Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista odontológica mexicana
versão impressa ISSN 1870-199X
Rev. Odont. Mex vol.18 no.2 Ciudad de México Abr./Jun. 2014
Original research
Similarities of elemental chemistry and morphology of cements type mineral trioxide aggregate and Portland cement through the use of scanning electronic microscopy and electron dispersion spectroscopy
Raúl Luis García Aranda* y Margarita García Garduño§
* Vice-president, Mexican Endodontics Commitee, Professor of Endodontics, National School of Dentistry.
§ Professor, Faculty of Sciences.
National University of Mexico (UNAM).
ABSTRACT
Currently, studies on similarities among Portland cement and cements type mineral trioxide aggregate have shown that these compounds exhibit similar performance. This can be due to the fact that they are made based on Portland cement. With the aim of assessing percentages and components of Portland cement and commercial cements type mineral trioxide aggregate ProRoot® white and Pro-Root® grey, Angelus® and CPM®, five 8 x 4 mm samples of each material were processed. These samples were subjected to a study of surface texture. This study was conducted with the help of a scanning electron microscope as well as a energy dispersive spectrometry analysis. Results: Upon comparison, Portland cement and mineral trioxide aggregate cements showed great similarities. The main difference was than in mineral trioxide aggregate there was absence of Fe, Mg, Na and K; in mineral trioxide aggregate cements O, C, Si, Ca, Al, Cl and Bi were found regularly. Ba presence was only detected in CP®. Conclusions: The present study established the presence of great similarities among chemical components of Portland cement and mineral trioxide aggregate cements of all commercial brands.
Key words: MTA type cements, Portland cement, MTA morphology, MTA elementary chemistry.
INTRODUCTION
The material known as trioxide aggregate (MTA) was first developed at the Loma Linda University for surgical use in retro-fillings.1 This material was patented by Torabinejad2 in 1995.
MTA Pro-Root® powder (Dentsply Tulsa, Ok USA)consists of small-sized hydrophylic particles, with presence of tricalcium silicate, tricalcium aluminate and silica oxide.1 This material has been studied in three phases: powder phase, crystalline phase and MTA powder hydration phase, forming a colloidal gel which sets with a pH of 10.2 and 12.5 after a three hour mixing process.1
Komabayashi et al3 concluded that white ProRoot MTA contained smaller particles, with smaller size range than grey Pro-Root MTA. MTA Angelus exhibited less circular, larger ranked particles which were less homogeneous than both white and grey ProRoot.
Setting time represents another problem which has arisen with MTA use. Torabinejad4 mentioned that setting time was less than four hours, whereas Kogan et al5 informed it was 50 minutes when mixed with sterilized water. Differences depended upon the method used to determine the setting process. An example could be the use of a Gilmore needle versus vacant Chng et al technique.6 When following methods established by the International Organization for Standardization, it was reported that setting process, from beginning to end was between 70 and 175 minutes, respectively.
Several methods have been used in order to determine MTA properties. Among them we can count scanning electron microscopy (SEM),7 spectroscopy8 and X-ray diffraction.9 Studies conducted with SEM provide images; nevertheless, they only allow morphological evaluation of the specimens' topographical characteristics.
Estrela10 and Funteas et al,11 among others, have reported similarities between MTA and Portland Cement (PC) and their basic components. Camilleri12 reported the production of calcium hydroxide as resulting product to PC and MTA hydration. Holland et al13 proposed the theory that PC and MTA mechanisms of action were very similar. In an energy dispersive spectrometry (EDS) analysis, Camilleri et al12 showed that the components' elements were the same.
With respect to bio-tolerance, Ribeiro DA et al14 indicated that grey and white MTA Pro-Root were not genotoxic and thus did not cause cellular death. In a similar manner to Camilleri et al15 informed that in biocompatibility studies, MTA extracts did not elicit grey MTA-derived cytotoxic reactions. They also informed that the addition of bismuth oxide to Portland cement did not interfere with biotolerance.
MATERIALS AND METHODS
The five cements used in the present study were divided into groups in the following manner: Group 1. Portland Cement, Group 2. White Pro-Root MTA(Dentsply, Tulsa, Ok USA), Group 3. Grey Pro-Rootcement (Dentsply Tulsa, Tulsa OK USA), Group 4. White MTA Angelus (Angelus, Londrina, Parana, Brazil), and Group 5. MTA CPM (Medix Mexico DF, Mexico).
All cements were mixed with the liquid provided by the manufacturer. Manufacturers' instructions were strictly followed. Bi-distilled water was used with Portland Cement. Five samples were made of all groups. Samples measured 8 mm diameter by 4 mm thickness. All samples were placed in a Hanau oven at 95% humidity at 37.5 ± 5 oC for 24 hours.
