INTRODUCTION
Abalones are economically important aquaculture resources in the international market and are highly valued in Asian countries where the demand for these products is increasing (Hartmann et al., 2015). Abalone meat is sold either whole or in pieces and may be canned, fresh, dehydrated or frozen. In addition to the meat, the shell, mantle, viscera and gonad are also commercialized (Cook, 2019).
Since the 1970s, abalone production worldwide has decreased considerably from 20,000 t in 1970 to 6,500 t in 2017 (Vega-García et al., 2016; Cook, 2019). This drop in production has been caused by overfishing, illegal fishing, climatic variations and diseases caused by various pathogens such as Perkinsus olseni Lester & Davis, 1981, Xenohaliotis californiensisFriedman et al. 2000 and Vibrio spp. (Guzmán del Proó et al., 2013; Dang & Miller, 2016). As an alternative to fishing, abalone crops have been developed in countries such as China, Korea, Australia and Mexico (Cook, 2019). In addition to focusing on abalone growth and crop sales, these operations have directed their efforts towards applying technological advances to improve production. Examples of these operations include those in China with Haliotis discus Reeve, 1846, and Haliotis diversicolor Reeve, 1846, Haliotis laevigata Donovan, 1808 (Zhang et al., 2004) as well as those in Korea with H. discus Ino, 1953 (Park & Kim, 2013), Australia with H. asinina Linnaeus, 1758 (Amin et al., 2020), and the Philippines with H. asinina (Santizo-Taan, 2020).
The main problem facing abalone cultivation is food availability (e.g., fresh Eisenia arborea Areschoug, 1876 and Macrocystis pyrifera (Linnaeus) C. Agardh, 1820 along the Baja California peninsula) and a reduction in biomass due to seasonal meteorological phenomena (Edwards & Hernández-Carmona, 2005; SAGARPA, 2017). In recent years, formulated foods have been developed and studied to ensure that the necessary nutrients are provided to farmed abalone to optimize growth (Fitzgerald, 2008; Meng et al., 2019). Of note, hydrolyzed ingredients have been found to promote efficient nutrient (peptides, amino acids, monosaccharides, and oligosaccharides) absorption by the digestive system, which favors faster growth (i.e., weight and size) (Benítez et al., 2008; Meng et al., 2019). In addition, hydrolyzed ingredients favor immunostimulant activity and function as attractants that improve food intake (Hou et al., 2017).
A wide variety of attractants that improve food intake and favor development exist for abalone diets and include amino acids, biogenic amines, nucleotides, algae extracts, and carbohydrates (Britz et al., 1994; Dunstan et al., 2002). Currently, the aim of abalone cultivation is to improve quality by providing foods with novel ingredients that benefit development (Lee et al., 2016; Meng et al., 2019; Tan et al., 2020). Among these food items are brown algae, which contain polysaccharides (e.g., alginate, fucoidan, and laminaran) and amino acids that directly benefit the development of farmed organisms, as has been shown in fish and shrimp species (Peso-Echarri et al., 2012; Ringø et al., 2012). Therefore, it is important to evaluate the effects of algae hydrolysates on the feeding parameters of cultivated abalone.
In Mexico, the green abalone Haliotis fulgens Philippi, 1845 is an aquaculture product of great economic importance because a substantial portion of the total production is exported to Asian countries (FEDECOOP, 2022; SAGARPA, 2017). In Baja California Sur, green abalone has been successfully farmed in the Laboratory of Abalone Production of the fishing cooperative Sociedad Cooperativa Progreso de Producción Pesquera S.C. de R.L., which continues to lead the world in H. fulgens commercialization (FEDECOOP, 2022). Despite advances that have improved the formulations of abalone diets and the cultivation of H. fulgens, thus far it has not been possible to formulate a diet that optimizes H. fulgens development in Mexico, where they are currently fed fresh algae. In addition, no information is available (globally) regarding the manner in which brown algae hydrolysates are incorporated either individually or when combined in the feed of farmed abalone. The present study aimed to formulate diets with hydrolyzed algae as the main ingredient and to evaluate the acceptability (i.e., attractability and food intake) of these diets in juvenile H. fulgens. Our results can be used to improve culture conditions to ensure the continuous supply of juvenile green abalone from Mexico.
