Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.12 no.7 Texcoco Set./Nov. 2021 Epub 22-Mar-2022
https://doi.org/10.29312/remexca.v12i7.2539
Articles
Germination and vigor of seeds of horticultural species, inoculated with biofertilizers and saline solutions
1Campo Experimental Centro Altos de Jalisco-INIFAP. Av. Biodiversidad núm. 2470, Tepatitlán de Morelos, Jalisco. CP. 47600. AP. 56. Tel. 800 0882222, ext. 84515.
2 Campo Experimental Santiago Ixcuintla-INIFAP. Entronque carretera internacional México-Nogales km 6, Colonia Centro, Santiago Ixcuintla, Nayarit. Tel. 800 0882222, ext. 84427. (sanchez.roberto@inifap.gob.mx).
3 Centro Nacional de Recursos Genéticos-INIFAP. Boulevard de la Biodiversidad núm. 400, Rancho las Cruces, Tepatitlán de Morelos, Jalisco. CP. 47600. Tel. 800 0882222, ext. 84823. (chavez.fernando@inifap.gob.mx; zelaya.lily@inifap.gob.mx).
4Universidad Autónoma de Nayarit-Unidad Académica de Agricultura. Carretera Tepic-Compostela km 9, Xalisco, Nayarit. Tel. 311 2110128. (rvald.uan@gmail.com).
This research work was carried out in Tepatitlán de Morelos, Jalisco, at the facilities of the Centro Altos de Jalisco Experimental Field. Seeds of horticultural species (chilaca chili pepper, melon and cucumber) were used inoculated with biofertilizers (Azospirillum brasilenses and Glomus intraradices) and subjected to different concentrations of potassium chloride (KCl). The objective was to evaluate the effect of biofertilizers and KCl on germination and vigor in seeds of horticultural species. The treatments evaluated under laboratory conditions were through the inoculation of biofertilizers, combination of biofertilizers plus saline solution at different concentrations, chemical treatment and absolute control, the above to observe the physiological effect on the seeds through the variables evaluated: standard germination, average plumule length, vigor and dry weight. The information obtained from each of the variables was analyzed by means of a completely randomized design with factorial arrangement and with four repetitions, the experimental unit being the tacos (substrate between paper). Highly significant differences between treatments and variables were detected, this due to the results obtained by inoculation of biofertilizers in combination with saline concentrations, they physiologically promoted the horticultural species under study, being treatments T7 and T8, with better response with respect to the absolute control; that is, they have a positive effect on germination and vigor in the species studied.
Keywords: biofertilizers and horticultural species; vigor
El presente trabajo de investigación se realizó en Tepatitlán de Morelos, Jalisco, en las instalaciones del Campo Experimental Centro Altos de Jalisco. Se utilizaron semillas de especies hortícolas (chile chilaca, melón y pepino) inoculadas con biofertilizantes (Azospirillum brasilenses y Glomus intraradices) y sometidas a diferentes concentraciones de cloruro de potasio (KCl). El objetivo fue evaluar el efecto de los biofertilizantes y KCl, en la germinación y vigor en semillas de especies hortícolas. Los tratamientos evaluados bajo condiciones de laboratorio fueron mediante la inoculación de biofertilizantes, combinación de biofertilizantes más solución salina en diferentes concentraciones, tratamiento químico y testigo absoluto, lo anterior para observar el efecto fisiológico en las semillas mediante las variables evaluadas: germinación estándar, longitud media de plúmula, vigor y peso seco. La información obtenida de cada una de las variables se analizó mediante un diseño completamente al azar con arreglo factorial y con cuatro repeticiones, siendo la unidad experimental los tacos (sustrato entre papel). Se detectaron diferencias significativas entre tratamientos y variables, esto debido a los resultados por inoculación de biofertilizantes en combinación con las concentraciones salinas, promovieron fisiológicamente las especies hortícolas en estudio siendo tratamientos T7 y T8, con mejor respuesta con respecto al testigo absoluto; es decir, tienen un efecto positivo en germinación y vigor en las especies estudiadas.
