Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias pecuarias
versão On-line ISSN 2448-6698versão impressa ISSN 2007-1124
Rev. mex. de cienc. pecuarias vol.11 no.4 Mérida Out./Dez. 2020 Epub 02-Mar-2021
https://doi.org/10.22319/rmcp.v11i4.5171
Reviews
Metabolic origin and bioactive properties of odd and branched-chain fatty acids in ruminants’ milk. Review
a Instituto de Investigación en Ciencias de la Alimentación (CSIC-UAM), Nicolás Cabrera, 9. Universidad Autónoma de Madrid, 28049 Madrid, España.
Milk odd and branched-chain fatty acids (OBCFA) are a group of lipids that represents less than 5 % of the total fatty acids (FA) and that includes a group of molecules, among which the most abundant are the isomers of the pentadecanoic (15:0, iso-15:0 and anteiso-15:0), hexadecanoic (iso-16:0), and heptadecanoic (17:0, iso-17:0 and anteiso-17:0) FA. OBCFA are synthesized by rumen microorganisms from the molecules produced during feed fermentation processes. Recent research indicates the possibility of endogenous synthesis of some odd (15:0 and 17:0) and branched-chain (iso-1:0 and anteiso-17:0) FA. The presence of these FA in milk is influenced by dietary factors, mainly the starch vs fiber proportion, forage to concentrate ratio, and the supplementation with fat sources that change the lipid metabolism, which modifies the OBCFA profile of milk. Milk and dairy products are the main and almost only source of OBCFA in the human diet. Despite their low concentration, OBCFA possess bioactive properties that have been shown in different investigations. This article reviews the metabolic origin, bioactive properties, and most recent nutritional strategies directed to manipulate the contents and profiles of OBCFA in milk fat.
Key words Ruminant; Fatty acids; Milk; Dairy products; Rumen; Lipids
Los ácidos grasos de cadena impar y ramificada (AGCIR) son un grupo de lípidos en la leche que no supera el 5 % de los ácidos grasos (AG) totales, y que agrupa a un conjunto de moléculas entre las cuales los isómeros de los AG pentadecanoico (15:0, iso 15:0 y anteiso 15:0), hexadecanoico (iso 16:0) y heptadecanoico (17:0, iso 17:0 y anteiso 17:0) son los más abundantes. Los AGCIR son sintetizados por microorganismos ruminales a partir de moléculas producidas durante los procesos de la fermentación de alimentos. Investigaciones recientes señalan la posibilidad de síntesis endógena de algunos AG de cadena impar (15:0 y 17:0) y ramificada (iso 17:0 y anteiso 17:0). La presencia de estos AG en la leche está influenciada por factores dietéticos, principalmente con la proporción de almidón vs fibra, la relación forraje/concentrado y la suplementación con fuentes de grasa que generan cambios en el metabolismo lipídico, que inducen modificaciones en el perfil de AGCIR de la leche. La leche y los productos lácteos son la principal y casi única fuente de AGCIR de la dieta humana. A pesar de su baja concentración, los AGCIR representan propiedades bioactivas que han sido puestas de manifiesto en distintas investigaciones. Este trabajo revisa el origen metabólico, las propiedades bioactivas, así como las más recientes estrategias alimenticias dirigidas para manipular los contenidos y perfiles de AGCIR en la grasa láctea.
Palabras clave Rumiante; Ácidos grasos; Leche; Productos lácteos; Rumen; Lípidos
Introduction
The lipids in milk are physically in the form of globules, which form an emulsion with the aqueous phase of milk. Inside these globules reside the triglycerides (TG), which are molecules of esterified glycerol with three FA. TG (more than 95 % of total lipids), and thus, they are mainly responsible for the properties of milk lipids, and their characteristics vary in function of the FA composition. Although milk fat has more than 400 different FA1, only 30 or 40 are present at concentrations higher than 0.1 %. The FA profile of milk and dairy products is mainly related to dietary factors, followed by ruminant species, and, to a lesser extent, genetic factors, milk yield, and lactation status.
Based on their structure, FA are classified as saturated or unsaturated FA. Most saturated FA have an even number of C atoms, ranging from 4 up to 20 C. Although the most abundant are those with a chain length of 10 to 20 C atoms, the ruminant milk fat is characterized by significant amounts of short-chain FA, especially 4:0 and caproic acid (6:0). Among the unsaturated FA, which can have one to four bonds, the most abundant (15 to 20 %) is oleic acid (cis-9 18:1). The presence of small amounts of linoleic (2 %) and α-linolenic (0.5 %) acids in milk derives from the diet, and since both are not synthesized in tissues, they are considered essential FA.
Ruminant milk also contains odd and branched-chain FA (OBCFA). Those with an odd number of C atoms represent 2 % of the total FA; 15:0 and 17:0 are the most abundant and representative (Table 1). Branched-chain FA represent a similar proportion and include a higher number of molecules, classified as iso and anteiso, with variable concentrations in dairy products. Although the concentration of OBCFA in fat milk is lower than 5 %, their presence is of great relevance because they work as indicators of ruminal function and, in humans, as indicators of the intake of dairy products. Branched-chain FA, especially anteiso, have lower melting points than their unbranched counterparts; this allows them to contribute to the fluidity of milk fat.
