Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Agrociencia
versão On-line ISSN 2521-9766versão impressa ISSN 1405-3195
Agrociencia vol.50 no.7 Texcoco Out./Nov. 2016
Plant protection
Handling of the white grub complex (Coleoptera: melolonthidae) associated with amaranth (Amaranthus hypochondriacus L.) crops in Puebla, Mexico
1 Benemérita Universidad Autónoma de Puebla, Centro de Agroecología, Maestría en Manejo Sostenible de Agroecosistemas, Instituto de Ciencias. Ciudad Puebla. (agustin.aragon@correo.buap.mx).
2 Instituto de Ecología, A. C. Red de Biodiversidad y Sistemática. 91000. Apartado Postal 63. Xalapa, Veracruz, México. (miguel.moron@inecol.edu.mx).
3 Fitopatología, Campus Montecillo, Colegio de Postgraduados. 56230. Km 36.5. Carretera México-Texcoco. Montecillo, Estado de México, México. (rojas@colpos.mx).
Amaranth (Amaranthus hypochondriacus L.) is grown in dryland farming areas of Puebla, Mexico. One of the main factors that contributes to production losses is the white grub complex; vast quantities of insecticides are used to control it, but they do not manage to reduce damages. This study focuses on the effect of the agroecological handling of the white grub complex associated with amaranth crops (32 800 m2) and a population density diagnosis was carried out. For this purpose, a plot was divided as follows: an 8000 m2 (treatment); a 16 800 m2 barrier section; and an 8000 m2 control section; after the land was prepared for sowing, a mercury light trap with a sheet was placed in the first one. After the diagnosis, 280 third-stage larvae of the Paranomala and Strigoderma genus were collected. The adult melolonthidae flight period began on May 9 and ended on June 22. Seventy percent of the sample was composed by the Phyllophaga genus, while Phyllophaga ilhuicaminai and Ph. Ravida were the most abundant species. Soil sampling was carried out on February, 2014, and 417 larvae of the white grub complex in all were collected, of the Phyllophaga, Paranomala, Strigoderma and Diplotaxis genera. The statistical analysis showed a significant difference (p<0.001) in the amount of larvae and a greater output (26.79%) in the plants of the treatment section, compared with those in the control section.
Key words: Phyllophaga ilhuicaminai; Ph. ravida; white grub; physical control; larva
El amaranto (Amaranthus hypochondriacus L.) se cultiva en zonas agrícolas de secano en Puebla, México, y entre los factores que ocasionan pérdidas en su producción está el complejo gallina ciega; para su control se aplican cantidades abundantes de insecticidas sin lograr disminuir los daños. En este estudio se evaluó el efecto del manejo agroecológico del complejo gallina ciega asociado a amaranto en una parcela de 32 800 m2, y se diagnosticó la densidad poblacional. El manejo se realizó en una parcela dividida en secciones: en una (tratamiento) de 8000 m2, después del barbecho se colocó una trampa de luz mercurial tipo pantalla; otra de 16 800 m2, como barrera; el testigo fue una parcela de 8000 m2. Después del diagnóstico se recolectaron 280 larvas, del tercer estadio, de los géneros Paranomala y Strigoderma. El periodo de vuelo de los melolóntidos adultos fue del 9 de mayo al 22 de junio. El género más abundante, con 70 % de la muestra, fue Phyllophaga, y las especies más abundantes fueron Phyllophaga ilhuicaminai y Ph. ravida. Los muestreos de suelo se realizaron en febrero del 2014 y mostraron un total de 417 larvas del complejo gallina ciega, de los géneros Phyllophaga, Paranomala, Strigoderma y Diplotaxis. El análisis estadístico mostró diferencia significativa (p≤0.001) en el número de larvas y rendimiento mayor (26.79 %) en las plantas de la sección de tratamiento respecto al testigo.