MATERIALS AND METHODS
Production of hydrolyzed algae paste
Samples of two species of brown macroalgae (Macrocystis pyrifera and Eisenia arborea) were harvested in May 2021 from La Bocana in Baja California Sur and subsequently dried. The dried samples were ground, sieved (400 µm), and placed in a water bath in a ratio of 1:2 (macroalgae: water). The algae were hydrolyzed in the water bath (90 °C) by means of an alkaline process for 2 h at pH 10, which was adjusted with 10% sodium carbonate (Hernández-Carmona et al., 2012). The resulting hydrolyzed algae paste was refrigerated until use. The proportion of hydrolyzed algae used for formulating the abalone feed was 26% (260 g Kg-1; Table 1).
Table 1 Formulations and proximal chemical compositions of the diets evaluated in this study.
| Dietary treatment | |||||
| MP | EA | MPEA | ABKELP (control) | Fresh E. arborea (negative control) | |
| Ingredients (g Kg-1) | |||||
| M. pyrifera (s)a | 270.0 | --- | 135 | --- | --- |
| E. arborea (s)a | --- | 270.0 | 135 | --- | --- |
| M. pyrifera (hi)b | 260.5 | --- | --- | --- | --- |
| E. arborea (hi)b | --- | 260.7 | --- | --- | --- |
| Mp-Ea (hi)b | --- | --- | 260.7 | --- | --- |
| Spirulinac | 100.0 | 100.0 | 100.0 | --- | --- |
| Fish flourd | 360.4 | 360.1 | 360.1 | --- | --- |
| PMC e | 9.1 | 9.1 | 9.1 | --- | --- |
| Chemical composition (%) | |||||
| Protein | 30.0 ± 0.24 | 30.0 ± 0.13 | 30.0 ± 0.10 | 25.0 ± 0.01 | 11.1 ± 0.12 |
| Ether extract | 2.8 ± 0.14 | 2.7 ± 0.05 | 2.6 ± 0.18 | 1.5 ± 0.30 | 0.2 ± 0.43 |
| Carbohydrates | 30.9 ± 0.06 | 30.1 ± 0.70 | 30.5 ± 0.45 | 32.0 ± 0.21 | 41.4 ± 0.01 |
| Fibre | 5.0 ± 0.06 | 4.1 ± 0.09 | 4.9 ± 0.32 | 4.5 ± 0.14 | 11.9 ± 0.2 |
| Ash | 31.3 ± 0.11 | 33.1 ± 0.71 | 31.9 ± 0.31 | 19.2 ± 0.12 | 22.5 ± 0.67 |
| Moisture | 4.99 ± 0.01 | 5.93 ± 0.01 | 5.01 ± 0.52 | 4.89 ± 0.23 | 4.69± 0.38 |
Abbreviations: Mp (Macrosystis pyrifera), Ea (Eisenia arborea), hi (hydrolyzed)
--- ingredient not included; ± standard deviation. Seaweeds were dried. aE. arborea and M. pyrifera by Algas Pacific®. bHydrolyzed E. arborea, M. Pyrifera, and mixture prepared in the Laboratorio de Algas Marinas of CICIMAR-IPN. cSpirulina produced by Estrella del Sur. dFish meal produced by PEGUSA, Mexico. ePolymethyl carbamide (PMC) product Acuabond® produced by Grupo Norel, Mexico. Amino acids provided by the algae include tryptophan, valine, alanine, leucine, methionine, cysteine, glycine, and threonine. Composition of ABKELP determined by Algas Marinas, S.A. (Ensenada, Baja California, Mexico) and includes wheat flour, seaweed flour, green algae flour, fish meal, soybeans, cereals and by-products, natural algae compactors (NKB), fish oil, oil vegetable, amino acids, minerals and vitamins from seaweed, vitamin E and C, and yeast and fungal inhibitors.
