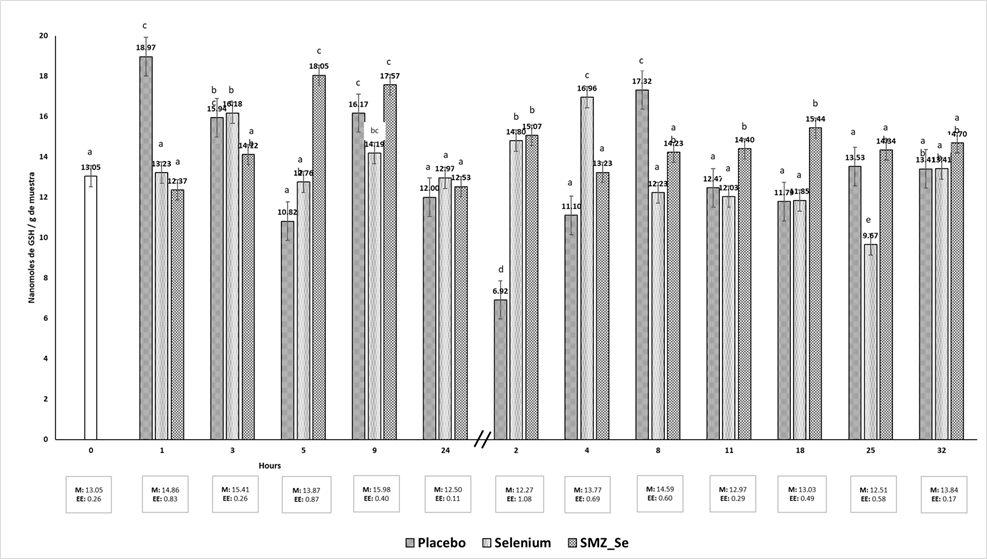
GSH levels did not show relevant significant differences between groups. The placebo group had the lowest (6.92 nmol) and the highest (18.97 nmol) GSH content at 2 d and 1 h, respectively. Selenium group had a range of 9.57 nmol (25 d) and 16.96 nmol (4 d). Se-SMZ group had a range of 12.42 nmol (1h) and 17.57 (9 h) (Figure 3).
Discussion
Boluses containing Se and Se-SMZ increased blood plasma Se levels for up to 24 h, followed by a gradual decrease over the next 32 d with no significant difference observed between the three study groups (P>0.05). The placebo group had levels less than 0.1 ng/g Se in plasma at 4, 11 and 25 d. Some authors suggest that the adequate level of Se in goat blood is 0.11-0.12 ng/g33,34. However, Field Pavlata et al35 reported levels of 0.07-0.01 ng/g without observing signs of deficiency. Se levels of 0.02-0.03 ppm are considered inadequate since they cause nutritional muscular dystrophy33. The animals in this study showed no signs of Se deficiency during the clinical examination. After taking Se supplements, no signs of toxicity were observed in the got kids. Plasma Se values are lower than those in erythrocytes but show greater variability over time due to immediate tissue distribution35,36. According to the study by Field Stefanowicz et al37, the amount of selenium in the plasma is commonly used as a reliable indicator of its levels in the body. Although, the selenium content in the whole blood is also measured, it should be noted that the risk of hemolysis can lead to false readings in the plasma. This is because selenium is present in red blood cells. Therefore, it is important to exercise caution while collecting samples from animals38. On the other hand, boluses are an alternative to supplement Se to ruminants. In this study, the bolus remained active in kids for up to 32 d, reaching maximum levels of 0.8 ng/g of Se in plasma. Other studies in sheep indicate that boluses with Se maintained an active release for up to 3 mo with hematic Se levels of 148 and 350 ng/g Se, respectively39,40. The difference perhaps was due to the types of materials that were used in the manufacture of intraruminal boluses since the density of the boluses influences the permanence of the bolus in the reticulo-rumen avoiding regurgitation. There is little information associated with the release of Se, the thickness and the types of materials used to design the boluses.
TBARS concentration is a method used to estimate oxidative stress. In this study, there was wide variability in plasma TBARS levels without showing a trend between treatments. All groups decreased TBARS levels up to 32 d without significant difference (P>0.05). Elsheikh et al31, supplemented Se in goats and measured oxidative stress biomarkers. They observed that TBARS concentration was reduced, and antioxidant activity improved in the animals. On the other hand, Chung et al41 supplemented Se in goats, indicating a lack of significant differences in the concentrations of TBARS in the intestine, serum, liver and muscle, between the supplemented groups and the control data similar to the present study. Various factors can increase lipoperoxidation in goats, such as peripartum, lactation, diet, antioxidant supplementation19,34. The presence of pathologies, exposure to toxins or administration of drugs, increase the levels of TBARS in the animal organism42. It is essential to mention that reactive oxygen species are formed in a normal physiological state, a consequence of the metabolic process24,30,43, where the variations depend on the metabolism of each organism, a significant increase in TBARS is associated with a deficiency in the antioxidant system. Shi et al34 report a higher concentration of TBARS in selenium deficient animals than animals supplemented with different sources of Se. In this study, the experimental units did not present pathologies associated with stress, and an apparent effect was not observed in animals supplemented with Se and the concentrations of TBARS.
GSH levels also did not show relevant significant differences between treatments. Celi19 mentions that in Se deficiency, the synthesis of GSH increases, a physiological process where the requirement of cysteine is increased until it is exhausted, once depleted, the GSH decreases.
A decrease in GSH in the blood has been reported in diseases44-46. Reduction of GSH levels in the blood is also associated with increased GSH-Px activity and utilization in the GST-catalyzed reaction46. Gresakova et al47 supplemented Se in small ruminants with a history of deficiency, observing a correlation between Se concentration and GSH-Px activity; the decrease in the activity of this enzyme can lead to oxidative stress. The lack of variation in the estimation of GSH, perhaps due to an increase in the levels of oxidative stress, due to the low levels of Se in plasma, triggering a more significant cellular peroxidation, tends to increase the levels of GSH48. For example, in old and Se-deficient animals the erythrocyte GSH increases28.
Conclusions and implications
Selenium boluses and Se-SMZ increased plasma Se levels. There was wide variability in plasma TBARS levels, showing no trend between treatments. There were no pathologies associated with stress, and an apparent effect on supplemented Se and TBARS concentrations was not observed. GSH levels also did not show relevant significant differences between treatments. The boluses during nine weeks were an excellent alternative to increase Se to ruminants.











 texto em
texto em