Palabras claves: biofertilizantes y especies hortícolas; vigor
Introduction
The soil is the habitat of a great variety of microorganisms, plants host in their root system a great variety of these microorganisms that interact with them in a positive or negative way. The dominant groups are fungi, bacteria, and nematodes (Manoharachary and Mukerji, 2006). Most of these interactions occur in an area known as the rhizosphere, a center of physical, chemical and biological activities barely 1 mm thick, which surrounds the living architecture of a plant’s root system and is influenced by root exudates (Cardon and Whitbeck, 2007).
Microorganisms have a great diversity of mechanisms through which they promote plant growth. Based on these mechanisms, four large groups of microorganisms that promote plant growth are recognized: a) microorganisms that incorporate nitrogen into the plant-soil system through biological nitrogen fixation; b) microorganisms that increase nutrient and water uptake; c) microorganisms that increase the availability of nutrients found in the soil in non-assimilable forms; and d) microorganisms that have antagonistic activities against phytopathogenic agents (Aguado, 2012).
The use of microorganisms that live in exchange with plants is one of the areas of study that has most impacted agriculture in the last two decades, because they are an emerging alternative to chemicals, to increase fertility and crop production in sustainable agroecosystems (Franco-Correa, 2009; Rueda et al., 2009).
Salinity is a limitation in horticultural crops, which causes alterations in growth, low absorption and distribution of nutrients to different organs of the plant and changes in quality. The potassium chloride fertilizer constituent of the greatest richness in potassium, good percentage of solubility and is also low cost in our country (Ayala et al., 2019). That is why this research work aims to evaluate the effect of biofertilizers and potassium chloride (KCl) on germination and vigor in seeds of horticultural species.
In today’s agriculture, new high-yielding varieties demand better growing conditions and high levels of fertilization, and consequently, it results in environmental deterioration that can outweigh the economic, social and environmental benefit. Hence the importance of understanding the effects or mechanisms of microorganisms that live in association with plants. Microorganisms (biofertilizer) exert a beneficial effect on the germination, development and control of other pathogenic microorganisms (biocontroller) (Franco-Correa, 2009; Rueda et al., 2009).
Microbial inoculants or biofertilizers currently have great ecological and economic relevance in agriculture, so their importance has increased within the conservation and fertility of soils. Arbuscular mycorrhizal fungi (AMF) and plant growth-promoting bacteria (PGPB) are among the most studied microorganisms (Adesemoye and Kloepper, 2009; Hungary et al., 2010; Sharma et al., 2012).
Classification of biofertilizers. Biofertilizers can be classified according to the mechanism(s) used by the bacterium to promote plant growth (nitrogen fixers, phosphate solubilizers or organic matter disintegrators) or according to the type of microorganisms used in their formulation, bacteria, fungi or a combination of both.
Bacterial inoculants. The rhizobacteria most applied as inoculants in agriculture include nitrogen-fixing bacteria (diazotrophic) and phosphate-solubilizing bacteria. Diazotrophic organisms employ the enzyme complex of nitrogenase to convert atmospheric nitrogen into ammonium, a compound assimilable by plants and can be free-living (Acetobacter, Azospirillum, Azotobacter, Bacillus, Beijerinckia, Enterobacter, Herbaspirillum, Klebsiella, Pseudomonas, Serratia and the cyanobacteria Anabaena and Nostoc) (Bloemberg and Lugtenberg, 2001). In particular, the genus Azospirillum has been studied for its ability to promote plant growth and has become an excellent model for studying plant-microorganism interaction.
The results that have been reported on the association Azospirillum-plant are: a) increases in germination percentage and rate; b) increases in height, total fresh weight, root length, increased number of hairs and adventitious and secondary roots; c) increases in leaf area, resulting in higher photosynthesis rates and consequently higher production; d) increases in flowering, tasseling and fruit set; and e) increase in nitrogen levels, expressed in protein in foliage and grain (Rueda et al., 2009) and production of auxins in the root (Star et al., 2012).
Fungal inoculants. The fungi that have been most intensively studied as fungal inoculants for their benefits in plant nutrition are mycorrhizae, mycorrhizae are divided into ectomycorrhizae and endomycorrhizae. In the case of ectomycorrhizae, these symbiotic associations typically form between the roots of woody plants and fungi belonging to the phylum Basidiomycota, Ascomycota and Zygomycota. While ectomycorrhizae colonize approximately 10% of plant families including pines, birches, eucalyptus, oaks and beeches, among others. Ectomycorrhizae can be visualized macroscopically as the fungus surrounds the root and forms a fungal layer or mantle (Elo et al., 2000).