Palabras clave: Phyllophaga ilhuicaminai; Ph. ravida; gallina ciega; control físico; larva
Introduction
Agricultural activity satisfies human needs, but food is the one that requires a bigger surface and causes greater territorial impacts (López and Llorente, 2010). The industrialization of export crops for livestock feed and the growing demand for the production of biofuels have strengthened monoculture farming; however, this has a negative impact on health, ecosystem integrity, and food quality (Holt-Giménez and Patel, 2009). Monoculture specialization has various consequences, including the frequent and abundant use of agrochemicals related to environmental problems and the increase of insect infestations (Gliessman, 1998). The knowledge about the environmental, social and cultural impact of certain agricultural practices has led us to consider that changing towards a sustainable agricultural model is necessary (Gliessman, 2002; Sarandón, 2002).
According to Muñoz (2004), the best known aspect of the agroecological food production is the substitution of agrochemicals with less pollutant and aggressive practices. Martínez (2002) propose an ecological approach to agriculture, using a new theoretical framework to analyze agricultural processes. Koohafkan and Altier(2010) show models to promote biodiversity, support output without agrochemicals, and promote ecological integrity. Agroecological production systems are biodiverse, resilient, energy efficient, socially fair, and they make up a strategy for energy, production, and food sovereignty (Gliessman, 1998). Pest control in agroecological farming is carried out by means of practices that contribute to keeping low the population of harmful organisms. Therefore, control is preventive and occurs before the pest manifestation (Villalobos, 1995).
The preparation for sowing and the raking of the plots has a double purpose: besides preparing the plot for the sowing, the larvae and the pests are eliminated from the soil where they live, such as pre-pupae and motionless pupae, which are more sensitive to predators (birds); the desiccation by temperature and the overwhelming action of the tools of cultivation; this reduces the populations’ annual and biannual cycle. Aragón et al. (2001) consider that the species’ identity and their life cycle is a key point to establish the adequate time to prepare the plot for sowing or raking. Later, Aragón et al. (2008) proposed collecting adult Melolonthidae, using light traps in agricultural plots, since this reduces the number of adults in the crops and, in consequence, the larvae population.
Owing to the increase of human population and the increasing demand for agricultural products, the Food and Agriculture Organization of the United Nations (FAO), the World Health Organization, and the National Academy of Science (NAS) are interested in crops with appropriate nutritional and protein values that meet the food needs of the global population, like amaranth (A. hypochondriacus L.), which is one of the most promising pseudocereals for human consumption.
Amaranth stands out due to its high content of lysine and other amino acids, proteins, vitamins and minerals, such as calcium (Mapes et al., 1997); in addition, it adapts to different conditions (agricultural, altitude, climate, and soil type). Amaranth is an important source of jobs for the agricultural, industrial, commercial, and services sectors; it also provides earnings to the farmers and, therefore, an increasing number of producers are interested in growing amaranth in Mexico (Hernández and Herrerías, 1998).
More than 70% of the amaranth sowing in Mexico is carried out in the state of Puebla, with an average output of 1.4 Mg ha-1, using conventional handling (SIAP, 2014). Amaranth crops are invaded and harmed by insect infestations, such as beetles (Melolonthidae), colaspis larvae (Colaspis sp.), cucumber beetles (Diabrotica balteata; Chrysomelidae), wireworms (Agriotes sp.; Elateridae), and fall armyworms (Spodoptera sp.; Noctuidae). The most abundant species which cause the greatest harm to the root system include the white grub complex, made of larvae of the Phyllophaga, Diplotaxis, Macrodactylus, Paranomala, and Cyclocephala genera which, along with borer and defoliator insects, are responsible for 65% of those losses (Aragón and Tapia, 2009). According to Aragón and López-Olguín (2001), Aragón et al. (2004 and 2005), and Pérez-Torres et al. (2005), this complex is one of the most important phytosanitary problems that this crop faces.
The larvae of agricultural crops are not fully understood; therefore, identifying species and studying this complex’s eating habits in every region is necessary to solve the problem (Pinzón, 2006). In addition, making a diagnosis is necessary in order to find out the infestation degree, from sowing to harvesting, with the aim of developing actions to handle the pest and to find out its ecological importance. The problems created by the white grub complex in each region depend on the handling and the weather conditions. The species that live in the amaranth crops in the agricultural zone of San Jerónimo Coyula, Atlixco, Puebla are unknown, although the farmers declare that these pests cause losses.
