Introduction
Hypoxia-inducible factor (HIF) is a heterodimer that increases the transcription of several genes by binding to DNA in consensus 5’-RCGTG-3 ‘regions (R = A or G)1. This transcriptional factor it’s made up of two proteins called HIF-α and HIF-β, also known as ARNT (hydrocarbon receptor nuclear that translocated), which have at their amino terminal b-HLH domain (basic-Helix loop) and two PAS domains (period clock hydrocarbon receptor nuclear that translocated single minded). The b-HLH domain regulates the dimerization of HIF-1 that which controls its binding to DNA, while the PAS domain participates in the selection of the target gene and the expression of HIF-β; the activity of HIF-1 can be regulated only by the expression of HIF-α. When situation of normoxia (normal oxygen concentration ~ 20-50 μM), HIF-1α is not detected and its half-life (t½) is 5 min, but in a state of hypoxia (O2 concentration 1% or 12.5 μM), its t ½ increases considerably up to 30 min. HIF-1α undergoes a degradation when in contact with oxygen and becomes sensitive (O DDD: oxygen-dependent degradation domain) that regulates its stability2. Under situation normoxia, this domain a hydroxylated in proline 402 and 564 by HIF-1α prolyl-4-hydroxylases, this allows its interaction with amino acids 549-572 of the domain of the von Hippel-Lindau protein (p VHL). The p VHL is a component of the E3 complex ubiquitin ligase that is responsible for ubiquitinated (process chain addition ubiquitin to a labeled protein for degradation) to the HIF-1 leading it to be degraded by the proteasome interaction between p VHL and the specific domain of HIF-1α which are regulated by the hydroxylation of the proline residue (HIF-1α P564) by an enzyme called HIF-1α prolyl hydroxylase (HIF-PH), requiring iron and oxygen.
On the other hand, if HIF-1α is not degraded, its transcriptional activity blocked when asparaginyl-aspartyl hydroxylases hydroxylate asparagine 803, which is found in the transactivation domain (CAD). Hydroxylases play an important role in regulating HIF-1 activity, however, under hypoxia, it is not clear how their activity decreases. Both enzymes require various substrates to catalyze the hydroxylation of HIF-1α3. Under hypoxic conditions, molecular O2 is not available, and therefore, the enzymes are inactive, which implies an increase in HIF-1α values. However, HIF-1α is not hydroxylated and, therefore, degraded, causing its accumulation in the heterodimerized form with the beta-subunit (HIF-β). This heterodimer migrates to the nucleus, where it will bind to specific DNA sequences, activating genes involved in adaptation to hypoxia, cell survival, angiogenesis, and metastasis, such as vascular endothelial growth factor (VEGF), the transforming growth factor-alpha (TGF-α), glucose transporter 1 (GLUT-1), or carbonic anhydrase IX (CA9), among many others4,5. The HIF complex plays a fundamental role in the process of neurogenesis, which involves various stages, such as proliferation of neuronal stem cells, migration, differentiation, survival of new neurons, as well as their integration into neurons and existing neural circuits. The first findings in the discovery of the hypoxia-inducible factor that indicated the formation of new neurons derive from the studies carried out by Altman and Cols in the 1970s, when hippocampal incorporation of tritiated thymidine (a marker of cell proliferation that integrates with deoxyribonucleic acid) was quantified; a dogma defended by neuroscience held that neurons lost their ability to reproduce and regenerate after birth and that those that died were irreplaceable. However, it was shown that in some areas of the brain, there was a neuron production process, that is, neurogenesis; but that said neuronal proliferation is restricted to certain areas called specialized neurogenic niches. The formation of these new neurons is regulated by multiple factors, which can positively or negatively influence neurogenesis. One of them is the presence of certain growth factors, which function as signaling molecules when they act on target cells; such as the vascular endothelial growth factor and erythropoietin6. To date, HIF is the only known protein that responds in a specific way to hypoxia, while the rest of the transcription factors previously described lack this specificity, being involved in the regulation of other physiological processes independent of the degree tissue oxygenation.