Table 1 Content of odd and branched-chain fatty acids (g/100 of total fatty acids) in dairy products
| Fatty acid |
Milk | Butter | Yogurt | Cream | Cheese | ||
|---|---|---|---|---|---|---|---|
| 7 | 58 | 59 | 43 | ||||
| iso 13:0 | 0.04 | ||||||
| iso 14:0 | 0.05-0.13 | 0.09 | 0.17 | 0.12-0.13 | 0.00-0.05 | 0.00-0.22 | |
| iso 15:0 | 0.14-0.22 | 0.22 | 0.10 | 0.14-0.15 | 0.00-0.11 | 0.02-0.42 | |
| iso 16:0 | 0.21 | 0.34 | 0.29-0.30 | 0.24 | 0.00-1.18 | ||
| iso 17:0 | 0.27 | 0.31 | 0.16-0.25 | 0.27-0.30 | 0.05-0.30 | ||
| iso 18:0 | <0.01 | 0.00-0.04 | <0.01 | 0.00-0.09 | |||
|
anteiso 13:0 |
0.08 | ||||||
| anteiso 15:0 | 0.32-0.45 | 0.46 | 0.63 | 0.62-0.63 | 0.46-0.49 | 0.38-0.88 | |
|
anteiso 17:0 |
0.50 | 0.38 | 0.56-0.59 | 0.36-0.37 | 0.29-0.61 | ||
| 15:0 | 0.84-1.31 | 0.89 | |||||
| 17:0 | 0.45-0.66 | 0.52 | 0.55-0.90 | ||||
Fievez et al7; O´Donnell-Megaro et al58; Shingfield et al59; Ran-Ressler et al43.
Although a great proportion of the OBCFA in milk fat is synthesized during the fermentative processes in the rumen, recent studies have suggested that a small amount could be endogenously synthesized (e.g., mammary gland). Moreover, in the last decade, increasing evidence suggests that OBCFA could have an important role in human health. Therefore, their presence in dairy products should be viewed positively, as these products are almost the only source of these components in the diet. This review aimed to update the information about the origin and synthesis of these FA in ruminants, reviewing the influence of the type of feed on their milk content, and compile evidence on the nutritional benefits of OBCFA in humans.
Origin of odd and branched-chain fatty acids
Ruminal synthesis of OBCFA
The fat in ruminant milk has higher concentrations of OBCFA than the milk of other mammals. Vlaeminck et al2 compiled data from numerous studies about the composition of OBCFA in milk and showed that the main OBCFA are isomers of the tetradecanoic (iso-14:0), pentadecanoic (15:0, iso-15:0 and anteiso-15:0), hexadecanoic (iso-16:0), and heptadecanoic (17:0, iso-17:0 and anteiso-17:0) FA.
The OBCFA are mainly synthesized during the microbial fermentation processes in the rumen. Rumen bacteria contain between 50 and 90 g/kg of lipids in their dry matter, and approximately 5 % of these lipids are OBCFA, which are preferentially located in the membranes3. Protozoa have less total OBCFA than bacteria (110 vs 160 g/kg of total FA), although they possess a higher proportion of iso 16:0 and anteiso 17:04.
The precursors of the microbial synthesis of branched-chain FA in the rumen are leucine, isoleucine, and valine, branched-chain amino acids obtained from the diet (Figure 1). Initially, the rumen microbiota transforms these amino acids into short branched-chain carboxylic acids; isovaleric, 2-methylbutyric, and isobutyric, respectively; linked to Coenzyme A. Subsequently, the microbial FA synthase (FAS) elongates the FA chains. The even-numbered iso FA originate from the isobutyric acid; the odd-chain iso and anteiso FA originate from the isovaleric and 2-methylbutyric acids, respectively. The precursor of medium odd-chain FA (13:0, 15:0, and 17:0) in the rumen is propionic acid, which results from the fermentation of specific ration components, although the 15:0 and 17:0 FA can also originate by α-oxidation from the 16:0 and 18:0 FA in the lipids in the diet.
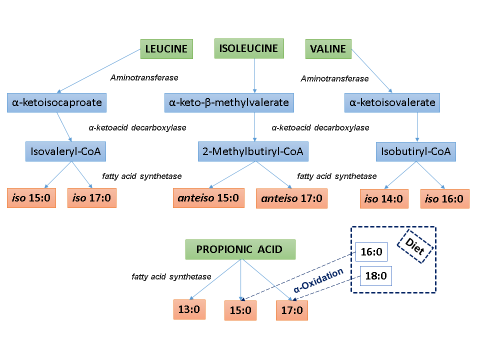
Adapted from Vlaeminck et al2
Figure 1 Synthesis of odd and branched-chain fatty acids by the rumen microbiota
After ruminal digestion, the OBCFA profile is strongly associated with the activity of the microorganisms in this digestive cavity2. Thus, the OBCFA profile variation reflects the relative abundance of the different microbial species in the rumen ecosystem5,6. Cellulolytic bacteria, those with enzymes that hydrolyze cellulose, such as Ruminococcus flavefaciens, Ruminococcus albus, or Butyrivibrio fibrisolvens, possess significant contents of iso OBCFA7. Higher proportions of anteiso-15:0 would indicate the presence of bacteria specialized in the fermentation of pectin and sugars8, such as Prevotella spp., Lachnospira multiparus, and Succinovibrio dextrinosolvens. Amylolytic bacteria, such as Succinivibrio dextrinosolvens, Succinimonas amylolytica, Ruminobacter amylophilus, Selenomonas ruminantium, and Streptococcus bovis, have lower proportions of branched-chain FA, but higher proportions of odd-chain FA.
Transfer of OBCFA from the intestinal tract to the mammary gland
The preponderant role of rumen microorganisms in the presence of OBCFA in dairy products is well known9. However, recent reports2,10 have questioned the strictness of the correlation between the content of OBCFA in the intestinal fluid and the milk fat. Theoretically, disarrangements could occur during the transfer of these FA from the intestinal tract to the internal tissues, particularly in the mammary gland. These disarrangements could occur during the intestinal absorption process or during the transport through the bloodstream. Like other FA that reach the small intestine, OBCFA are absorbed in the jejunum. Apparently, the intestinal absorption of microbial FA would be higher11, but the few available data are not enough to render a definitive conclusion.