Once set, the samples were placed in the sample tray which had a carbon film to which the samples became adhered to. A scanning electron microscope (Jeol Model 5900LV, Tokio Japan) was used to make observations. The microscope possessed magnification range of 18 X to 300,000X. Magnifications used were 500X, 1000X and 2000X.
An elemental chemical analysis was conducted in an Oxford equipment, ISIS model, with 133 eV resolution and detection of elements from carbon to uranium. For the study, magnifications of 500, 1000 and 2000X were used at four points previously determined in all samples, at 2 Sigma, as data variability measure, implying thus it was found to be within 95% of real value.
Comparisons were established among all 5 elements shared in Portland type cement and the four elements found in MTA type cement. Student t test was applied.
At a later point a Kruskal-Wallis non-parametric variance analysis was applied in order to compare all elements of the five cements.
RESULTS
All five elements in shared Portland type cement were compared, as well as all four elements shared in MTA type cement. Significant differences were found with exception of CK elements (Tables I and II).


The aforementioned results were obtained applying a Student t test, where a comparison was established between MTA cements versus Portland type cement.
A Kruskall-Wallis non-parametric variance analysis was later applied in order to compare all elements of the five cements. K = 0.244, p = 0.993.
In the analysis conducted with the help of the SEM, an irregular and porous micro-structure was observed in hydrated and hardened Portland cement (Figure 1). In hydrated grey and white Pro-Root, as well as in Angelus and CPM, a more homogeneous, irregular porous image was observed, with some loose granules identified as bismuth, and in CPM bismuth and barium were found (Figure 1).

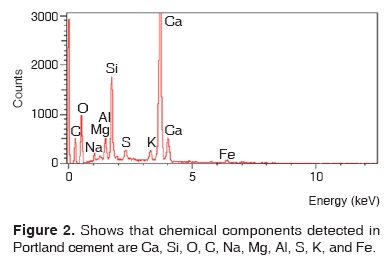
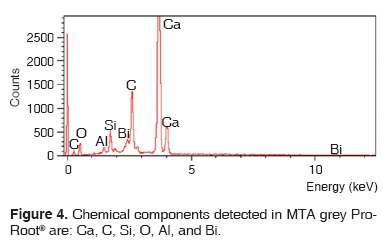
Results obtained with energy dispersive spectrometry (EDS) show that in Portland cement, Group 1, there was presence of Fe, Mg, Na and K (Figure 2 and Table III). These aforementioned elements were absent in the other groups. In groups 2, 3, 4 and 5, O, C, Si, Ca, Cl and Bi were regularly detected (Figures 3, 4, 5 and Tables IV, V, VI). Ba and S were only detected in Group 5 (Figure 6 and Table VII).








DISCUSSION
Many studies have been published on Portland cement, and grey and white MTA with respect to their chemical composition, superficial structural characteristics, sealing abilities, biocompatibilities and capacity to regenerate and repair original tissue.
From the surface analysis perspective, some reports state that there is a material with irregular consistence with granulated areas resembling coral.16 These characteristics are in agreement with our study, except in the case of white Pro-Root MTA, whose surface is less rough and porous when compared to the surfaces of other studied cements.
Asgary et al17 reported the fact that MTA presented significantly lesser amounts of ferric oxide, as well as aluminum oxide and manganeseoxide. In our EDS study, no presence of Fe and Mg and aluminum oxide was detected, no significant differences were observed although, regularly, amounts were always smaller than those found in Portland cement.
In the SEM study conducted by Oliveira et al18 it was reported that they found chemical components which were very similar among all studied materials. Percentages only exhibited minimal differences. Bismuth was the only additional element. Our study agrees with this report; bismuth was the additional element. Only in the CPM cement, additional elements were bismuth and barium. Our study also agreed with Oliveira's study with respect to surface analysis: when using scanning electron microscope, differences were observed in textures and in each material's particles.
With respect to bismuth percentages, Funteas et al16 reported that this material was insoluble, and was incorporated to the Pro-Root® MTA formulation in order to provide radio-opacity to the material. He reported an average 9.2 bismuth percentage. In our study, this percentage waslower, since 4.46% was detected in white Pro-Root®, 2.51% was detected in grey Pro-Root®, 1.61% was detected in Angelus® and 6.96% was detected in CPM. General average was 3.10%. Barium percentage detected in CPM® was 19.87.
J. Camillleri19 reported in his study that MTA was aluminum-deficient. He suggested the material had been prepared in rotary ovens, as is habitual for Portland cement manufacturing. In the process of hydration, this affects ettringite and monosulfate production, which are usually formed during hydration of Portland cement. Bismuth affects the material's hydration mechanism in MTA type cements, it is part of the C-S-H structure and also affects precipitation of calcium hydroxide in the hydrated paste. He also states that MTA possesses a more fragile microstructure when compared to Portland cement.