Endomycorrhizae. These make up a highly variable group of fungi that are classified into arbuscular, ericoid, arbutoid, monotropoid, and orchidioid (Peterson et al., 2004). The associations of arbuscular mycorrhizae or AM (previously known as vesicular-arbuscular or VAM) are formed only by fungi belonging to the phylum Glomeromycota. Arbuscular mycorrhizae constitute the most important group of symbiotic fungi from an agricultural and ecological point of view. They are currently the group of fungi most used in the formulation of biofertilizers and are strong candidates for the biocontrol of phytopathogens through their competitive capabilities for the available spaces in the roots (Aguado, 2012).
Some of the benefits of mycorrhizae in plants, control of soil pathogens (Whipps, 2001) and stabilization of soil structure through an increase in its aggregation (Miller and Jastrow, 2000). Composite inoculants. There is a synergism in promoting plant growth when two or more stimulating microorganisms are used.
However, the formulation of compound biofertilizers that are effective in the field requires meticulous studies to be carried out to know and understand the nutritional and environmental requirements of each of the microorganisms to be used in the formulations and the results of their interaction in physiological and ecological terms to be able to formulate biofertilizers that are compatible and synergistic in terms of their effects on the agronomically important variables of the crops, in field or greenhouse (Aguado, 2012).
For several years now, the use of phosphorus-solubilizing bacteria and fungi has been a common agricultural practice in Russia, which has yielded good results. It has also been shown that the combination of different strains of growth-promoting rhizobacteria produces a beneficial effect on rice (Nguyen et al., 2002) and barley (Belimov et al., 1995). It has also been found that the combination of plant growth-promoting rhizobacteria and arbuscular mycorrhizae may be useful for increasing wheat growth in low-fertility soils (Galal et al., 2003). In this same connection, it is mentioned that the co-inoculation of legumes with arbuscular fungi and Rhizobium results in better plant growth than that obtained using each of these microorganisms separately (Barea and Azcon-Aguilar, 1983; Bagyaraj, 1984).
Although the main benefit of dual inoculants is to increase the absorption of nutrients from fertilizers and the soil itself (Belimov et al., 1995; Bashan et al., 2004), other synergyms may be the result, for example, of an increase in nutrient uptake and control of phytopathogenic agents. Despite the benefits mentioned above regarding the use of mixed, dual or multiple biofertilizers, it is important to carry out studies in order to determine the compatibility of the microorganisms to be applied since antagonisms between some growth-promoting microorganisms have been found (Aguado, 2012).
Materials and methods
The present research work was carried out under laboratory conditions, in the facilities of the Centro Altos de Jalisco Experimental Field, seeds of horticultural species (chilaca chili pepper, melon and cucumber) inoculated with biofertilizers (Azospirillum brasilenses and Glomus intraradices) were used and subjected to different concentrations of potassium chloride (KCl).
The treatments used are the following: T1= absolute control (seeds without inoculation and without saline concentrations). T2= chemical treatment GA3 (gibberellic acid) at 3 ppm. T3= inoculated seeds Azospirillum brasilenses (Az) + Glomus intraradices (Gl). T4= seeds inoculated with Az. T5= seeds inoculated with Gl. T6= seeds inoculated Az +Gl + 5 dS m-1 of KCl. T7= inoculated seeds. Az +Gl + 10 dS m-1 of KCl. T8= inoculated seeds Az +Gl + 15 d Sm-1 of KCl.
For this experiment, 800 seeds of each species were used, of which 500 seeds were inoculated with biofertilizers (in the case of treatments 3, 4, 5, 6, 7 and 8), 0.428 g of Azospirillum brasilenses and 0.802 g of Glomus intraradices were required, for each gram of seed, for each of the species studied in this experiment, in the case of the chemical treatment (T2), the incorporation was important because its function is to stimulate germination and therefore the emergence in the field, this was used at the recommended dose at 3 ppm.