After being absorbed, the OBCFA and remaining FA are esterified in the glycerol by the intestinal epithelial cells to form TG and phospholipids (PL), and transported, first to the lymphatic system and then to the bloodstream, where they form part of macromolecular complexes, such as chylomicrons and very low density lipoproteins (VLDL). Chylomicrons and VLDL contain different types of lipids (TG, PL, cholesterol esters (CE), and free fatty acids), but each one differs in composition since each type of FA selectively binds to the different fractions. The transfer of the FA from the bloodstream to the cytoplasm of mammary gland cells occurs after their release from these macromolecules by the lipoprotein lipase enzyme (Figure 2).
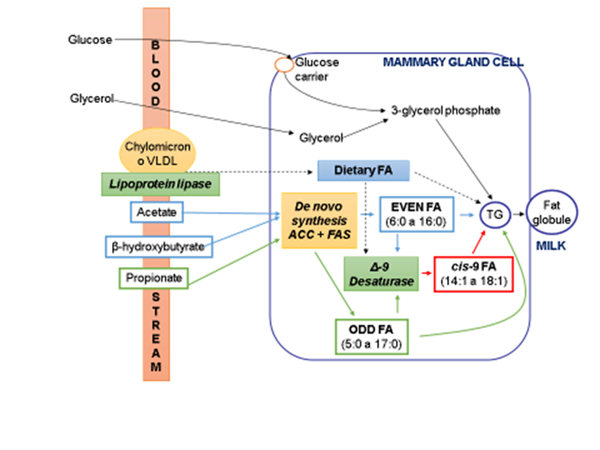
ACC: acetyl-CoA carboxylase; FAS: fatty acid synthase; FA: fatty acid; TG: triglycerides; VLDL: very low-density lipoproteins.
Figure 2 Metabolic pathways of fatty acid synthesis in the mammary gland cells of ruminants
The main targets of the lipoprotein lipase are the FA of the TG. On the contrary, the characteristic FA of the CE and PL fractions are more poorly transferred to milk fat because this enzyme has a low affinity for these FA. Fievez et al7 reported that the branched-chain FA are more abundant in the CE and TG than in the PL or free fatty acids. However, these last two fractions are richer in odd-chain FA. Nevertheless, the available literature about the distribution of OBCFA between the different types of plasma lipids is still too scarce to predict trends or forecast consolidated metabolic behaviors. Therefore, it would be worth exploring the metabolic processes in the mammary gland cells in detail to find the mechanisms responsible for the differences in the OBCFA profiles between rumen fluid and milk.
Endogenous synthesis of OBCFA
Most of the saturated FA with an even number of C atoms in the milk fat are synthesized de novo in the epithelial cells of the mammary gland12. Their synthesis occurs from the blood-circulating acetate and β-hydroxybutyrate molecules generated in the rumen during the fermentation of carbohydrates in the diet. Acetyl-CoA carboxylase (ACC) and FAS are the two enzymes responsible for this de novo synthesis in the mammary gland (Figure 2). The first step in the synthesis consists of the activation of acetate to acetyl-CoA, followed by the condensation of two acetyl-CoA molecules to form malonyl-CoA. This step is catalyzed by the ACC. Subsequently, FAS regulates the chain elongation of the FA synthesized de novo. If the initial substrate instead of acetate was propionate, methylmalonate, or volatile branched-chain FA (isovaleric, isobutyric, and 2-methylbutyric), then the final products of the de novo synthesis would be odd-chained FA, non-terminal methyl-substituted FA, or iso and anteiso, respectively, as it occurs in the rumen (Figure 1).
The first studies in this field demonstrated that 15:0 and 17:0 could be synthesized de novo in the mammary gland of ruminants using propionyl-CoA instead of acetyl-CoA as the primer molecule13. The elongation of this molecule, catalyzed by FAS, would explain the presence in milk of 5:0, 7:0, 9:0, and 11:0, as well as the increase in the amounts of 13:0, 15:0, and 17:0 compared to those already generated in the rumen and transferred from the duodenum. The importance of this endogenous synthesis was confirmed in subsequent studies10,14,15. Theoretically, these odd-chained FA (13:0, 15:0, and 17:0) could also be metabolized to cis-monounsaturated by the delta-9 desaturase enzyme, However, only the conversion from 17:0 to cis-9 17:1 seems to be of quantitative importance16 (Figure 2).
In contrast to odd-chained FA, the mammary synthesis of iso and anteiso FA did not respond to the increase in the availability of its biological precursors, the isovaleric, 2-methylbutyric, and isobutyric FA13,14. This observation would indicate that the FAS might not be active in the elongation process, and thus, the de novo synthesis would not occur in extraruminal tissues. However, these results would contradict the increased content of iso 17:0 and anteiso 17:0 in milk fat, compared to the intestinal fluid, reported by other researchers2,10,17.
Fievez et al7 postulated that the lowest values of the iso 15:0/iso 17:0 and anteiso 15:0/anteiso 17:0 ratios in milk, compared to those in the duodenal fluid, could be explained if the chain elongation of the iso 15:0 and anteiso 15:0 molecules was demonstrated to be viable after being absorbed into the bloodstream. In this sense, it seemed striking that the secretion in the milk of iso 15:0 + iso 17:0 and anteiso 15:0 + anteiso 17:0 was very similar to the sum of these FA in the duodenum7. These data corroborated the hypothesis about the existence of an extraruminal elongase activity on the iso and anteiso FA with 15 C atoms; it also supported the idea of an almost complete transfer of total branched FA from the duodenum to the milk. In a subsequent study, Vlaeminck et al15 observed higher levels of iso 17:0 and anteiso 17:0 in milk fat than in the duodenal fluid (Table 2). This fact, along with lower iso-15:0/iso-17:0 and anteiso 15:0/anteiso 17:0 ratios in milk, would rule out the postruminal de novo synthesis of these FA and confirm the predominant role of postabsorption elongases, which would exert their activity on the iso 15:0 and anteiso 15:0 FA. The lowest value of the iso 15:0/iso 17:0 and anteiso 15:0/anteiso 17:0 ratios in the plasma TG, regarding the duodenal fluid samples, would also indicate that the elongation could be taking place in tissues other than the mammary gland.