CONCLUSIONS
There is a great similarity of chemical components when comparing Portland Cement and MTA type cements of all commercial brands, with exception of chemical components which provide opacity such as bismuth oxide and barium oxide. Nevertheless, further studies are required geared to researching processes of decreasing setting time and increasing compressive forces in order to then be able to consider them suitable restorative materials.
REFERENCES
1. Torabinejad M, Hong CU, McDonald F, Pitt Ford TR. Physical and chemical properties of a new root-end filling material. J En-dod. 1995; 21: 349-353. [ Links ]
2. Torabinejad M, White DJ. Tooth filling material and use. US Patent Number, 5,769,638; May 1995. [ Links ]
3. Takashi K, Spangberg SW. Comparative analysis of the particle size and shape of commercially available mineral trioxide aggregates and Portland cement: a study with a flow particle image. Analyzer J Endod. 2008; 34: 94-98. [ Links ]
4. Torabinejad M, Chivian N. Clinical applications of mineral trioxi-de aggregate. J Endod. 1999; 25: 197-205. [ Links ]
5. Kogan P, He J, Glickman GN, Watanabe I. The effects of various additives on setting properties of MTA. J Endod. 2006; 32: 569-572. [ Links ]
6. Chng HK, Islam I, Yap AU, Tong YW, Koh ET. Properties of a new root-end filling material. J Endod. 2005; 31: 665-668. [ Links ]
7. Abdullah D, Ford TR, Papaioannou S, Nicholson J, McDonald F. An evaluation of accelerated Portland cement as a restorative material. Biomaterials. 2002; 23: 4001-4010. [ Links ]
8. Bozeman TB, Lemon RR, Eleazer PD. Elemental analysis of cristal precipitate from gray and white MTA. J Endod. 2006; 32: 425-428. [ Links ]
9. Song JS, Mante FK, Romanow WJ, Kim S. Chemical analysis of powder and set forms of Portland cement, gray ProRoot MTA, white ProRoot MTA, and gray MTA-Angelus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 102: 809-815. [ Links ]
10. Estrela C, Bammann LL, Estrela CR, Silva RS, Pecora JD. Antimicrobial and chemical study of MTA, Portland cement, calcium hydroxide paste, Sealapex and Dycal. Brazi Dent J. 2000; 11: 3-9. [ Links ]
11. Funteas UR, Wallace JA, Fochtman EW. A comparative analysis of mineral trioxide aggregate and Portland cement. Aust Dent J. 2003; 29: 43-44. [ Links ]
12. Camilleri J, Montesin FE, Brady K, Sweeney R, Curtis RV, Pitt Ford TR. The constitution of mineral trioxide aggregate. Dental Materials. 2005; 21: 297-303. [ Links ]
13. Holland R, de Souza V, Nery MJ, Otoboni-Filho JA, Bernabe PF, Dezan-Junior E. Reaction of rat connective tissue to implanted dentin tubes filled with mineral trioxide aggregate or calcium hydroxide. J Endod. 1999; 25: 161-166. [ Links ]
14. Ribeiro DA, Sugui MM, Matsumoto MA, Duarte MA, Márquez ME, Salvadori DM. Salvadori ex vivo biocompatibility tests of regular and white forms of mineral trioxide agrégate. Oral Surg Oral Med Oral Path Oral Radio. 2006; 101 (2): 258-261. [ Links ]
15. Camilleri J, Montesin FE, Di Silvio L, Pitt-Ford TR. The chemical constitution and biocompatibility of accelerated Portland cement for endodontic use. Int Endod J. 2005; 38 (11): 834-842. [ Links ]
16. Diamanti E, Kerezoudis NP, Gakis DB, Tsatsas V. Chemical composition and surface characteristics of grey and new white ProRoot MTA. J Endod. 2003; 36: 946-947. [ Links ]
17. Asgary S, Parirokh M, Eghbal MJ, Brink F. Chemical differences between white and gray mineral trioxide aggregate. J Endod. 2005; 31: 101-103. [ Links ]
18. de Oliveira MG, Xavier CB, Demarco FF, Pinheiro AL, Costa AT, Pozza DH. Comparative chemical study of MTA and Portland cements. Braz Dent J. 2007; 18 (1): 3-7. [ Links ]
19. Camilleri J. Hydration mechanisms of mineral trioxide aggregate. Int Endod J. 2007; 40: 462-470. [ Links ]
Note This article can be read in its full version in the following page: http://www.medigraphic.com/facultadodontologiaunam Mailing address:
Mailing address:
Raúl Luis García Aranda, MD
E-mail: rlga@unam.mx











 texto em
texto em 