Neurogenesis
Adult neurogenesis is a complex process, beginning with the division of a precursor cell and leading to the functional integration of newborn neurons into a pre-existing circuit. Neurogenesis occurs in two brain structures throughout life, the olfactory bulb and the hippocampus. Two types of interneurons are found in the olfactory bulb, generated from a population of dividing precursor cells in the subventricular zone (SVZ)7,8. The continuous addition of interneurons that modulate the spatial and temporal coding of olfactory information could provide a substrate to adapt to environmental changes9. In the dentate gyrus of the hippocampus, new granule cells are continually generated from precursor cells in the subgranular zone10. The neurogenesis of the hippocampus includes different steps from a stem cell similar to a radial glia (a cell with a bipolar shape that spans the entire width of the cortex in the developing central nervous system [CNS]) and that serves as a primary progenitor capable of generating neurons, astrocytes, and oligodendrocytes presumably bipotent with properties astrocytic to transient amplifying progenitor cells determined according to its function, to mature neurons and post-mitotic (those that are not renewed, such as neurons) early11. The newly generated cells mature into functional neurons, which are structurally integrated into a pre-existing network; they receive synaptic information from local interneurons and the entorhinal cortex (located in the middle temporal lobe and functions as a hub in an extended network for memory and orientation) and extend axons to target cells in the CA3 region12. The structural integration of neurons generated in the adult brain is the prerequisite for functional synaptic integration. Throughout life, there is a progressive reduction in the neurogenesis of the hippocampus in the adult.
Among the many intrinsic and extrinsic molecular signals that regulate the production of new neurons from adult progenitor cells in the central nervous system (CNS), hypoxic damage plays an important role in the maintenance and function of stem cells in development and disease. HIF-1α is a transcription factor known for its role in the regulation of metabolic and angiogenic responses to hypoxia. Although HIF-1α normally undergoes proteasomal degradation under non-hypoxic conditions, it is constitutive stabilized in multiple stem cell types in vivo, including bone marrow hematopoietic stem cells, adult subventricular zone (SVZ) mesenchymal stem cells, and of the subgranular zone (SGZ) of the hippocampus13.
Hypoxia-inducible factor (HIF) and neurogenesis
The investigation of the molecular mechanisms of one of the most surprising responses to hypoxia was the induction of hematopoietic growth hormone erythropoietin (EPO), which allowed the first identification of a hypoxia-inducible transcription factor14,15. When the oxygen content in the blood is reduced in anemia or at high altitude, the production of EPO in renal interstitial fibroblasts is rapidly activated. Studies of DNA-protein interactions in the EPO gene enhancer 30 identified a protein complex that only bound in hypoxia. Semenza and Wang designated it hypoxia-inducible factor 1 (HIF-1). HIF has been characterized as a key regulator in various mammalian cell responses in response to hypoxia. Changes in gene expression regulated directly or indirectly by HIF extend to more than 100 genes16. An important factor involved is the hypoxia-inducible factor (HIF) and has found that neural stem cells favoring mild hypoxia and hypoxia is a potent stimulator of in vitro expansion of neural stem cells. Hypoxia can also promote the in vitro differentiation of neural stem cells into neurons, particularly dopaminergic neurons. In the adult brain, neural stem cells have been found at high densities in the subventricular zone of the forebrain (SVZ) and the dentate gyrus (DG) of the forebrain. We can also find that in the hippocampus and the increase in neurogenesis in vivo participate in the regulation of learning, memory, repair of brain injuries, and the fight against depression. As mentioned above, the treatment of hypoxia promotes angiogenesis, gliogenesis, and neurogenesis in vivo,17,18 it has also been found that exposure to hypoxia can induce the expression of HIF-1α in neurogenic regions of the brain including the dentate gyrus (DG) and the subventricular zone (SVZ). However, almost all existing data regarding cerebral oxygen tension during hypoxia exposure were indirect. It is not clear whether external hypoxia affects the actual oxygen content in the SVZ and DG in the brain as a means of promoting neurogenesis. Actual oxygen concentrations in vivo provide a significant index of the metabolic levels of tissues in different physiological or pathological states19. Oxygen demand, metabolic activity, and brain function vary according to the needed region of the brain. Oxygen supply must be precisely controlled in the brain in response to local demands induced by metabolic activity. In particular, HIF-1 mediates the developmental and physiological pathways that deliver O2 to cells allowing them to survive O2 deprivation20. Low oxygen concentrations in vitro increase proliferation and reduce cell death of CNS precursor cells21. In normoxia, HIF-1α is hydroxylated by an oxygen-dependent reaction that allows the binding of the von Hippel-Lindau protein, then HIF-1α accumulates and forms dimers but also with HIF-1b. HIF-1α/HIF-1b binds to hypoxia-responsive elements in the regulatory sequence of various genes22. The conditional deletion of the HIF-1α gene demonstrates that HIF-1α is necessary for normal brain development and for the proliferation and survival of precursor cells derived from the embryonic midbrain23,24. Focal cerebral ischemia activates the HIF-1 transcription factor. A well-known target gene for HIF-1 is vascular endothelial growth factor (VEGF). In fact, HIF-1α and VEGF are localized after cerebral ischemia. HIF can induce a wide range of gene products that control energy metabolism, neovascularization, survival, pH, and cell migration, and has become an important promoter of tumor growth. This pro-oncogenic feature is only one facet of HIF’s dual action. In addition to being a “gatekeeper” of oxygen homeostasis, HIF is capable of inducing pro-apoptotic genes that lead to autophagy and cell death, which can be characteristic of hypoxic tissues. In this sense, HIF functions as a master gene that controls nutritional stress, angiogenesis, tumor metabolism, invasion, and autophagy/cell death25.