Diet formulation
Three diets were formulated from two species of brown algae: 1) M. pyrifera (MP), 2) E. arborea (EA), and 3) M. pyrifera and E. arborea (MPEA). The diet were formulated as suggested by Viana (2002), Viana et al. (2007), and Durazo-Beltrán et al. (2014) using NUTRION v. 10.55 (Table 1). The formulation of the MP, EA, and MPEA diets was adjusted to ensure that all had similar nutritional content. During the experiment, the commercial brand ABKELP (Algas Marinas, S.A., Ensenada, Mexico) was used as a positive control, and fresh E. arborea (harvested in May 2021 from La Bocana in Baja California Sur) was used as a negative control.
Diet preparation
The resulting hydrolyzed algae were mixed with dry ingredients (spirulina, fish meal, polymethyl carbamide, and dried M. pyrifera and E. arborea, proportion used Table 1) in an MX1200 stand mixer (BLACK & DECKER, New Britain, USA) until the mixture was homogenized. The food mixture was prepared using a manual pasta extruder (Markwind, Walnut, USA). The resulting paste was formed into 5 g square bars (2.5 x 2.5 x 0.5 cm). The bars were dried in a DX312 convection oven (Yamato, Chuo City, Japan) at 55 °C for 48 h and subsequently placed in a desiccator for 24 h. The control diets were not processed in any way.
Proximal chemical analysis
A proximal chemical analysis was performed in triplicate for the formulated diets (MP, EA, and MPEA), the ABKELP® control, and the negative E. arborea control (Table 1) following the guidelines established by the Association of Official Analytical Chemists (AOAC, 2019). The analyses were conducted at the Centro Universitario de Ciencias Biológicas y Agropecuarias (CUCBA, Mexico).
Toughness and stability
The toughness and stability tests were performed in triplicate using rectangular 10,800 L tanks (6 x 2 x 0.9 m) with aeration and continuous seawater flow (15 water changes per hour). The diet distribution among trials was random.
The toughness (i.e., breaking point) of the food bars (g cm-2) was determined using a Nikan-Sui texture analyzer. Three food bars (15 g) of each diet (MP, EA, MPEA, and ABKELP®) were placed individually in the center of the plate of the texture analyzer and weight was added until a break was generated in a bar. Measurements were taken every hour over 6 h, and then again at 9, 12 and 24 h.
The stability of the three food bars (three per diet) was individually determined by immersing them in water and subsequently drying them in the DX312 convection oven (Yamato) at 55 °C for 24 h. After which, the bars were weighed. The measurement time was the same as that of the toughness analysis. Stability was determined with the following formula (Pérez-Estrada, 2006):
where, Fr is the food recovered and Fp is the food provided.
The stability of fresh algae was calculated at 24 h to determine food intake.
Acceptability (attractability and food intake)
The acceptability of the diets was evaluated in 900 H. fulgens juveniles (32.8 ± 0.3 mm, 4.67 ± 0.8 g) under commercial culture conditions (temperature: 18.4 ± 0.7 °C, pH: 7.8 ± 0.4, dissolved oxygen: 7.5 ± 0.6 mg/mL, photoperiod: 24 h darkness) at the facilities of the Sociedad Cooperativa Progreso de Producción Pesquera S.C. de R.L. which are located in La Bocana, Baja California Sur (26° 46´ N. 113° 42´ W). Prior to the acceptability tests, the organisms were acclimated for 30 days under commercial culture conditions during which time they were fed a fresh seaweed mix. After which, the organisms were fasted for two days before initiating the acceptability tests.
The acceptability tests were performed in triplicate with a total of 300 organisms per tank (600 L) based on the methodology of Dunstan et al. (2002) with some modifications. The three formulated diets plus the positive and negative controls were placed inside each tank in the devices described in Figure 1a. The device consisted of a metal mesh base (10 x 10 cm) and a plastic cover (20 x 20 cm; Fig. 1a). The devices contained 15 g (three bars) of each diet under evaluation and were randomly yet equidistantly distributed in the tanks. The tests were conducted for 24 h in the dark.