The aim of this research was to evaluate the agroeconomical handling of the white grub complex associated with amaranth, a handling that farmers can affords and which effectively increases the grain output, without causing environmental damage.
Materials and methodology
The study was carried out in the 2012 and 2013 agricultural cycles, in San Jerónimo Coyula, Atlixco, at an altitude of 1903 m. In order to determine the species density of the white grub complex, larvae samples were taken in a 32 800 m2 plot, when the amaranth stumps from the previous agricultural cycle (November 2012 and February 2013) were still there. To take the samples, three 310 meter-long transects were traced, with a 20 m separation between them; 20 sample sites were selected along each one, and they had 15 m separations between them. A 30x30x30 cm cube of soil was obtained from each site, and the larvae were manually separated from them.
The collected larvae were placed in plastic containers and were taken for analysis to the Entomology lab of the Agroecology Center of the Instituto de Ciencias of the Benemérita Universidad Autónoma de Puebla (CENAGRO-ICUAP). In the lab, the larvae were separated in accordance with the morphological characters proposed by Morón (1986). Thirty percent of the collected population was fixed in Pampel’s fluid; 4 d later, they were transferred to a 70 % alcohol solution (Morón and Terrón, 1988) and then they were identified according to the dichotomous keys proposed by Morón (1986) and Aragón et al. (2005).
The remainder of the population was kept alive, in 500 mL polyethylene containers, with soil from the site of collection, inside a brood chamber, with a 26±2 °C temperature and a 70(10 % relative humidity, in order to verify its taxonomic identity and to determine the larvae-adult ratio. Adults were left in the same recipients for a month, until they reached their sexual maturity. The dichotomous keys for the Coleoptera:
Scarabaeoidea fauna proposed by Morón (1986; 1994) were used to corroborate the larvae-adult identity and the taxonomic identification of the adults. Larvae and adults were added to the CENAGRO collection.
In order to carry out an agroecological handling of the white grub complex, amaranth was planted, during the agricultural cycle 2013, in the study plot where the diagnosis was made; the plot was divided in three sections: 1) an 8000 m2 treatment section; 2) a 16 800 m2 barrier; and 3) a 8000 m2 control section. With the purpose of destroying the larvae and the pupae in the treatment section, the soil was prepared for sowing to a depth of 30 cm, on February 25, 2013, and on April 22, 2013; in both cases third stage larvae and pupae were found; they were exposed to the effect of their natural predators and solar irradiance; in addition, to catch and recover the beetles, a mercury light trap with a sheet (with its own portable generator) was placed in the plot, and it turned on from 8:00 pm to 10:00 pm. The samples were taken in the adults’ flight season, from April 27 to June 30. The taxonomic determination of the adults was made with the keys proposed by Morón (1986; 1994) and they were compared with the collections of CENAGRO-ICUAP’s Entomology lab.
The effectiveness of agroecological handling in the larvae population density was detected by means of the systematic soil sampling in the treatment and control sections; 100 amaranth plants and 30x30x30 soil samples were randomly taken from each sections. Sampling was carried out during February 2014. White grub complex larvae were identified using the same methodology that was used for diagnosis. Statistical analysis included a comparison test between two independent samples, in order to evaluate if there was any difference (p≤0.05) between the number of larvae in the treatment and the control sections.
In order to determine the connection of soil factors that regulate the population of white grub species, soil samples were laid on paper, and were kept 48 hours in the shade, before being put through a sieve with 2 mm holes. The following elements were evaluated: 1) pH, using a potentiometer in a watery suspension (1:2); 2) texture, using a Bouyoucos hydrometer; and 3) organic matter, using the Walkley-Black method. Additionally, the effect on amaranth production of the preparation for sowing and adult collection with the light trap (agroecological handling) was determined. With this purpose, production was determined in 100 plants, from the treatment, control, and barrier sections, randomly chosen before harvest (measured in kg, and kg per ha-1 output was calculated).
Results were analyzed with ANOVA and a Tukey test (p≤0.05), using the Statgraphics Centurion XVI statistics software (Statgraphics, 2010), and a 95 % trust level.