The seeds were impregnated with adherent and mixed with the biofertilizers in a Petri dish, once the seeds were inoculated, the germination test was carried out according to ISTA (2004) rules, for which sheets of anchor paper, distilled water (in the case of the absolute control) were used. During the germination test, the tacos (substrate between paper) were watered with their respective dose of KCl (5, 10 and 15 dS m-1), while for the treatments (T1 to T5), they were watered only with distilled water, on the fourth and seventh day after sowing, being a total of seven days for evaluations.
Once the experiment was established, the variables analyzed in the experiment were as follows: standard germination or ‘PN’ (normal seedlings ‘PN’, abnormal seedlings ‘PA’ and non-germinated seeds ‘SSG’), the evaluation is carried out on the seventh day after sowing. For these purposes, germination is defined as the emergence and development of those essential structures that come from the embryo and that manifest the ability of the seed to produce a normal plant under favorable conditions. Normal seedlings are considered all those that possess the essential structures to produce normal plants under favorable conditions of water, light and temperature.
Abnormal seedling. They are those plants that present deficiency in the development of their essential structures, which prevents them from their normal development under favorable conditions of water, light and temperature. Non-germinated seeds. For this classification, it is important to consider that for the seeds that do not germinate, there are different reasons, such as the following: fresh seeds, hard seeds, dead seeds and dormant seeds, their expression is in percentage. Vigor (V), evaluation on the fourth day, only normal seedlings are considered and their expression is in percentage. Average radicle length (ARL), average plumule length (APL). Seedling growth tests are performed when the seedlings in a germination test show all their essential structures and a balanced development, they are considered normal seedlings, at the end of the test, the number of plumules that are in each parallel is counted.
Lines are given a value of (0-2) 1, (2-4) 3, (4-6) 5, (6-8) 7, (8-10) 9, (10-12) 11 and (12-14) 13 cm value from the midpoint of each parallel to the centerline. The number that is in each line is multiplied by the corresponding distance and added together, dividing the total length by the number of seeds (25). And dry weight (DW), seedlings are evaluated discarding the abnormal ones, normal seedlings are dried for 24 h at 80 °C, after removing the seed or cotyledons, after drying they are weighed down to mg, the total dry weight of normal seedlings by repetition and it is divided by the number of plants included, reporting the result in g seedling-1 (ISTA, 2004).
Statistical analysis. The information obtained from each of the variables studied in this research was analyzed with the SAS 9.4 statistical program, a completely randomized design was used, with the tacos (substrate between paper) being the experimental unit, the treatments had factorial arrangement, with four repetitions, which consisted of inoculations of biofertilizers (Az and Gl) and in combination with three levels of KCl (5, 10 and 15 dS m-1), an analysis of variance (Anova) was performed and in the response variables, where a statistically significant difference was observed, a comparison of means was made (Tukey p≤ 0.05).
Results and discussion
For this section, it will be discussed according to the variables and factors under study, as the main thing, we will analyze the effect on germination with respect to the variables and the effect on the vigor and growth of the plant with respect to the variables.
Effect on germination. In Table 1, it is analyzed that there are highly significant differences with respect to the species used corresponding to standard germination, abnormal seedlings and non-germinated seed, with regard to the treatment and its interaction treatment*species, there is no significance in germination and in non-germinated seeds, contrary to abnormal seedlings, where there is a high significance, which means that in all treatments and combinations, the behavior regarding its effect on germination, a totally independent response occurred.
Table 1 Mean squares of the variables analyzed.
| Source of variation | DF | G (%) | PA (%) | SSG (%) |
| Treatment | 7 | 52.23 ns | 47.02** | 9.18 ns |
| Species | 2 | 6743.04** | 486.54** | 3650.79** |
| Treatment*species | 14 | 41.51 ns | 33.39** | 24.64 ns |
| SE | 72 | 30.93 | 10.02 | 20.81 |
| Mean | 85.35 | 4.79 | 9.85 | |
| CV | 6.515821 | 66.08 | 46.3 |
* and **= significant values with p≤ 0.05 and p≤ 0.01, respectively; ns= not significant; DF= Degrees of freedom; G= germination; PA= abnormal seedlings; SSG= non-germinated seeds.