Table 2 Fatty acid proportion in cow blood plasma
| Fatty acid | Assay | Blood plasma | P-value | |||
|---|---|---|---|---|---|---|
| Duodenum | Milk | TG | FFA | |||
| g/100 g of total odd and branched-chain fatty acids | ||||||
| iso-15:0 | 1 | 12.87d | 7.20b | 9.13c | 6.22a | <0.001 |
| 2 | 10.45c | 5.42a | 6.80b | 7.54b | <0.001 | |
| anteiso 15:0 | 1 | 26.98d | 14.54b | 19.07c | 12.47a | <0.001 |
| 2 | 33.57c | 13.22a | 15.00ab | 19.06b | <0.001 | |
| iso 17:0 | 1 | 5.76a | 8.85b | 10.02c | 13.45d | <0.001 |
| 2 | 5.79a | 6.64a | 9.54b | 10.09b | <0.001 | |
| anteiso 17:0 | 1 | 7.32a | 13.24b | 14.21bc | 15.50c | <0.001 |
| 2 | 9.90a | 16.18bc | 14.41b | 17.63c | <0.001 | |
| C15/C17 ratios | ||||||
|
iso 15:0/iso 17:0 |
1 | 2.28c | 0.83b | 0.92b | 0.48a | <0.001 |
| 2 | 1.82b | 1.18ab | 0.78a | 0.77a | <0.001 | |
|
ant 15:0/ant 17:0 |
1 | 3.98b | 1.10a | 1.37a | 0.82a | <0.001 |
| 2 | 3.73a | 0.84b | 1.10b | 1.13b | <0.001 | |
TG= triglycerides; FFA= free fatty acids.
a-d Values in a row with different superscripts are different (P<0.05).
Source: Vlaeminck et al15.
Overexpression of the gene that codifies the ELOVL6 elongase in ruminants is described in mammary epithelial cells18, and, more recently, an in vitro study evaluated for the first time the role of this enzyme in the regulation of FA elongation19. Upregulation of ELOVL6 increases the elongation indices of 16:0 and 18:0, which suggests an important role of this enzyme in controlling the chain length of FA in the mammary gland. However, the effects on branched FA are yet to be investigated.
Influence of the cattle diet on the OBCFA contents in milk
The chemical composition of the ration, the proportion of starch and fiber, the forage to concentrate ratio (F/C), and the lipid profile in the diet exert a significant influence on the type of ruminal microbial populations and the microbial synthesis of FA; therefore, the proportion of OBCFA that reaches the small intestine reflects the composition and quantity of rumen microbiota2,3,20.
Effects of the basal diet
Among the different diet components, the starch to fiber ratio has an important role in the production of OBCFA through its influence on the microbial ecosystem, particularly on the proliferation of cellulolytic bacterial strains21,22. An increase in starch in the rations limits the growth of cellulolytic microorganisms, promoting the proliferation of amylolytic bacteria. As previously described, cellulolytic bacteria possess mainly branched iso FA in their membranes7 and are sensitive to low ruminal pH values, which are characteristic of feeds with a high starch content23. Vlaeminck et al2 observed that starch-rich diets, characterized by a higher proliferation of amylolytic bacteria, decreased the levels of iso 14:0, iso 15:0, and iso 16:0 (Table 3).
Table 3 Mean content (g/100 of total fatty acids) of odd and branched-chain fatty acids in the milk of ruminants fed with different ingredients
| Fatty acid | Shingfield et al 60 | Vlaeminck et al 2 | Patel et al 24 | Li et al 25 | Cívico et al 27 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| GS | CS | GS | CS | HGS | LGS | HF | LF | HF | HS | |
| iso 13:0 | 0.03 | 0.04 | 0.02 | 0.01 | ||||||
| iso 14:0 | 0.08 | 0.06 | 0.09 | 0.05 | 0.08 | 0.07 | 0.14 | 0.13 | 0.07 | 0.03 |
| iso 15:0 | 0.21 | 0.18 | 0.24 | 0.17 | 0.21 | 0.18 | 0.32 | 0.23 | 0.17 | 0.14 |
| iso 16:0 | 0.21 | 0.23 | 0.18 | 0.16 | 0.26 | 0.26 | 0.19 | 0.15 | ||
| iso 17:0 | 0.74 | 0.91 | 0.19 | 0.23 | 0.47 | 0.33 | 0.38 | 0.49 | 0.27 | 0.23 |
| iso 18:0 | 0.03 | 0.01 | 0.05 | 0.04 | ||||||
| anteiso 13:0 | 0.05 | 0.07 | ||||||||
| anteiso 15:0 | 0.39 | 0.37 | 0.46 | 0.46 | 0.42 | 0.39 | 0.49 | 0.45 | 0.30 | 0.22 |
| anteiso 17:0 | 0.46 | 0.55 | 0.89 | 0.76 | 0.29 | 0.26 | ||||
| 11:0 | 0.20 | 0.22 | ||||||||
| 13:0 | 0.09 | 0.09 | ||||||||
| 15:0 | 1.22 | 0.78 | 0.95 | 1.21 | 1.06 | 0.94 | 1.00 | 0.98 | 0.82 | 0.62 |
| 17:0 | 0.63 | 0.54 | 0.48 | 0.55 | 0.67 | 0.53 | 0.73 | 0.68 | ||
GS= grass silage; CS= corn silage; HGS= high grass silage; LGS= low grass silage; HF= high-fiber; LF= low-fiber; HS= high-starch.