Results and discussion
In the soil sampling carried out before the sowing (November 2012-Feburary 2013), 203 third stage Paranomala larvae and 33 larvae from unidentified genera were detected. After the preparation for sowing was carried out, 31 Paranomala and 8 Strigoderma larvae were detected, with a 0-8 larvae gap per rootball. This result showed that the number of larvae diminished by 80.93% as a result of the said preparation process.
The nocturnal flight of Melolonthidae was recorded in 32 collection activities, carried out between May 9 and June 22 (Figure 1); most of them were registered on May 30 (677 adult specimens). Paranomala foraminosa was the first species to appear; it began its flight on May 9; its maximum abundance was recorded on May 15 and May 30 (29 and 28 individuals, respectively); afterwards, it gradually diminished, and its activity stopped on June 21. This species was found during the collection period, except for rainy and windy days. These results match the findings of Castro-Ramírez et al., (2004) who pointed out that Melolonthidae do not fly under adverse environmental conditions.
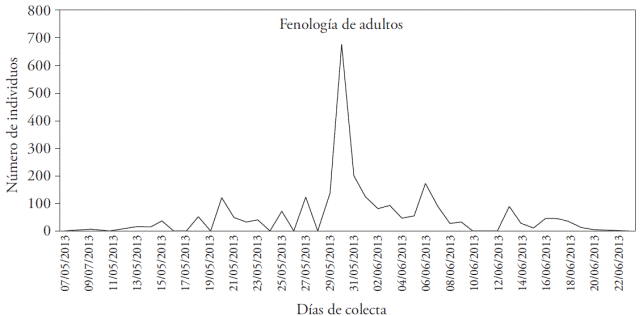
Figure 1 Variation of melolonthidae adults captured in the agricultural zone of San Jerónimo Coyula, Atlixco, México.
Overall, 2591 adult specimens from 32 species were collected, including 13 genus and 7 Scarabaeoidea subfamilies; 78.1 % of the species were represented by few specimens (<15) (Table 1). The specific richness and abundance of species in this study contrast with the findings of Aragón et al., (2008), in a maize crop at Santa Cruz Alpuyeca, Puebla; these researchers collected 91 486 specimens, from 19 species out of 14 genus, 6 subfamilies, and four families, using black fluorescent lamps (20W). The results also differed from those documented by Lugo-García et al. (2011), who collected 61 198 adult specimens, using a 20W black fluorescent funnel trap, in a maize crop, at Sinaloa, and which belonged to 8 species, seven genus, and four subfamilies; in the last case, despite the abundance of individuals, the richness of species was low, in comparison with the variety found in our study.
Table 1 List of species and relative abundance of Coleopteora: Lamellicordia found in San Jerónimo Coyula, Atlixco, México (20013).
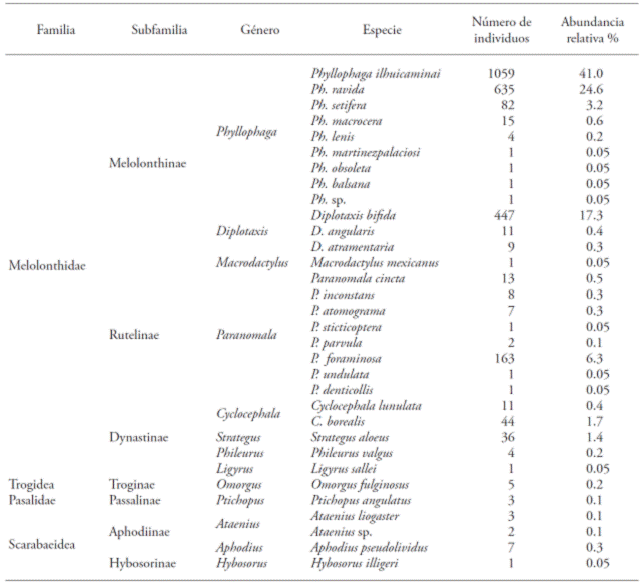
In the Melolonthidae species of the white grub complex, the Ph. ilhuicaminai, Ph. ravida, P. foraminosa, Cyclocephala lunulata, C. borealis, S. aloeus, and D. bífida adult females showed positive phototaxis (Table 2). This result matches the findings of Castro-Ramírez et al. (2003) and Aragón et al. (2008), who pointed out that the positive phototaxis of Phyllophaga ravida females stood out in collecting activities carried out using light traps. More males were found in Ph. setifera; only one male represented Phyllophaga sp., Ph. martinezpalaciosi, Ph. obsoleta, Ph. balsana, and Paranomala atomograma; in contrast, Macrodactylus mexicanus, Paranomala sticticoptera, P. undulata, P. denticollis, Ligyrus sallei, Hybosorus illigeri, and Ataenius sp. were only represented by one female. This may mean that not all species are attracted to mercury light traps and that using only light traps for collections is a relevant factor, since these may only attract one sex.