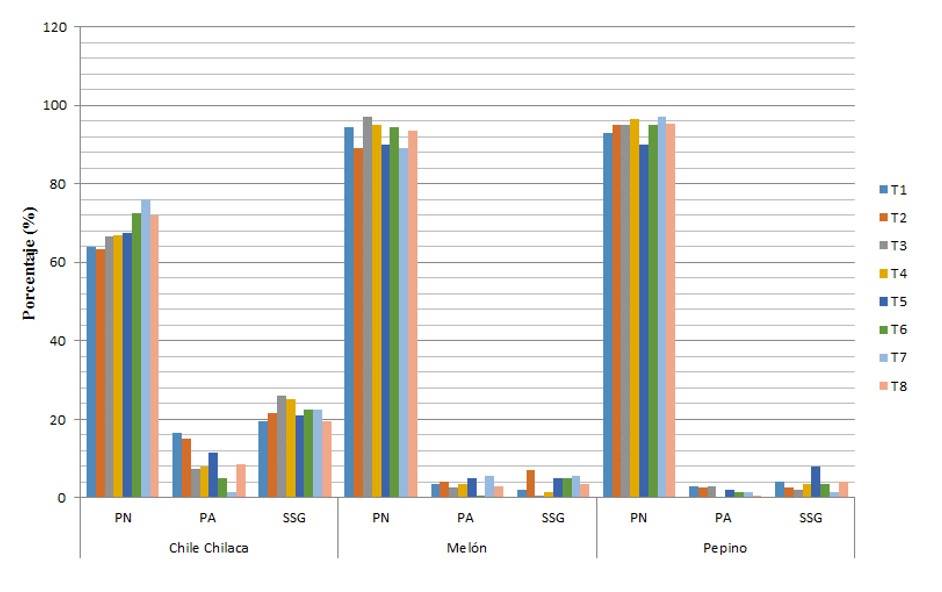
In Figure 1, it is observed that in the case of chilaca chili pepper, the best result based on germination with respect to treatments with different levels of salinity and combination with biofertilizers, obtaining 8% more germination compared to the absolute control and 7.5% with treatment 2, which indicates that this species tolerates salinity, while the melon is not so tolerant to salinity and the highest percentage of germination was obtained in treatment 3 (AZ+Gl) and finally, the cucumber had a better percentage of germination with treatment 7, which indicates that it tolerates salinity.
The lowest percentage of abnormal seedlings or germination (%) in chilaca chili pepper is for treatment 7, the result is reversed with respect to the germination result; that is, the higher the percentage of germination, the lower percentage of abnormal seedlings or in its case SSG, for melon, the one that had the lowest percentage of abnormal seedlings was treatment 6 and for cucumber, it was treatment 4.
Regarding the chilaca chili pepper, the lowest percentage of non-germinated seeds was observed in treatments 1 and 8, in the case of melon, it was treatment 3 in which there was a low percentage of non-germinated seeds, and regarding cucumber, treatment 7 was the one that had a minimum percentage of non-germinated seeds, achieving a synchrony in results with the percentage of germination, which is what was expected (Figure 1).
Effect on the vigor and growth of plants. In Table 2, it is observed that there are highly significant differences regarding the development of seedlings between the treatments, considering APL and ARL, likewise and as indicative of a physiological component in the seeds, the V and DW show that there are also differences, these similar results are found in the interaction treatment*species, while in the species evaluated, it is perceived that there are highly significant differences in all the variables analyzed.
Table 2 Mean squares of the variables analyzed, vigor and growth of plants.
| Source of variation | DF | ARL (cm) | APL (cm) | V (%) | DW (g) |
| Treatment | 7 | 4.97** | 39.45** | 245.27** | 0.08** |
| Species | 2 | 542.82** | 935.31** | 13311.16** | 6.42** |
| Treatment*species | 14 | 3.03** | 13.95** | 228.11** | 0.01** |
| SE | 72 | 1.03 | 0.58 | 26.06 | 0.005 |
| Mean | 11.82 | 12.57 | 81.47 | 0.64 | |
| CV | 8.61 | 6.08 | 6.26 | 11.05 |
* and **= significant values with p≤ 0.05 and p≤ 0.01, respectively; ns=not significant; DF= degrees of freedom; ARL= average radicle length; APL= average plumule length; V= vigor and DW= dry weight of seedling.
When analyzing Figure 2a, it is observed that, for the three species studied, there is a better root system in treatment 3; however, there is a numerical difference between them, we can emphasize that the performance of fused biofertilizers is still reflected, followed by treatment 4 and 5, biofertilizers are undoubtedly present, but individually.