Subsequent studies have confirmed the idea that the fiber and starch ratios influence the content of milk OBCFA (Table 3). Patel et al24 reported that an increase in fiber resulting from the presence of grass silage in the rations increased the milk contents of iso 15:0, iso 17:0, 15:0, and 17:0; while substituting fiber to the detriment of starch in the diet increased the content of iso 15:0 in the milk25 and rumen26. These responses were associated with a higher abundance of cellulolytic versus amylolytic bacteria. Moreover, Cívico et al27 measured higher levels of iso 14:0, iso 17:0, and 15:0 in milk fat when the diet was enriched with fiber and low on starch (Table 3).
The F/C ratio in the rations could modify the contents of OBCFA in dairy products. Vlaeminck et al2 concluded that a greater proportion of forage in the basal diet contributed to a selective increase of specific OBCFA, such as iso 14:0 and iso 15:0. However, the levels of anteiso 15:0 were less affected. These results are explained by changes in the ruminal ecosystem induced by the variation in the F/C ratio of the diets. An increase in the concentrate would favor the proliferation of amylolytic bacteria which could increase anteisos and odd-chain FA. In this line, researchers10 observed lower levels of 15:0 and 17:0 in the milk of cows fed diets with an elevated F/C ratio.
The analysis of the digestive fluids extracted from goats with duodenal cannulation confirmed that increasing the F/C ratio in the ration increases all the OBCFA synthesized de novo by the bacteria5. A similar experiment in cows28 had similar results. More recently, Zhang et al29confirmed that the OBCFA profiles in the digestive fluids of bovines are drastically affected by the F/C ratio in the basal diet. The concentrations of 11:0, 13:0, iso 15:0, iso 16:0, iso 17:0, and 17:0 were higher when the proportion of forage in the ration was higher. They also observed that only the anteiso 15:0 and 15:0 increased with higher proportions of the concentrate.
Effects of lipid supplementation
The levels of OBCFA in dairy products show a significant decrease when they come from animals whose diet has been supplemented with lipid sources. This pattern, observed in the milk of bovines6,30 and small ruminants31,32, is characteristic of supplementation with oilseeds rich in unsaturated FA.
These results could be explained by the inhibitory effect of the polyunsaturated fatty acids (PUFA) on the gut microbiota. The severity of the effect of the FA incorporated into the diet on the viability of rumen bacteria is greater as the number of unsaturations increases. The effects would be more pronounced if the geometric configuration of the double bonds is of the cis type2,3. Furthermore, not all microorganisms would be affected in the same way by the lipid supplementation of the diet. Previous studies have observed that cellulolytic and Gram-positive bacteria are more sensitive to the lipids in the diet than amylolytic and Gram-negative bacteria20,33,34.
Branched-chain fatty acids as bioactive components
Neonatal gut microbiota
Recent studies have highlighted the role of branched-chain FA as health-protective bioactive components. The presence of branched-chain FA is very low in adult human tissues; however, they are essential bioactive components in the digestive tract at the final stages of fetal development and after delivery35.
Approximately 30 % of the total FA in the vernix caseosa are branched-chain FA, with a great variety of molecular structures, among which iso 14:0 and iso 16:0 stand out35. The vernix is a waxy material with a cheese-like texture that coves the skin of the fetus and newborn. It consists of a mixture of fatty secretions originated from the 18th week of gestation from the sebaceous glands. The vernix avoids water loss, protecting the skin of the fetus from dehydration, preventing its hardening, and reducing friction and cracking. Moreover, it helps regulate the temperature of the fetus by acting as an insulating layer. There is no other land mammal that produces vernix-covered neonates; however, the fetuses of aquatic mammals present this same fatty film composed of branched FA36.
A complementary hypothesis postulates that the vernix may have antibacterial activity. This idea is based on the fact that vernix particles are detached from the skin during the last months of pregnancy and pass into the amniotic fluid, increasing its turbidity. In the last trimester, the fetus ingests a significant part of the amniotic fluid, and thus, its intestine impregnates with the branched-chain FA in the vernix35.
Moreover, the significant amount of branched-chain FA in the meconium (the first feces of the newborn) constitutes a sufficiently relevant indication of the type of microorganisms that begin to colonize the intestinal tract of the newborn, and that would be favored by the presence of these non-fermentable prebiotics35. As previously described, branched-chain FA are among the most important molecules in the membrane of several microorganisms, particularly of most species of the genus Bacilli37. A previous report indicated that the substitution of the dietary fat with branched-chain FA in newborn rat pups modifies their microbiome. These changes translate into an increase of the microorganisms that can incorporate branched-chain FA into their membranes, and a simultaneous reduction of the incidence of necrotizing enterocolitis38, one of the major causes of mortality in preterm infants. Furthermore, in vitro studies have demonstrated that branched-chain FA reduce mortality and virulence of pathogens such as Pseudomonas aeruginosa39.
High concentrations of branched-chain FA, as a consequence of the presence of vernix in the intestinal lumen of the fetus, may have an important role in the growth and metabolism of enterocytes, as well as in intestinal health and regulation. Recent studies have observed that branched-chain FA can be incorporated into the membrane PL of enterocytes, conferring them an anti-inflammatory activity40,41. Liu et al42 postulated that this incorporation of branched-chain FA to the PL would contribute to the modulation of the biophysical properties of membranes. Branched-chain FA are assigned biophysical functions comparable to monounsaturated FA with cis configuration, but they have the advantage of presenting greater oxidative stability due to the absence of double bonds in their structure. Moreover, the lower melting points of branched-chain FA compared to their linear homologous would be associated with the fluidity of cell membranes43.