Table 2. Sex ratio of eight Melolonthidae species in San Jerónimo Coyula, Atlixco, Puebla, Mexico.
| Especie | Número de individuos | Total | Proporción | ||
| ♀ | ♂ | ♀ | ♂ | ||
| Phyllophaga ilhuicaminai | 693 | 366 | 1059 | 1.89 | 1 |
| Phyllophaga ravida | 385 | 261 | 646 | 1.48 | 1 |
| Phyllophaga setifera | 39 | 43 | 82 | 0.91 | 1 |
| Diplotaxis bifida | 241 | 206 | 447 | 1.17 | 1 |
| Paranomala foraminosa | 84 | 79 | 163 | 1.06 | 1 |
| Cyclocephala lunulata | 8 | 3 | 11 | 2.67 | 1 |
| Cyclocephala borealis | 31 | 13 | 44 | 2.38 | 1 |
| Strategus aloeus | 22 | 14 | 36 | 1.57 | 1 |
More females were collected than males (60.4% vs. 39.6%); therefore, this will have reductive repercussions in the larvae population in the next cycle. In this regard, Aragón et al. (2005) pointed out that each oviposit female lays 18-23 eggs on the ground. However, Méndez-Aguilar et al. (2005) stated that taking into account the abundance of females with regard to males is not enough to determine the effect of the kind of collection. Therefore, in order to make a diagnosis of agricultural important species and their ratio, capture methods must be combined, in order to optimize a response from most species.
A sampling carried out in February 2014, has proven the effectiveness of agroecological handling in the soil’s larvae population. Ninety-four white grub larvae were found in the treatment section. Based on larvae development, adults collected, and their identification, the following number of larvae was found: 3 Ph. brevidens, 7 Ph. ilhuicaminai, 11 Paranomala hoepfneri, 13 P. affinis binotata, 32 P. foraminosa, 9 Paranomala sp., and 19 Diplotaxis sp. Meanwhile, 323 larvae were found in the control section, including: 7 Ph. brevidens, 5 Ph. ilhuicaminai, 16 Strigoderma costulipennis, 29 Paranomala hoepfneri, 4 P. affinis binotata, 168 P. foraminosa, 36 Paranomala sp., and 58 Diplotaxis sp. Not all Melolonthidae species have positive phototropism, such as the case of the Strigoderma genus, which, according to Alcázar-Ruiz et al. (2003) and Morón et al. (1997, 1998) is likely diurnal, and probably P. affinis binotata also.
The comparison test between two independent samples showed a significant difference between the number of larvae in the treatment and control sections (p<0.001). The number of larvae per rootball had a 0-9 range in the control area, and a 0-3 range in the treatment area. Of all the adults captured, Ph. ilhuicaminai and P. foraminosa matched the number of larvae collected; therefore, they belong to the white grub complex associated with amaranth crops in San Jerónimo Coyula.
Paranomala was the richest genus. Morón (1983) pointed out that the larvae of this genus have optional rhizophagus habits, and that they seem only to feed on roots in soils with scarce organic matter, when they are facing inter or intra-specific competition. There may have been a problem in the plot caused by this optional genus which dominates the edaphic Melolonthidae samples. Therefore, understanding the feeding habits of the white grub complex species is necessary, because some studies mistakenly generalize the Phyllophaga genus species as a pest, when in fact a genus and species complex may be present (some of which may have potentially-damaging rizophagus habits), while others (with saphrophagus habits) are actually beneficial for soil conservation (Ramírez-Salinas et al., 2001).