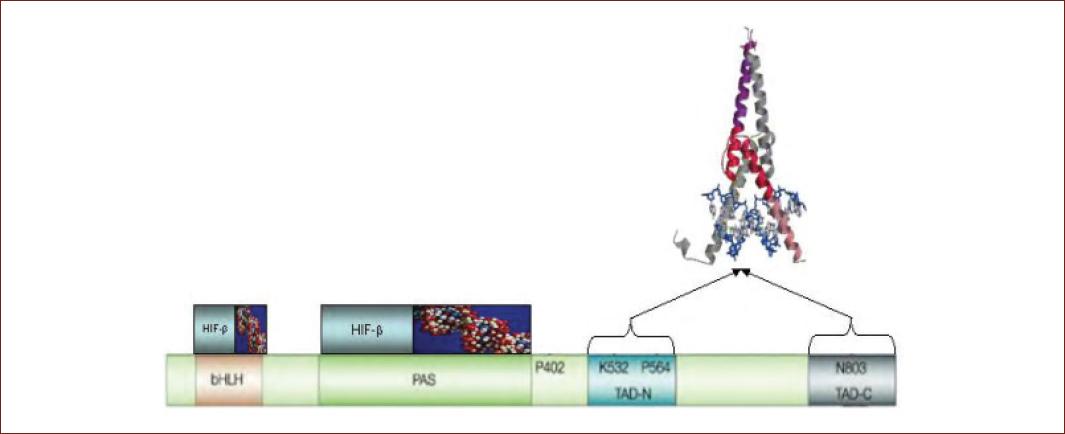
Figure 1 HIF-1α protein domains. The domains of HIF-1α are represented, as well as the fundamental amino acids in the oxygen-dependent regulation of the protein. Interactions with HIF-β, DNA-binding sites, and domains involved in transcriptional activity are shown41. bHLH: helix-loop-helix basic domain. PAS: dimming auxiliary domain. TAD-N: amino terminal transactivation domain. TAD-C: carboxyl terminal transactivation domain. P402 and P564: Proline at positions 402 and 564. K: 532: lysine at position 532. N803: asparagine at position 803.
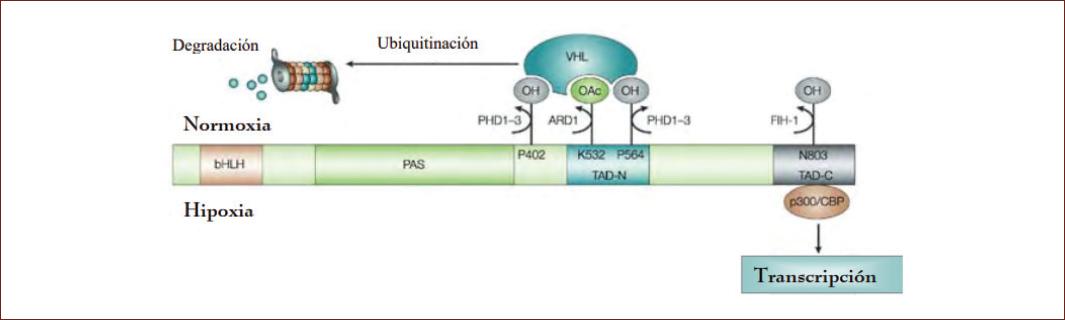
Figure 2 Oxygen-dependent regulation of HIF-1α. The oxygen-dependent post-translational regulation mechanism is shown. The activity of PHDs in normoxia leads to the hydroxylation of proline 402 and 564 of HIF-1α, which marks the protein for its interaction with VHL and its ubiquitination and subsequent degradation in the proteasome. This protein-protein interaction is increased by the action of ARD1 acetylase, which acetylates lysine at position 532. In hypoxia, inhibition of FIH-1 asparaginyl hydroxylase activity blocks hydroxylation, which of asparagine residue 803, which allows interaction of HIF-1α with the transcriptional coactivator p300/CBP42.