Other bioactive properties of branched-chain fatty acids
Besides their positive effects on the composition of gut microbiota, branched-chain FA in the diet could help prevent different diseases. The first study that attributes anti-cancer activity to branched-chain FA was published at the beginning of this century44. This study describes the inhibitory effects of iso 15:0 on cell proliferation and apoptosis induction in prostate cancer, leukemia, and adenocarcinoma cell lines. More recently, Cai et al45 reported that iso 15:0 could contribute to human lymphomas inhibition. Other studies46,47 determined that branched-chain FA could also induce apoptosis in breast cancer cells and inhibit tumor development in cell cultures and animal models.
Moreover, a recent study in overweight humans48 reported for the first time the possibility that the abundance of iso branched-chain FA in blood serum could be inversely correlated with the presence of TG and negatively associated with other characteristic indicators of inflammatory processes. In any case, the beneficial effects of this group of FA require more research to help clarify the mechanisms underlying the prevention of these pathologies.
Odd-chain fatty acids as bioactive components
Different recent studies have demonstrated that 15:0 and 17:0, the most abundant odd-chain FA in dairy products, could benefit human health49,50. For example, there is an inverse association between the concentration of these FA in plasma and the risk of developing type 2 diabetes51-53. This result has also been observed in European populations subjected to different diets54. Even several prospective studies on cardiovascular diseases have shown that the plasma concentration of these FA would be associated with a lower risk of developing cardiovascular diseases55-57. However, more detailed research is needed to help elucidate the metabolic pathways involved in these health effects.
Conclusions
Milk and dairy products are the most significant sources of OBCFA in the human diet. Despite their low concentrations, recent investigations have demonstrated their potential as bioactive components and their nutritional importance. Although they derive mainly from the microbial activity in the rumen, there is recent evidence that their formation is not limited to the biochemical processes that occur in the digestive tract of ruminants. The ability of other tissues to endogenously synthesize specific OBCFA must be carefully considered and may encourage very promising lines of research in the future.
Literatura citada
1. Schroeder M, Vetter W. Detection of 430 Fatty acid methyl esters from transesterified butter sample. J Am Oil Chem Soc 2013;90:771-790. [ Links ]
2. Vlaeminck B, Fievez V, van Laar H, Vlaeminck B, Fievez V, Cabrita ARJ, Fonseca AJM et al. Factors affecting odd- and branched-chain fatty acids in milk: A review. Anim Feed Sci Technol 2006;131:389-417. [ Links ]
3. Buccioni A, Decandia M, Minieri S, Molle G, Cabiddu, A. Lipid metabolism in the rumen: New insights on lipolysis and biohydrogenation with an emphasis on the role of endogenous plant factors. Anim Feed Sci Technol 2012;174:1-25. [ Links ]
4. Or-Rashid MM, Odongo NE, McBride BW. Fatty acid composition of ruminal bacteria and protozoa, with emphasis on conjugated linoleic acid vaccenic acid, and odd-chain and branched-chain fatty acids. J Animal Sci 2007;85:1228-1234. [ Links ]
5. Bas P, Archimède H, Rouzeau A, Sauvant D. Fatty acid composition of mixed-rumen bacteria: effect of concentration and type of forage. J Dairy Sci 2003;86:2940-2948. [ Links ]
6. Baumann E, Chouinard PY, Lebeuf Y, Rico DE, Gervais R. Effect of lipid supplementation on milk odd- and branched-chain fatty acids in dairy cows. J Dairy Sci 2016;99:6311-6323. [ Links ]
7. Fievez V, Colman E, Castro-Montoya JM, Stefanov I, Vlaeminck B. Milk odd- and branched-chain fatty acids as biomarkers of rumen function-An update. Anim Feed Sci Technol 2012;172:51-65. [ Links ]
8. Bessa RJB, Maia, MRG, Jerónimo E, Belo AT, Cabrita ARJ, Dewhurst RJ, Fonseca AJM. Using microbial fatty acids to improve understanding of the contribution of solid associated bacteria to microbial mass in the rumen. Anim Feed Sci Technol 2009;150:197-206. [ Links ]
9. Keeney M, Katz I, Allison J. On the probable origin of milk fat acids in rumen microbial lipids. J Am Oil Chem Soc 1962;39:198-201. [ Links ]
10. Dewhurst RJ , Moorby JM, Vlaeminck B, Van Nespen T, Fievez V. Apparent recovery of duodenal odd- and branched-chain fatty acids in milk. J Dairy Sci 2007;90:1775-1780. [ Links ]
11. Schmidely P, Glasser F, Doreau M, Sauvant D. Digestion of fatty acids in ruminants: a meta-analysis of flows and variation factors. 1. Total fatty acids. Animal 2008;2:677-690. [ Links ]
12. Palmquist DL. Milk fat: Origin of fatty acids and influence of nutritional factors thereon. In: Fox PF, McSweeney PLH editors. Advanced dairy chemistry, vol 2, Lipids, 3rd ed. New York, USA: Springer; 2006:43-92. [ Links ]
13. Massart-Leën AM, Roets E, Peeters G, Verbeke R. Propionate for fatty acid synthesis by the mammary gland of the lactating goat. J Dairy Sci 1983;66:1445-1454. [ Links ]