The soil’s 7.3 pH was optimum for plant growth. The organic matter content in the cultivated plot was very low (0.31 %), mainly owing to the intense cultivation and high-density crop rotation in the plot, which leave behind very few residues because they are extracted to be used as stubble for livestock. The soil had a high permeability level (sand: 82 %; slime: 12 %, and clay: 6 %) with regard to water and nutrients (washed-away and inert soils).
Since the Paranomala genus includes the most frequent and abundant species found in the samples collected in San Jerónimo Coyula, and that organic matter is very scarce in the area under study, the larvae likely feed from roots and consequently harm them.
The production of amaranth seeds was different (p≤0.05) in each section (Table 3). The treatment section had a greater production (26.79 %), compared with the control, which showed a lower production (p≤0.05) than the treatment and the barrier.
Table 3 Average production of amaranth, with three treatments in San Jerónimo Coyula, Puebla, México.
| Secciones | Promedios* + Error estándar (kg ha-1) | Incremento de producción con relación al testigo (%) |
| Producción kg ha-1 | ||
| Testigo | 1400.6 ± 39.01 a | ___ |
| Barrera | 1577.0 ± 9.07 b | 12.59 |
| Tratamiento: barbecho/ colecta de adultos | 1775.8 ± 23.22 c | 26.79 |
*Mean values with different letter indicate significant differences (Tukey; p≤0.05).
Handling the white grub complex with cultural practices, within a sustainable agricultural framework, has the benefit of increasing the output, without using chemical products. These practices are healthy alternatives for humans, are beneficial for the environment, and improve biodiversity, due to their minimal impact. Agroecological treatments, such as the one employed in this study, may be repeated in other sites.
Conclusions
Preparing the land for sowing and collecting adult Melolonthidae with light traps during their flight season is a green and inexpensive alternative that reduces the larvae population of the white grub complex. Farmers from San Jerónimo Coyula, Puebla, use it as an alternative to increase the grain output per hectare. The integrated, non-pollutant control of white grub in amaranth increased the output by one third with regard to the control section.
Literatura citada
Alcázar-Ruiz, J. A., A. Morón-Ríos, y M. A. Morón. 2003. Fauna de Coleoptera Melolonthidae de Villa las Rosas, Chiapas, México. Acta Zool. Mex. (n.s.) 88: 59-86. [ Links ]
Aragón G., A., y J. F. López-Olguín. 2001. Descripción y control de las plagas de amaranto. Benemérita Universidad Autónoma de Puebla. Alternativas y Procesos de Participación Social. A. C. SIZA-CONACYT. Puebla, México. pp: 19-22. [ Links ]
Aragón G., A., y A. M. Tapia R. 2009. Amaranto orgánico. Métodos alternativos para el control de plagas y enfermedades. Benemérita Universidad Autónoma de Puebla. Alternativas de Procesos de Participación Social. A. C. SIZA-CONACYT. Puebla, México. 63 p. [ Links ]
Aragón G., A., M. A. Morón., J. F. López-Olguín, y B. C. Pérez T. 2001. Fundamentos para el manejo integrado de las especies del género Phyllophaga (Coleoptera: Melolonthidae) en Agricultura Sostenible. In: Ruiz, C. J., y E. Torres (eds). Manejo Sostenible de los Suelos. Avances en el Estudio de Suelos. Benemérita Universidad Autónoma de Puebla y el Instituto de Suelos de Cuba. Puebla, Puebla. México. pp: 135-144. [ Links ]
