Introduction
Aortic Regurgitation (AR) is a common form of valvular disease characterized by the backflow of blood from the aorta into the left ventricle (LV) during the diastole. AR is the result of various etiologies affecting the aortic valve leaflets or the aortic root1. The clinical presentation of patients with AR depends on the severity of regurgitation and differs whether AR develops acutely or progresses over a prolonged period, allowing the cardiac chambers to adapt.
AR has an estimated prevalence of 4.9% in the Framingham study and moderate to severe AR occurs in 0.5% of the study population. AR occurs in men in 13% and in women at 8.5%. The incidence and severity of AR increase with age with a peak presentation at 40-60 years2.Haga clic o pulse aquí para escribir texto.
Pathophysiologically, AR causes left ventricular volume overload. An increase in LV end-diastolic volume causes eccentric LV dilatation and hypertrophy. This allows the ejection of a larger systolic volume. In patients with AR, the total systolic volume ejected by the LV is the sum of the effective systolic and regurgitant volumes. Thus, AR is associated with an increase in preload. LV dilatation increases LV systolic strain according to Laplace’s law. This, in combination with the elevated systolic blood pressure resulting from increased total forward stroke volume, leads to increased afterload. LV function is compensated due to the combination of LV dilatation and hypertrophy. However, over time, wall thickening fails to keep pace with the hemodynamic load, leading to a decrease in systolic function and ejection fraction3.
Pure Native Aortic Valve Regurgitation (PNAVR) is defined as AR without aortic stenosis (AS). Many patients with mixed aortic valve disease with severe AS and at least moderate AR have been successfully treated with both balloon-expandable and self-expandable transcatheter aortic valve replace (TAVR), but AR without AS remains a contraindication for TAVR, and is not recommended in the predominant AR or non-calcified valve patient guidelines. There is limited experience of TAVR in patients with severe PNAVR4. Haga clic o pulse aquí para escribir texto.
Materials and Methods
We present the case of a 79-year-old male patient who has severe AR, with high surgical risk. Based on the cardiology team’s decision, a transfemoral TAVI was planned with the use of an Edwards SAPIEN 3 valve.
His past medical history was notable for Chronic Obstructive Pulmonary Disease (COPD) of 10 years of evolution, treated with bronchodilators, inhaled steroids, and use of supplemental oxygen. He had an episode of pulmonary embolism (PE) 3 years before current admission, and he was still in treatment with Rivaroxaban for that matter. He also had history of Atrial Flutter with high thrombotic risk and moderate bleeding risk in treatment with Amiodarone and Verapamil. The valve disease history began on 2017 with moderate PNAVR in for which he was treated with loop diuretics.
He reported an evolution of 18 months with general malaise, NYHA class II dyspnea, starting with a deterioration of functional capacity, limitation for walking at 60 m, presence of orthopnea, paroxysmal nocturnal dyspnea, bendopnea, and peripheral oxygen saturation of 84% despite the use of supplemental oxygen. A day before hospital admission, he started with intense oppressive chest pain, without irradiation with worsening dyspnea and lasting 10 min, for which he went to the emergency room.
Physical examination was relevant for apex palpation at 5° intercostal space sat the midclavicular line, without abnormal uprisings, single S1, clean systole, S2 with a physiological split with A2 component decreased in intensity, diastole with protomesodyastolic murmur, with grade III/VI intensity, irradiated to the apex, Corrigan’s pulse, Hill’s sign, and Quincke’s sign where present.
A 12-lead EKG with sinus rhythm, 1st degree AV block, complete right bundle branch block, left bundle branch anterior fascicular block, with secondary alterations in ventricular repolarization suggesting RV systolic overload (Fig. 1), paraclinical tests were requested with hs-Troponin T in 8 ng/dL, NT-Pro BNP 6172 pg/mL. Transthoracic echocardiogram reported LV concentric hypertrophy, left ventricular ejection fraction in 62%, severe AR, left ventricular end-diastolic diameter of 57 mm, and pulmonary artery systolic pressure 52 mmHg (Fig. 2). Complementary TE was made with evidence of dilatation of the aortic annulus, which conditioned lack of leaflet coaptation and severe central regurgitation (Fig. 3).