In Figure 2b, it is observed that in the case of chilaca chili pepper and melon, the best result regarding the development of plumula is given by treatment 6, since, despite the high concentration of salinity, biofertilizers performed the function adequately, while, for cucumber, the best result was obtained in treatment 8, which shows us once again that despite the high concentrations of salinity, biofertilizers act by making the seedlings physiologically present tolerances to salinity and vigor in the seed.
The development of seedlings is in accordance with what was reported by Cruz-Romero et al. (2016), who, in their research, inoculation with the strain 7A of Azospirillum and foliar spraying of honey, demonstrated that they have plant growth-promoting effects, therefore, constitute a promising and viable alternative for the production of vegetable seedlings. Considering that vigor is one of the most important parameters in seeds, it is observed that chilaca chili pepper and cucumber had a higher percentage of vigor in treatment 8, this indicates that these species are more tolerant to salinity and that the inoculation of biofertilizers is a very efficient alternative, showing difference with respect to the absolute control and chemical treatment. While melon had the highest percentage of vigor in all treatments even when the seed is inoculated (ranging between 87.5 and 98%), in treatments 3 and 4 inoculated with Az + Gl and Az, respectively, as shown in Figure 2c.
Likewise, Rojas and Ortuño (2007) conclude, in their research work, that the plants inoculated with mycorrhiza in combination with worm humus and poultry manure, evaluated in the onion crop, allowed the obtaining of high yields and a greater development of the plant (greater plant height, greater bulb diameter, greater root development, greater vigor and greater plant health) in the absence of chemical fertilizers.
By observing the results in Figure 2d, we deduce that in the case of chilaca chili pepper and melon, more dry matter was generated in treatment 7 and in the case of cucumber, the greatest amount of dry matter was obtained in treatment 6, emphasizing that the inoculation of biofertilizers considerably increased the dry matter of the seedlings, this result goes in parallel with what was obtained in APL, ARL, as well as the vigor of the seed, showing highly significant differences with respect to the control and showing a considerable result when the biofertilizers interact in combination with the potassium chloride (Mujica and Batlle, 2013).
By performing a comprehensive analysis of the growth and development indices of the plants evaluated in this test (plant height, lower and upper diameter of the stem, number of flowers and aerial dry mass), the effectiveness of the inoculation by capillarity of the Glomus species was verified, for all variables, the inoculated plants stand out in relation to the control.
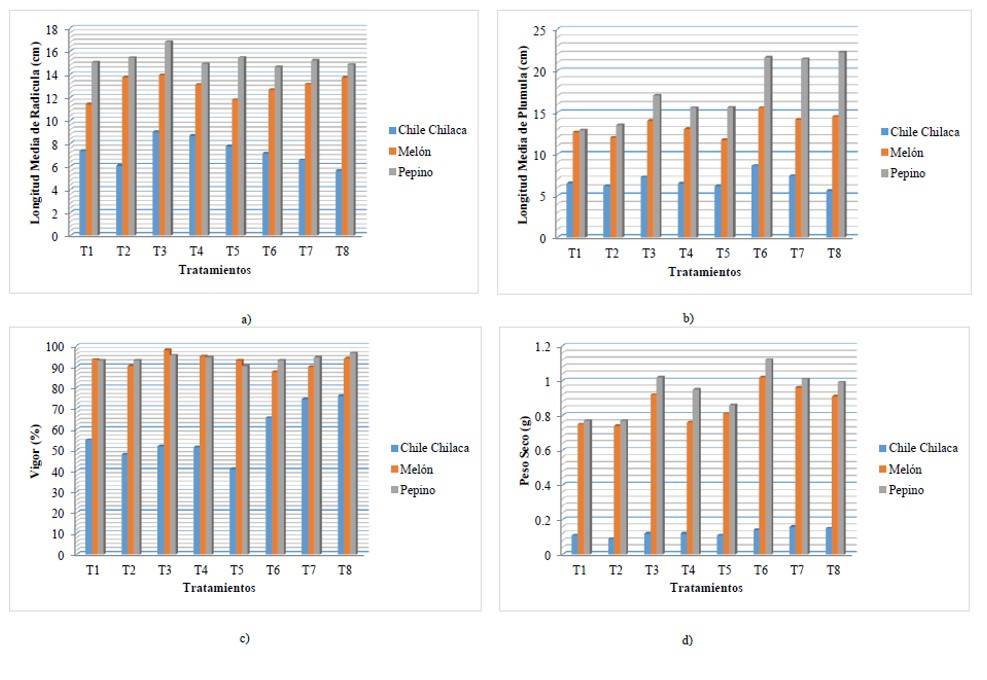
Figure 2 Comparison of means of treatments/species. a) average radicle length; b) average plumule length; c) vigor; and d) dry weight.