Aragón G., A., A. M. Tapia R., J. F. López-Olguín, y B. C. Pérez T. 2004. Insectos plaga del cultivo de amaranto (Amaranthus hypochondriacus L.) en el Valle de Tehuacán, Puebla, México. Segundo Encuentro de Transferencia y Tecnología Agropecuaria y Agroindustrial en el estado de Puebla. En la Región Altiplano del Estado de Puebla. Consejo Estatal de Ciencia y Tecnología de Puebla. Fundación PRODUCE Puebla, A. C. Pue. pp: 86-90. [ Links ]
Aragón G., A., M. A. Morón R., J. F. López-Olguín, y L. M. Cervantes P. 2005. Ciclos de vida y conducta de adultos de cinco especies de Phyllophaga Harris, 1827 (Coleoptera: Melolonthidae: Melolonthinae). Acta Zool. Mex. (n.s.) 12. 87-99. [ Links ]
Aragón G., A., C. D. Nochebuena T., M. A. Morón, y J. F López-Olguín. 2008. Uso de trampas de luz fluorescente para el manejo de la “gallina ciega” (Coleoptera: Melolonthidae) en maíz (Zea mays L.). Agrociencia 42: 217-223. [ Links ]
Badilla F., M. Chacón, y C. Sáenz. 1999. Utilización de trampas de luz para la captura de adultos de Phyllophaga spp., en caña de azúcar en Costa Rica. Manejo Integrado de Plagas 51: 59-65. [ Links ]
Castro-Ramírez, A. E., J. A. Cruz-López., C. Ramírez-Salinas., H. Perales Rivera, y J. A. Gómez. 2003. Manejo de la “gallina ciega” (Coleoptera: Melolonthidae) con trampas de luz en Chiapas, México. In: Onore, G., P. Reyes-Castillo y M. Zunino. (Comps). Escarabeidos de Latinoamérica: estado del conocimiento. Monografías tercer milenio Vol. 3, SEA, Zaragoza, España. pp: 81-86. [ Links ]
Castro-Ramírez , A. E., C. Ramírez-Salinas, y C. Pacheco Flores. 2004. Guía ilustrada sobre “gallina ciega” en la Región Altos de Chiapas. El Colegio de la Frontera Sur Benemérita Universidad Autónoma de Puebla. México. 48 p. [ Links ]
Gliessman S. R. 1998. Agroecology: Ecological Processes in Sustainable Agriculture. Ann Arbor Press, Chelsea, Michigan. 197 p. [ Links ]
Gliessman, S. R. 2002. Agroecología: Procesos Ecológicos en Agricultura Sostentable. Segunda edición. Editorial Universidade/ UFRGS. Porto Alegre, Brasil. 356 p. [ Links ]
Hernández G. , R., y G. Herrerías G. 1998. Amaranto: Historia y Promesa. Horizonte del Tiempo. Patrimonio Histórico de Tehuacán A. C. México. 529 p. [ Links ]
Holt‐Gimenez, E., and R. Patel. 2009. Food Rebellions: The Real Story of the World Food Crisis and what we can do about it. Fahumu Books and Grassroots International. Oxford. 260 p. [ Links ]
Koohafkan, P., and M. A. Altieri. 2010. Globally Important Agricultural Heritage Systems: A Legacy for the Future Food and Agriculture Organization of the United Nationas. (UN‐ FAO). Rome. 41 p. [ Links ]
López G., D., y Llorente S. M. 2010. Agroecología: Hacia un nuevo modelo agrario sistema agroalimentario, producción ecológica y consumo responsable. Ecologistas en acción. Madrid España, 34 p. [ Links ]
Lugo-García , G. A., L. D. Ortega-Arenas, H. González-Hernández, A. Aragón-García, J. Romero-Nápoles, R. Rubio-Cortés, y M. Á. Morón. 2011. Melolonthidae nocturnos (Coleoptera) recolectados en la zona agrícola agavera de Jalisco, México. Acta Zool. Mex. (n. s.) 27: 341-357. [ Links ]
Mapes C., F. Basurto, and R. Bey 1997. Ethnobotany of quintonil: Knowledge, use and management of edible greens Amaranthus ssp. (Amaranthaceae) in the Sierra Norte of Puebla, Mexico. Economic Bot. 5: 293-306. [ Links ]
Martínez C. R. 2002. Atributos Agroecológicos de Sustentabilidad, InterSedes, Rev. De las Sedes regionales, No. 5 Universidad de Costa Rica y Universidad Nacional de Costa Rica. pp: 25-45. [ Links ]