Hypoxia-inducible factor (HIF) and Wnt-catenin
The pathway activation Wnt/β-catenin is characterized by the 1) stabilization of β-catenin cytoplasmic after connection of the receiver by Wnt ligands; 2) nuclear translocation of the β-catenin, 3) interaction of β-catenin with lymphoid enhancer-binding factor 1/T-cell factor 1 transcription factors (LEF/TCF); and the 4) stimulation of target genes26,27. Different stem cell populations are known to reside in hypoxic niches during embryonic development and adult life. Hypoxia-inducible transcription factors (HIFs) directly regulate the Wnt/β-catenin pathway to ensure the maintenance of the two different types of adult and embryonic stem cells. The Wnt/-β-catenin signaling pathway is a branch of an extensive functional network that developed around a class of proteins, called armadillo; it is a specialized member of the larger armadillo protein family consisting of three subfamilies: the p120 subfamily, the beta subfamily (-β-catenin and plakoglobin), and the more distant alpha subfamily; the path of β-catenin signaling is involved in a wide range of biological systems, including stem cell biology, biology of systems development, and adult organs. The first discovery of network Wnt/-β-catenin was reported in 1982 with the identification of the proto-oncogene int-1 in mice28. Due to this, the importance in terms of functionality of the Wnt / -β-catenin pathway is evidenced in various systems and the development of some organs such as the cerebral cortex, hippocampus, eye, lens, spine, umbilical cord, teeth, neural crest, bone, extremities, intestine, lungs, heart, pancreas, liver, kidneys, the mammary glands, the hematopoietic system, and the reproductive system29. The key mediator of Wnt signaling, the armadillo protein-β-catenin, is found in different subcellular spaces, including junctions where it helps stabilize cell-cell contacts, the cytoplasm where -β-catenin levels are tightly controlled for stability protein a and regulatory processes in the nucleus, where β-catenin participates in transcriptional regulation and chromatin interactions. However, many other processes including hepatocyte growth factor, prostaglandins, PKA (protein kinase A), E-cadherin, and hypoxia30. They can also influence catenin levels itself. The functional interaction between members of this protein family is not well understood, but a role for p120 and plakoglobin in Wnt/-catenin signaling has been shown31. Other important mechanisms that regulate subcellular catenin thresholds are those that control its mobilization from adherent junctions and its translocation to the nucleus. One of the central end points of the Wnt/-β-catenin signaling pathway is the regulation of transcription through the binding of -β-catenin to members of the Tcf-1 transcription family/lymphoid enhancer factors (Lef-1)32 factors in the nucleus and the hypoxia-inducible factor. The activation of Wnt-mediated HIF1a promotes the maintenance of the activity of stem cells in both ESC (embryonic stem cells), and NSC (neural stem cells) is significantly decreased in the resulting differentiated cells of these stem cells33. These findings build on previous work that demonstrated that HIF mediates the activation of the Notch1 pathway and important stem cell genes34. Furthermore, the stabilization of HIF1a under hypoxic conditions is believed to play a key role in cancer stem cell function and during oncogenesis. The finding is to define a specific mechanism mediated by Wnt and HIF, all through the proliferation and renewal of ESC cells. It was found that the use of a glycogen synthase kinase (GSK) inhibitor can increase the activity of β-catenin (plus inhibitors of mitogen-activated protein kinase35,36; MEK and fibroblast growth factor signaling) to maintain levels of ESC. The role of HIF-mediated Wnt/β-catenin signaling also has implications for hematopoietic stem cell (HSC) niche biology. It has also been determined that HSCs can reside in two different niches: an endosteal niche and a vascular niche. How these two areas are connected and why HSCs can develop and/or transition to perivascular regions that are likely to be more normoxic37. Therefore, it is likely that the predominant HSC niche is the one located in the most hypoxic-anoxic region. A better characterization of the connection between hematopoietic niche elements and HSCs is the connections (including signaling mechanisms) between niche elements such as mesenchymal stem cells or stromal cells and HSCs38. Combining these new insights and methodologies with the analysis of hypoxia-mediated signaling will allow a clearer understanding of how stem cell niches are regulated in various microenvironments in vivo. Clearly, self-renewal does not operate through identical mechanisms in all stem cells39. However, conserved molecules and signaling pathways likely mediate similar functions such as self-renewal in multiple stem cells. Furthermore, hypoxia can protect the genome. If stem cells reside in niches with less exposure to reactive oxygen species and to should reduce the risk of mutagenesis in stem cell populations that serve as a source of differentiated cells and tissues for life of the organism. However, these pathways may also have a negative effect if hypoxia and Wnt signaling also contribute to the survival of cancer stem cells or inhibit DNA repair in stem cells, which increases the probability of one mutagenesis40.