In this regard, Parra and Cuevas (2001) indicate that the mechanism by which Azospirillum influences the development and productivity of plants is not clear and among the explanations, they point out its ability to fix atmospheric N in the soil through the increase in the activity of the nitrate reductase enzyme that stimulates the assimilation of nitrates by inoculated plants.
Conclusions
Based on the results obtained in the present research, we can conclude concretely that the inoculation of biofertilizers in the seeds under study has a positive effect regarding the variables studied.
The results obtained by inoculation of biofertilizers in combination with the saline concentrations physiologically promoted the horticultural species under study, being treatments T7 and T8 with greater response with respect to the absolute control, showing significant differences; that is, the combination of microorganisms and potassium chloride have a positive effect on germination and vigor in horticultural species.
Literatura citada
Adesemoye, A. O. y Kloepper, J. W. 2009. Plant-microbes interactions in enhanced fertilizer-use efficiency. Appl. Microbiol. Biotechnol. 85(1):1-12. [ Links ]
Ayala-Apaza, B. V.; Huanca-Chui, C. y Fernández-Chávez, C. M. 2019. Evaluación del cultivo de la lechuga (Lactuca sativa) en sistema hidropónico bajo dos niveles de cloruro de potasio. Rev. de Investigación e Innovación Agropecuaria y de Recursos Naturales. 6(2):66-71. http://www.scielo.org.bo/scielo.php?script=sci-arttext&pid=S2409-16182019000200009&lng=es&tlng=es . [ Links ]
Aguado, S. G. A. 2012. Introducción al uso y manejo de los biofertilizantes en la agricultura. In: Aguado-Santacruz, G. A. 1a (Ed.). INIFAP/SAGARPA. México, DF. 35-37. [ Links ]
Bagyaraj, D. J. 1984. Biological interaction with VA mycorrhizal fungi. In: Mycorrhizae, V. A. Powell, C. and Bagyaraj, D. (Ed.). CRC Press, Boca Raton, FL. 240 p. [ Links ]
Barea, J. M. and Azcón-Aguilar, C. 1983. Mycorrhiza and their significance on nodulating nitrogen fixing plants. Adv. Agron. 36:1-54. [ Links ]
Bashan, Y.; Holguin, G. and de-Bashan, L. E. 2004. Azospirillum-plant relationships: physiological, molecular, agricultural, and environmental advances. Can. J. Microbiol. 50(8):521-577. [ Links ]
Belimov, A. A.; Kojemiakov, A. P. and Chuvarliyeva, C. V. 1995. Interaction between barley and mixed cultures of nitrogen fixing and phosphatesolubilising bacteria. Plant Soil. 173:29-37. [ Links ]
Bloemberg, G. V. and Lugtenberg, B. J. 2001. Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr. Opin. Plant Biol. 4(4):343- 350. [ Links ]
Cardon, Z. G. and Whitbeck, J. L. 2007. The rhizosphere-an ecological perspective. Academic Press, San Diego, CA. 31-56 pp. [ Links ]
Cruz-Romero, W.; Barrios-Díaz, J. M.; Rodríguez-Mendoza, M. N.; Espinoza-Victoria, D. y Tirado-Torres, J. L. 2016. Producción de plántulas de hortalizas con Azospirillum sp. y aspersión foliar de miel de abeja. Rev. Mex. Cienc. Agríc. 7(1):59-70. http://www.scielo.org.mx/scielo.php?script=sci-arttext&pid=S2007-09342016000100059&lng=es&tlng=es . [ Links ]
Elo, S.; Maunuksela, L.; Salkinoja-Salonen, M.; Smolander, A. and Haahtela, K. 2000. Humus bacteria of Norway spruce stands: plant growth promoting properties and birch, red fescue and alder colonizing capacity. FEMS Microbiol. Ecol. 31(2):143-152. [ Links ]