Méndez-Aguilar , M. J., A. E. Castro-Ramírez, R. Alvarado Barrantes, C. Pacheco-Flores, y C. Ramírez-Salinas . 2005. Eficacia de dos tipos de recolecta para registrar la diversidad de Melolóntidos nocturnos (Coleoptera:Scarabaeoidea). Acta Zool. Mex. (n.s.) 21: 109-124. [ Links ]
Morón, M. A. 1983, Introducción a la sistemática y ecología de los Coleópteros Melolonthidae edafícolas en México. In: II Mesa Redonda sobre Plagas del Suelo. Sociedad Mexicana de Entomología. Chapingo, Estado de México. pp: 1-14. [ Links ]
Morón, M. A. 1986. El género Phyllophaga en México. Morfología, Distribución y Sistemática Supraespecífica. (Insecta:Coleoptera). Publ. No 20 Instituto de Ecología. México, D. F. 341 p. [ Links ]
Morón, M. A. 1994. La diversidad genérica de los Coleópteros: Melolothidae en México. Acta Zool. Mex. (n. s.) 61: 7-19. [ Links ]
Morón , M. A., y R. A. Terrón, 1988. Entomología Práctica. Publicación 22. Instituto de Ecología A. C. México, D. F. 534 p. [ Links ]
Morón, M. A., B. C. Ratcliffe, y C. Deloya, 1997. Atlas de los escarabajos de México. Coleóptera; Lamellicornia Vol. I Familia Melolonthidae. Publicación especial de la Sociedad Mexicana de Entomológica; A. C. y CONABIO. México. pp: 2-7. [ Links ]
Morón , M. A., C. Deloya, A. Ramírez C., y S. Hernández-Rodríguez. 1998. Fauna de Coleóptera Lamellicornia de la región de Tepic, Nayarit. Acta Zool. Méx. (n.s.) 79: 77-102. [ Links ]
Muñoz L., P. 2004. Productores orgánicos mexicanos: El trecho del dicho al hecho. CONABIO. Biodiversitas 55: 8-12. [ Links ]
Pérez-Torres, B. C., A. Aragón, G , A. M. Tapia, y J. F. López Olguín. 2005. Plaga de importancia económica en el sistema radicular del cultivo de amaranto y su control. Memorias del Tercer Encuentro de Transferencia y Tecnología Agropecuaria y Agroindustrial en el estado de Puebla. En la Región Mixteca del Estado de Puebla. Consejo Estatal de Ciencia y Tecnología de Puebla. Fundación PRODUCE Puebla, A. C. Pue., México (CD). [ Links ]
Pinzón F., O., P. 2006. Problemas Fitosanitarios en plantaciones forestales. In: Seminario establecimiento y manejo de plantaciones El Semillero. Colombia. p. 11. [ Links ]
Ramírez-Salinas, C., A. E. Castro-Ramírez, yM. A. Morón. 2001. Descripción de la larva y pupa de Euphoria basalis (Gory & Percheron, 1833) (Coleoptera:Melolonthidae:Cetoniinae) con observaciones sobre su biología. Acta Zool. Mex. (n.s.) 83: 73-82. [ Links ]
Sarandón, S. J. 2002. El desarrollo y uso de indicadores para evaluar la sustentabilidad de los agroecosistemas. In: Sarandón S., J. (ed). Agroecología. El Camino hacia una Agricultura Sustentable. Ediciones Científicas Americanas. La Plata. pp: 393-414. [ Links ]
SIAP (Servicio de Información Agroalimentaria y Pesquera). 2014. Producción anual de amaranto para México. http://www.siap.gob.mx/cierre-de-la-produccion-agricola-porestado/ (Accessed: August 2014). [ Links ]
Statgraphics. 2010. Statgraphics Centurion XVI User Manual. Stat Point Tecnologies, Incorporated (Inc.) E. S. A. 305 p. [ Links ]
Villalobos, F. J. 1995. El manejo sostenible de plagas del suelo: El caso de las larvas de Melolonthidae. In: Aragón, G. A. (ed). Control de plagas con Métodos Alternativos al Químico. Publicación Especial de la Sociedad Mexicana de Entomología Puebla, Puebla. México. pp: 69-89. [ Links ]
Received: June 2015; Accepted: May 2016











 texto em
texto em 