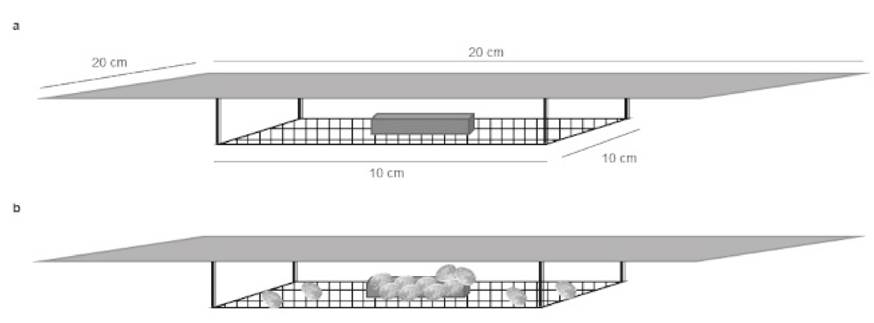
Figure 1 Device used to evaluate diet attractability (a). Device with interaction of abalone juveniles (b).
The attractability of each diet was determined by visual and photographic observations based on the frequency of approach and the interactions between the organisms and food bars at 1, 3, 6, 9, 12, and 24 h (Fig. 1b). Only the organisms that were found on top the food were counted. A device without food was also included in the attractability tests to see if its presence influenced the attraction of the organisms (e.g., the device acting as a refuge).
Food intake was evaluated 24 h after providing the food bars. The excess food was recovered, dried in a convection oven at 55 °C for 48 h, and weighed. Food intake was determined with the following equation (Uki & Watanabe, 1992):
where, G is the weight of the food provided (g), S is stability, and R is the weight (g) of the recovered food.
Statistical analysis
Comparisons were made between treatments in terms of physical parameters (toughness and stability) and acceptability (attractability) in R (R Core Team, 2021).
Shapiro-Wilk tests for normality and Fligner-Killeen tests for homoscedasticity were applied to the data sets (attractability, toughness, and stability) prior to subsequent analyses. Given the nature of the data, a Kruskal Wallis test was used to identify significant differences between diets. Wilcoxon post-hoc tests with Bonferroní-Holm adjustments were used to highlight the differences between diet groups. Comparisons of food intake among the formulated diets and controls were conducted with Student t-tests with a significance level of p < 0.05.
RESULTS
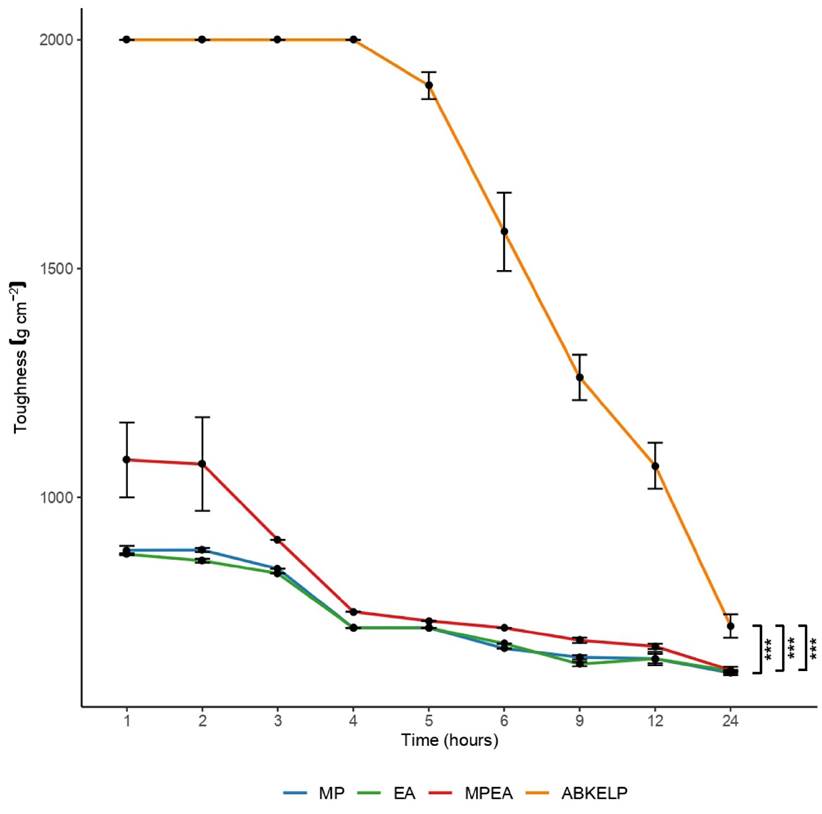
The ABKELP control diet exhibited the greatest toughness. During the first four hours, ABKELP show a toughness of 2000 g cm-2, which decreased with immersion time (700 g cm-2 at 24 h). The post-hoc test indicated that there were significant differences in toughness (p < 0.01) between the ABKELP® control diet and the formulated diets. At 24 h, the toughness of all formulated diet was similar (p > 0.01). The MP, EA, and MPEA diet exhibited toughness values of 615.2 g cm-2, 620.1 g cm-2 and 622 g cm-2, respectively (Fig. 2).