Franco-Correa, M. 2009. Utilización de los actinomicetos en procesos de biofertilización. Rev. Per. Biol. 16(2): 239-242. [ Links ]
Galal, Y. G. M.; El-Ghandour, I. A.; Osman, M. E. and Abdel, R. A. M. N. 2003. The effect of inoculation by mycorrhizae and rhizobium on the growth and yield of wheat in relation to nitrogen and phosphorus fertilization as assessed by 15N techniques. Symbiosis. 34:171-183. [ Links ]
Hungría, M. 2010 Inoculation with selected strains of Azospirillum brasilense and A. lipoferum improves yield of maize and wheat in Brazil. Plant and Soil. 331:413-425. [ Links ]
ISTA. International Seed Testing Association. 2004. International rules for seed testing. PO Box 308, 8303 Basserdorf, CH-Switzerland. ISBN: 3-906549-38-0. Chapter 3, 4, 5 y 9. [ Links ]
Manoharachary, C. and Mukerji, K. G. 2006. Rhizosphere biology-an overview. In: Mukerji, K. G.; Manoharachary, C. and Singh, J. (Ed.), microbial activity in the Rhizosphere. Springer, German. 1-38 pp. [ Links ]
Miller, R. M. and Jastrow, J. D. 2000. Mycorrhizal fungi influence soil structure. In: arbuscular mycorrhizas: physiology and function. Kapulnik, Y. and Douds, D. (Ed.). Kluwer Academic Publishers, Dordrecht, The Netherlands. . [ Links ]
Mujica, P. Y. y Batlle, S. J. 2013. Funcionamiento de la inoculación líquida con hongos micorrízicos arbusculares (HMA) en plantas de tomate (Solanum lycopersicum L.). Cultivos Tropicales. 34(4):5-8. http://scielo.sld.cu/scielo.php?script=sci-arttext&pid=S0258- 59362013000400001&lng=es&tlng=es . [ Links ]
Nguyen, T. H.; Kennedy, I. R. and Roughley, R. J. 2002. The response of field grown rice to inoculation with a multi-strain biofertilizer in the Hanoi district, Vietnam. In: biofertilizers in action. Kennedy, I. R. and Choudhury, A. T. M. A. (Ed.). Rural Industries Research and Development Corporation, Barton, ACT. 37-44 pp. [ Links ]
Parra, Y. y Cuevas, F. 2001. Revisión bibliográfica. Potencialidades de Azospirillum como inoculante para la agricultura. Cultivos Tropicales. 23(3):31-41. [ Links ]
Rojas, R. K. y Ortuño, N. 2007. Evaluación de micorrizas arbusculares en interacción con abonos orgánicos como coadyuvantes del crecimiento en la producción hortícola del Valle Alto de Cochabamba, Bolivia. Acta Nova. 3(4):697-719. http://www.scielo.org.bo/scielo.php? script=sci-arttext&pid=S1683-07892007000200005&lng =es&tlng=es. [ Links ]
Rueda, P. E.; Barrón, H. J. y Hallman, J. 2009. Bacterias promotoras del crecimiento vegetal. 1ª (Ed.). Plaza y Valdés. México. 141 p. [ Links ]
Sharma, S. 2012. Impact of application of biofertilizers on soil structure and resident microbial community structure and function. In: Maheshwari, DK. (Ed.). Bacteria in agrobiology: plant probiotics. New Delhi: Hauz Khas. 65-79 pp. [ Links ]
Star, L.; Matan, O.; Dardanelli, S. M.; Kapulnik, Y.; Burdman, S. and Okon, Y. 2012. The Vicia sativa spp. nigra Rhizobium leguminosarum bv. viciae symbiotic interaction is improved by Azospirillum brasilense. Plant Soil. 356:165-174. [ Links ]
Whipps, J. M. 2001. Microbial interactions and biocontrol in the rhizosphere. J. Exp. Bot. 52:487-511. [ Links ]
Received: May 01, 2021; Accepted: July 01, 2021











 texto em
texto em