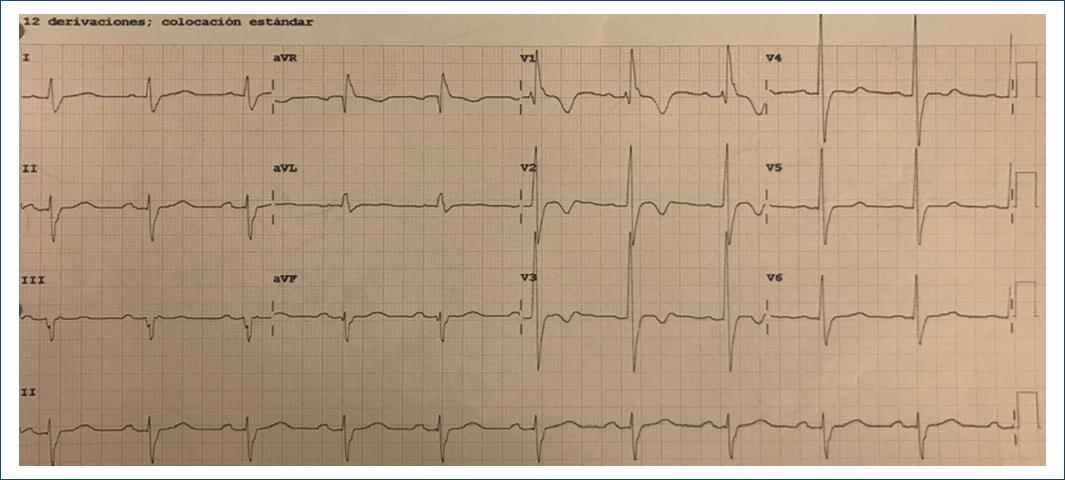
Figure 1 12-lead Echocardiogram in sinus rhythm, HR 60 bpm, 1st degree AV block, complete right bundle branch block, left bundle branch anterior fascicular block, with secondary alterations in ventricular repolarization suggesting RV systolic overload.
Coronary angiography showed left anterior descending (LAD) artery with 80% stenosis at the mid-segment, right coronary artery with 50% stenosis of mid-segment with negative iFR. PCI to LAD was performed placing two everolimus-eluting stents.
Results
Because the patient had severe COPD GOLD class IV, with heart failure symptoms, recent acute coronary syndrome, and very high surgical risk, the heart team decided to perform TAVR. Computed tomography showed maximum aortic diameter 30 mm, ring area 654 mm2, derived area 28.9 mm, without evidence of calcium in the aortic ring, diameter of the valsalva sinuses 40.8 mm, diameter of the sinotubular junction 41 mm, compatible with a 29 mm Edwards SAPIEN 3 Prosthetic Valve (Fig. 4), considering an additional expansion of 15% with extra 3 ml during balloon insufflation. Valvular prosthesis placement was performed, with control transthoracic echocardiogram without perivalvular leak (Figs. 5 and 6). A course with intermittent AV block which occurred after 72 h, so it was decided to place a definitive pacemaker. The patient improved clinically and was discharged home. During follow-up, the patient had considerable clinical improvement, with better quiality of life and NYHA II fucntional class. He presented atrial fibrillation for which he underwent pharmacological cardioversion and oral anticoagulant was indicated. At 14-month follow-up, the patient had a transthoracic echocardiogram perfomed with no significant gradient change and no perivalvular leak. (Fig. 7) The patient continued with better functional class, NYHA II. Unfortuntely at 15-month follow-up since valve implant, he had an hemorragic stroke for which he could not recover and passed away in the intensive care unit.

Figure 4 A: computed tomography TAVR protocol, maximum LVOT diameter documented at 30 mm. B: maximum diameter at the level of the sinus of valsalva at 42.2 mm. C: at the level of the aortic valve, the absence of calcifications was observed. D: height of sinus of valsalva at 19.9 mm of aortic valve.
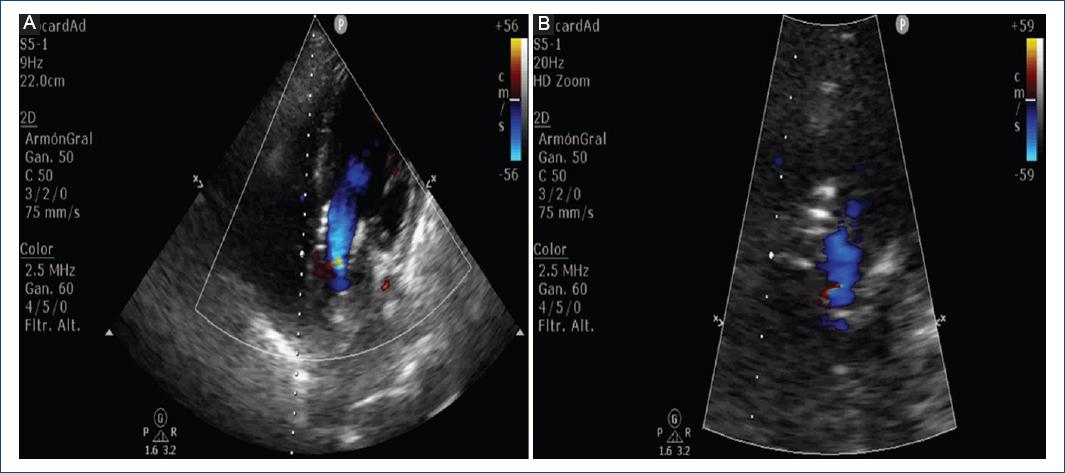
Figure 5 Post-procedural transthoracic echocardiogram: A: 5-chamber view, without evidence of a perivalvular leak. B: left ventricular outflow tract zoom without residual insufficiency.