14. French EA, Bertics SJ, Armentano, LE. Rumen and milk odd-and branched-chain fatty acid proportions are minimally influenced by ruminal volatile fatty acid infusions. J Dairy Sci 2012;95:2015-2026. [ Links ]
15. Vlaeminck B , Gervais R, Rahman MM, Gadeyne F, Gorniak, M, Doreau M et al. Postruminal synthesis modifies the odd- and branched chain fatty acid profile from the duodenum to milk. J Dairy Sci 2015;98:4829-4840. [ Links ]
16. Fievez V , Vlaeminck B , Dhanoa MS, Dewhurst RJ . Use of principal component analysis to investigate the origin of heptadecenoic and conjugated linoleic acids in milk. J Dairy Sci 2003;86:4047-4053. [ Links ]
17. Gervais R , Vlaeminck B , Fanchone A, Nozière P, Doreau M , Fievez V . Odd-and branched-chain fatty acids duodenal flows and milk yields in response to N underfeeding and energy source in dairy cows. J Dairy Sci 2011;94(Suppl 1):125-126. [ Links ]
18. Xu HF, Luo J, Zhao WS, Yang YC, Tian HB, Shi HB et al. Overexpression of SREBP1 (sterol regulatory element binding protein 1) promotes de novo fatty acid synthesis and triacylglycerol accumulation in goat mammary epithelial cells. J Dairy Sci 2016;99:783-795. [ Links ]
19. Shi HB, Wu M, Zhu JJ, Zhang CH, Yao, DW, Luo J et al. Fatty acid elongase 6 plays a role in the synthesis of long-chain fatty acids in goat mammary epithelial cells. J Dairy Sci 2017;100:4987-4995. [ Links ]
20. Enjalbert F, Combes S, Zened A, Meynadier A. Rumen microbiota and dietary fat: a mutual shaping. J Appl Microbiol 2017;123:782-797. [ Links ]
21. Vlaeminck B , Dewhurst RJ , Demeyer D, Fievez V . Odd and branched chain fatty acids to estimate proportions of cellulolytic and amylolytic particle associated bacteria. J Anim Feed Sci 2004;13:235-238. [ Links ]
22. Cabrita ARJ , Vale JMP, Bessa RJB , Dewhurst RJ , Fonseca AJM . Effects of dietary starch source and buffers on milk responses and rumen fatty acid biohydrogenation in dairy cows fed maize silage-based diets. Anim Feed Sci Technol 2009;52:267-277. [ Links ]
23. Sun YZ, Mao SY, Zhu WY. Rumen chemical and bacterial changes during stepwise adaptation to a high-concentrate diet in goats. Animal 2010;4:210-217. [ Links ]
24. Patel M, Wredle E, Bertilsson J. Effect of dietary proportion of grass silage on milk fat with emphasis on odd- and branched-chain fatty acids in dairy cows. J Dairy Sci 2013;96:390-397. [ Links ]
25. Li F, Li Z, Li S, Ferguson JD, Cao Y, Yao J et al. Effect of dietary physically effective fiber on ruminal fermentation and the fatty acid profile of milk in dairy goats. J Dairy Sci 2014;97:2281-2290. [ Links ]
26. Li F, Yang XJ, Cao YC, Li SX, Yao JH, Li ZJ et al. Effects of dietary effective fiber to rumen degradable starch ratios on the risk of sub-acute ruminal acidosis and rumen content fatty acids composition in dairy goat. Anim Feed Sci Technol 2014;189:54-62. [ Links ]
27. Cívico A, Núñez Sánchez N, Gómez-Cortés P, De la Fuente MA, Peña Blanco F, Juárez M et al. Odd- and branched-chain fatty acids in goat milk as indicators of diet composition. Ital J Anim Sci 2017;16:68-74. [ Links ]
28. Vlaeminck B , Fievez V , Demeyer D , Dewhurst RJ . Effect of forage:concentrate ratio on fatty acid composition of rumen bacteria isolated from ruminal and duodenal digesta. J Dairy Sci 2006;89:2668-2678. [ Links ]
29. Zhang Y, Liu K, Hao X, Xin H. The relationships between odd- and branched-chain fatty acids to ruminal fermentation parameters and bacterial populations with different dietary ratios of forage and concentrate. J Anim Physiol Anim Nutr 2017;101:1103-1114. [ Links ]
30. Vazirigohar M, Dehghan-Banadaky M, Rezayazdi K, Nejati-Javaremi A, Mirzaei-Alamouti H, Patra AK. Effects of diets containing supplemental fats on ruminal fermentation and milk odd- and branched-chain fatty acids in dairy cows. J Dairy Sci 2018;101:6133-6141. [ Links ]
31. Gómez-Cortés P, Toral PG, Frutos P, Juárez M, De la Fuente MA, Hervás, G. Effect of the supplementation of dairy sheep diet with incremental amounts of sunflower oil on animal performance and milk fatty acid profile. Food Chem 2011;125:644-651. [ Links ]
32. Martínez-Marín AL, Gómez-Cortés P, Gómez CG, Juárez M, Pérez AL, Pérez HM et al. Animal performance and milk fatty acid profile of dairy goats fed diets added differently unsaturated plant oils on fatty acid profile of goat milk. J Dairy Sci 2011;94:5359-5368. [ Links ]
33. Maia MRG, Chaudhary LC, Figueres L, Wallace RJ. Metabolism of polyunsaturated fatty acids and their toxicity to the microflora of the rumen. Antonie van Leeuwenhoek 2007;91:303-314. [ Links ]
34. Yang SL, Bu DP, Wang JQ, Hu ZY, Li D, Wei HY, et al. Soybean oil and linseed oil supplementation affect profiles of ruminal microorganisms in dairy cows. Animal 2009;3:1562-1569. [ Links ]
35. Ran-Ressler RR, Devapatla S, Lawrence P, Brenna JT. Branched chain fatty acids are constituents of the normal healthy newborn gastrointestinal tract. Pediatr Res 2008;64:605-609. [ Links ]
36. Wang DH, Ran-Ressler R, St Leger J, Nilson E, Palmer L, Collins R et al. Sea lions develop human-like vernix caseosa delivering branched fats and squalene to the GI tract. Sci Rep 2018;8:7478. [ Links ]