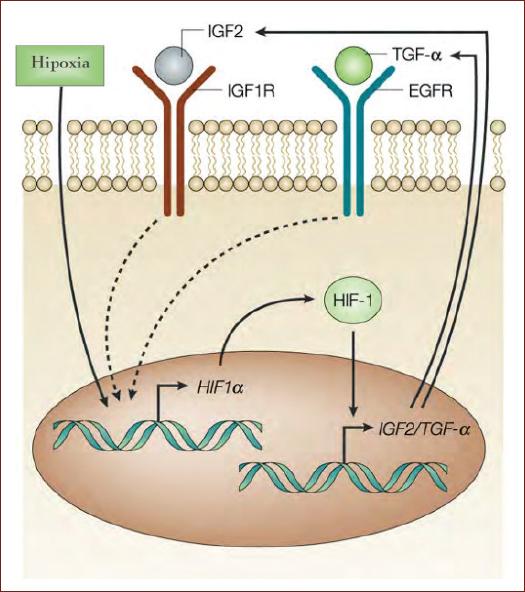
Figure 3 Under hypoxic conditions, the increase in HIF-1 results in the transcription of several genes, among which are IGF-2 or TGF-α, genes whose activity leads to an increase in the translation of HIF-1α, thus contributing to increasing the intracellular concentration of HIF-1α (modified from Semenza, 2003)43.
Conclusions
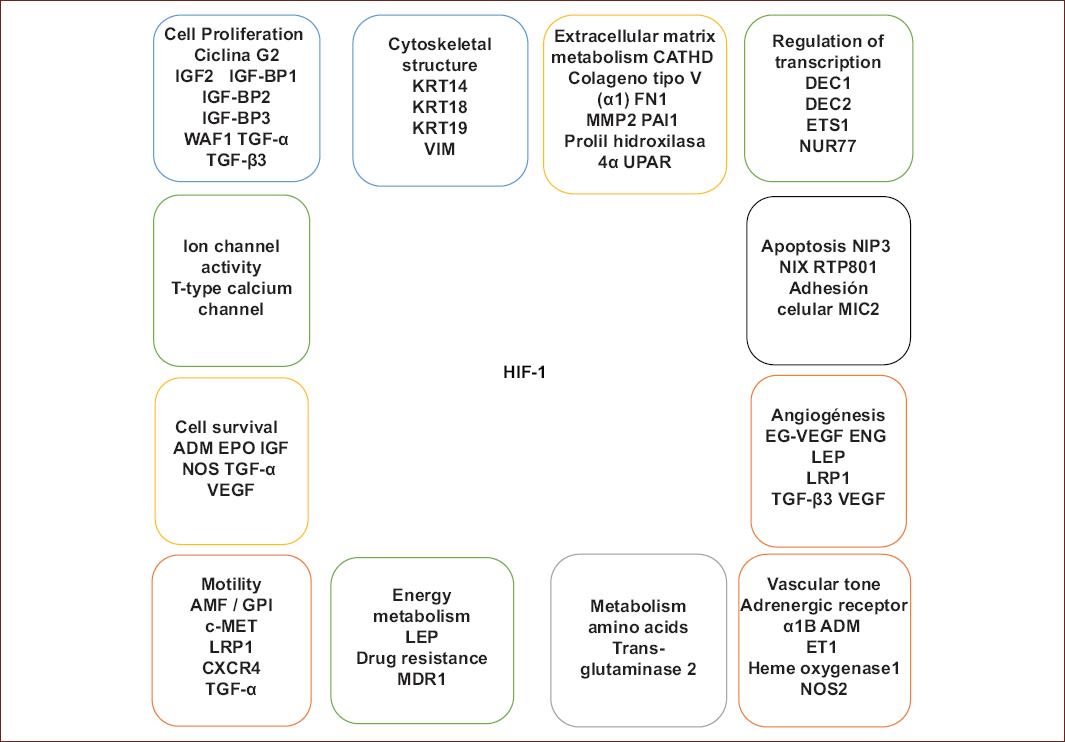
The possibility to stimulate neurogenesis in the adult brain may offer an exciting alternative for brain repair. Considering the described roles of neurogenesis in learning and memory, the hypoxia-induced activation of progenitor cells in the adult hippocampus may help to ameliorate the cognitive decline associated in neurodegenerative diseases. Based on the information provided in this review, we can conclude that HIF-1 increases the expression of certain isoforms of glycolytic enzymes to promote increased glycolysis and other processes (DNA transcription, cell migration, inhibition of the apoptosis, cell proliferation, cell immortalization, invasion, and metastasis).











 nova página do texto(beta)
nova página do texto(beta)