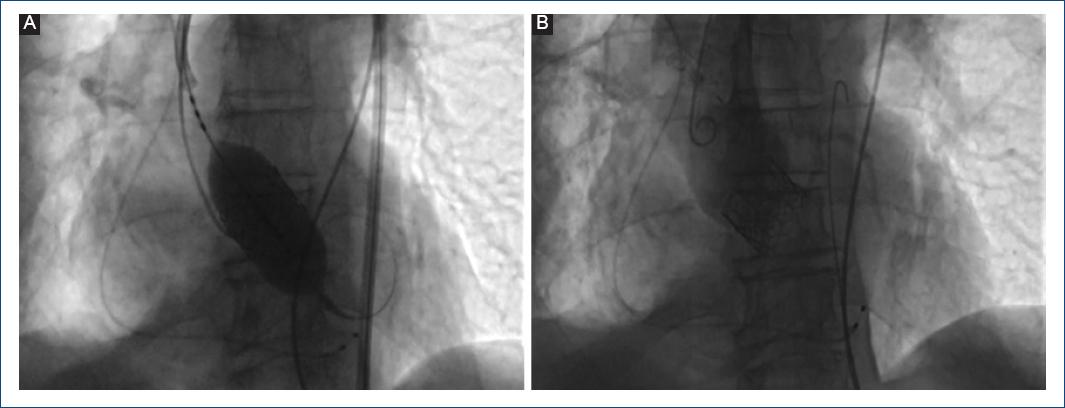
Figure 6 A: 29 mm Edwards SAPIEN 3 valve delivered with 3 ml extra contrasted media to achieve 15% overexpansion. B: aortic angiography demonstrating no residual aortic regurgitation.
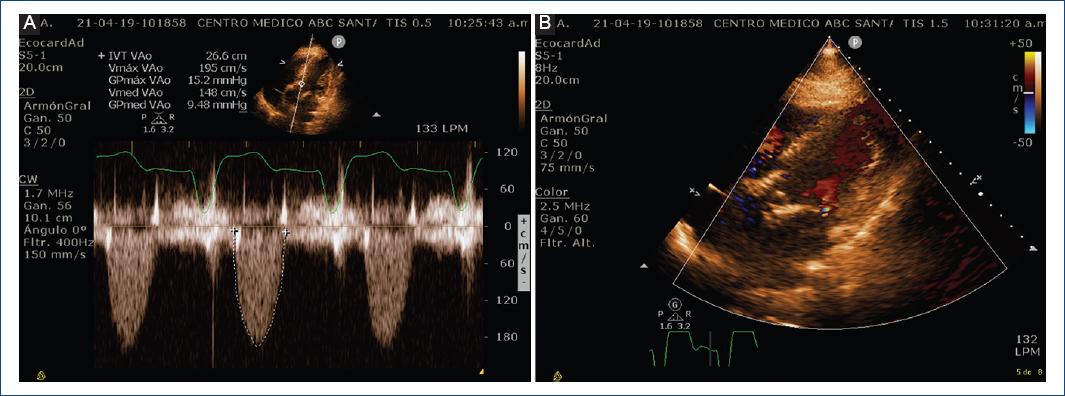
Figure 7 Fifteen months follow-up echocardiogram. A: 5 chamber view showing CW Doppler at the valve implant, no significant gradient change was noted. Max gradient 15 mmHg, Mean gradient 9.5 mmHg. Max peak velocity 1.95 m/s. B: color flow Doppler at LVOT in diastole with no evidence of perivalvular leak.
Discussion
Since the first TAVR procedure for severe AS back in 2002, and since the Food and Drug Administration approval for patients with severe AS with prohibitive surgical risk in 2011, there has been a trend in the use of percutaneous valve protheses for off-label indications specially in patients with high surgical risk, for example in patients with bicuspid aortic valve, moderate AS5, failing surgical bioprotheses6, and recently in the latest year for PNAVR7.Haga clic o pulse aquí para escribir texto.