37. Kaneda T. Fatty acids of the genus bacillus: An example of branched-chain preference. Bacteriol Rev 1977;41:391. [ Links ]
38. Ran-Ressler RR, Khailova L, Arganbright KM, Adkins-Rieck CK, Jouni ZE, Koren O et al. Branched chain fatty acids reduce the incidence of necrotizing enterocolitis and alter gastrointestinal microbial ecology in a neonatal rat model. PLoS One 2011;6:e29032. [ Links ]
39. Inoue T, Shingaki R, Fukui K. Inhibition of swarming motility of Pseudomonas aeruginosa by branched-chain fatty acids. FEMS Microbiol Lett 2008;281:81-86. [ Links ]
40. Yan Y, Wang Z, Greenwald J, Kothapalli KSD, Park HG, Liu R et al. BCFA suppresses LPS induced IL-8 mRNA expression in human intestinal epithelial cells. Prostaglandins Leukot Essent Fatty Acids 2017;116:27-31. [ Links ]
41. Yan Y, Wang Z, Wang D, Lawrence P, Wang X, Kothapalli KSD et al. BCFA-enriched vernix-monoacylglycerol reduces LPS-induced inflammatory markers in human enterocytes in vitro. Pediatr Res 2018;83:874-879. [ Links ]
42. Liu L, Wang Z, Park HG, Xu C, Lawrence P, Sub X et al. Human fetal intestinal epithelial cells metabolize and incorporate branched chain fatty acids in a structure specific manner. Prostaglandins Leukotr Essent Fatty Acids 2017;116:32-39. [ Links ]
43. Ran-Ressler RR, Bae S, Lawrence P, Wang DH, Brenna JT. Branched chain fatty acid content of foods and estimated intake in the USA. Br J Nutr 2014;112:565-572. [ Links ]
44. Yang Z, Liu S, Chen X, Chen H, Huang M, Zheng J. Induction of apoptotic cell death and in vivo growth inhibition of human cancer cells by a saturated branched-chain fatty acid, 13-methyltetradecanoic acid. Cancer Res 2000;60:505-509. [ Links ]
45. Cai Q, Huang H, Qian D, Chen K, Luo J, Tian Y et al. 13- methyltetradecanoic acid exhibits antitumor activity on T-cell lymphomas in vitro and in vivo by down-regulating p-AKT and activating caspase-3. PLoS One 2013;8:e65308. [ Links ]
46. Wongtangtintharn S, Oku H, Iwasaki H, Toda T. Effect of branched-chain fatty acids on fatty acid biosynthesis of human breast cancer cells. J Nutr Sci Vitaminol 2004;50:137-143. [ Links ]
47. Wongtangtintharn S, Oku H, Iwasaki H, Inafuku M, Toda T, Yanagita T. Incorporation of branched-chain fatty acid into cellular lipids and caspase in dependent apoptosis in human breast cancer cell line, SKBR-3. Lipids Health Dis 2005;4:29. [ Links ]
48. Mika A, Stepnowski P, Kaska L, Proczko M, Wisniewski P, Sledzinski M et al. A Comprehensive study of serum odd- and branched-chain fatty acids in patients with excess weight. Obesity 2016;24:1669-1676. [ Links ]
49. Jenkins B, West JA, Koulman A. A review of odd-chain fatty acid metabolism and the role of pentadecanoic acid (C15:0) and heptadecanoic acid (C17:0) in health and disease. Molecules 2015;20:2425-2444. [ Links ]
50. Jenkins BJ, Seyssel K, Chiu S, Pan PH, Lin SY, Stanley E et al. Odd chain fatty acids; New insights of the relationship between the gut microbiota, dietary intake, biosynthesis and glucose intolerance. Sci Rep 2017;7:44845. [ Links ]
51. Yakoob MY, Shi PL, Willett WC, Rexrode KM, Campos H, Orav EJ et al. Circulating biomarkers of dairy fat and risk of incident diabetes mellitus among men and women in the United States in two large prospective cohorts. Circulation 2016;133:1645-1654. [ Links ]
52. Pfeuffer M, Jaudszus A. Pentadecanoic and heptadecanoic acids: multifaceted odd-chain fatty acids. Adv Nutr 2016;7:730-734. [ Links ]
53. Risérus U, Marklund M. Milk fat biomarkers and cardiometabolic disease. Curr Opin Lipidol 2017;28:46-51. [ Links ]
54. Forouhi NG, Koulman A, Sharp SJ, Imamura F, Kroger J, Schulze MB et al. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol 2014;2:810-818. [ Links ]
55. Khaw KT, Friesen MD, Riboli E, Luben R, Wareham N. Plasma phospholipid fatty acid concentration and incident coronary heart disease in men and women: The EPIC-Norfolk prospective study. PLoS Med 2012;9:e1001255. [ Links ]
56. Otto MCD, Nettleton JA, Lemaitre RN, Steffen LM, Kromhout D, Rich SS et al. Biomarkers of dairy fatty acids and risk of cardiovascular disease in the multi-ethnic etudy of atherosclerosis. J Am Heart Assoc 2013;2:e000092. [ Links ]
57. Liang J, Zhou Q, Amakye WK, Su Y, Zhang Z. Biomarkers of dairy fat intake and risk of cardiovascular disease: A systematic review and meta analysis of prospective studies. Crit Rev Food Sci Nutr 2018;58:1122-1130. [ Links ]
58. O’Donnell-Megaro AM, Barbano DM, Bauman DE. Survey of the fatty acid composition of retail milk in the United States including regional and seasonal variations. J Dairy Sci 2011;94:59-65. [ Links ]
59. Shingfield KJ, Chilliard Y, Toivonen P, Kairenius P, Givens DI. Trans fatty acids and bioactive lipids in ruminant milk. Adv Exp Med Biol 2008;606:3-65. [ Links ]
60. Shingfield KJ, Reynolds CK, Lupoli B, Toivonen V, Yurawecz MP, Delmonte P et al. Effect of forage type and proportion of concentrate in the diet on milk fatty acid composition in cows given sunflower oil and fish oil. Anim Sci 2005;80:225-238. [ Links ]
Received: November 30, 2018; Accepted: October 23, 2019











 texto em
texto em 