Nowadays, there are limited studies that evaluate the use of TAVR in PNAVR. Current 2020 American Heart Association guidelines dictate a class III level of recommendation for the use of TAVR in patients with severe AR who are candidates to surgical valve replacement, but it does not mention patients who are considered at prohibitive surgical risk. It also states that patients who have LV systolic dysfunction, patients should be treated with heart failure GDMT8. Considering these recommendations, it is not strictly contraindicated to perform TAVR in patients with PNAVR considered not candidates for surgical intervention due to high risk.
Although it is viable, to perform TAVR in patients with PNAVR it is technically complicated. The procedure itself in this type of patients has several pitfalls. First, these patients have in the majority of cases aortic root dilatation with concomitant aortic annulus deformation and as stated in current guidelines, patients with aortic root dilatation of > 55 mm diameter without collagen related diseases or patients with Marfan syndrome, Ehlers-Danlos, bicuspid aortic valve, etc., have specific criteria for surgical intervention including aortic root and valve replacement9. Furthermore, there is concern regarding the possibility of aortic root continue to expand despite TAVR. Another point to consider is that in PNAVR, there is lack of annular calcium which limits valve anchoring and visualization during the procedure10. To overcome this problem, interventional cardiologists overexpand the valve to 10-20% to maximize radial force and ensure valve anchoring. In our case, a 29 mm Edwards SAPIEN 3 valve was chosen, and the balloon was filled with an extra 3 ml to achieve the desired overexpansion. Finally, high stroke volume with turbulent flow makes it difficult to control the device and rapid pacing is needed to diminish this. A device that can solve this problem is the Jena-Valve, due to its clip-fixation mechanism to the native valve’s leaflets, which makes it more suitable for patients with hemodynamically unstable AR11.
Because of all the previously mentioned pitfalls, that the evaluation of patients with PNAVR who are being considered to TAVR, need to be widely evaluated not only by conventional echocardiography, but also with multimodal imaging that includes angiotomography to evaluate with better spatial resolution, the aortic structures and to identify, quantify and localize the presence or absence of aortic valve apparatus calcium.
In 2013, Roy et al. published a multicenter registry of 43 patients with PNAVR that were taken to TAVR with Medtronic Core Valve. They documented a 30-day mortality of 9.23-23%, with a stroke rate of 0.7-4.7%, the need for pacemaker implant of 7.5-27.3%, and residual AR more than mild of 20.9-23%. The need for a second valve deployment was 18.6%, and the need for surgical conversion was 2.3%.
New generation devices, including specifically designed devices for this purpose, modified these numbers. Yoon et al. in 2017 published the largest cohort to date evaluating the efficacy, safety, and clinical outcomes of the use of TAVR in PNAVR. In this study, 331 patients with high surgical risk calculated by STS score and EUROSCORE II, where divided in two groups, treated with first generation devices (CoreValve, Sapien XT) and new generation devices (Sapien 3, Evolut R, Jena Valve, Direct Flow, J-Valve, Engager, Portico, Acurate, Lotus). Treatment with first generation devices was associated with more peri-procedural complications. The use of new generation devices was associated with better procedural outcomes, with less need for second valve implantation, and less residual AR more than moderate. The device with best overall performance was Edwards Sapien 3 (p = 0.002), including 0% residual AR (p = 0.003 compared to CoreValve and p < 0.001 in the single analysis in group comparisons). The need for permanent pacemaker implantation was similar in both groups (p = 0.71)12.
Conclusions
Management of patients with severe symptomatic AR with high surgical risk continues to be a special challenge. They have high mortality if left untreated with valvular change, despite medical treatment. That is why there has been special interest in TAVR use por patients with PNAVR with prohibitive surgical risk. This is our center’s first experience, and it is to our knowledge the first reported case in our country of TAVR in a patient with PNAVR, in a patient with important pulmonary and cardiovascular comorbidities that limited surgical approach. The excellent procedural outcome with no residual AR, good inpatient and outpatient evolution, and current lifestyle improvement makes this case relevant for the medical community to consider that, even though it is strictly off-label, this might be a reasonable solution for a select type of patients that we can meet on the daily consult. We consider that TAVR devices will continue to evolve as well as interventional techniques and this will make that in a near future, percutaneous treatment for pnaVr will be a feasible option to solve this problem on inoperable patients.











 nova página do texto(beta)
nova página do texto(beta)