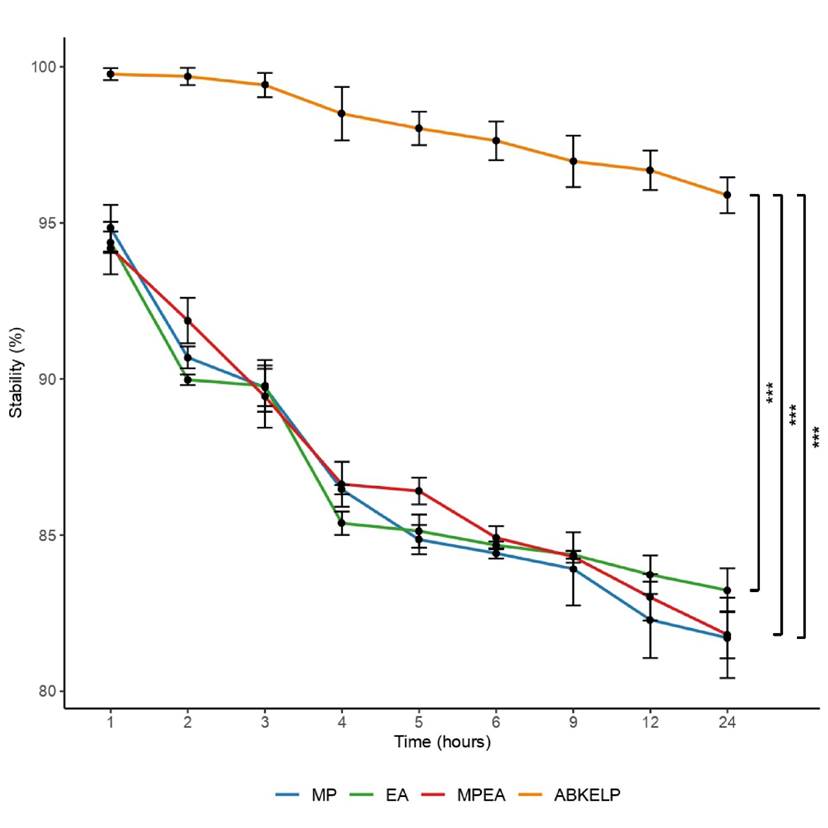
The stability of the formulated diets (MP, EA, and MPEA) showed similar trends with the values ranging from 94-81% (Fig. 3), although ABKELP® exhibited the greatest stability (99-95%; Fig. 3). After 24 h, the stability values of the MP, EA, MPEA, and ABKELP control diets were 81.7 ± 1.3%, 83.2 ± 0.7%, 81.8 ± 0.8%, and 95.9 ± 0.6%, respectively. Furthermore, the stability of the ABKELP control diet was significantly higher (i.e., 13.7% more stable, p < 0.01) than those of the formulated diets (Fig. 3).
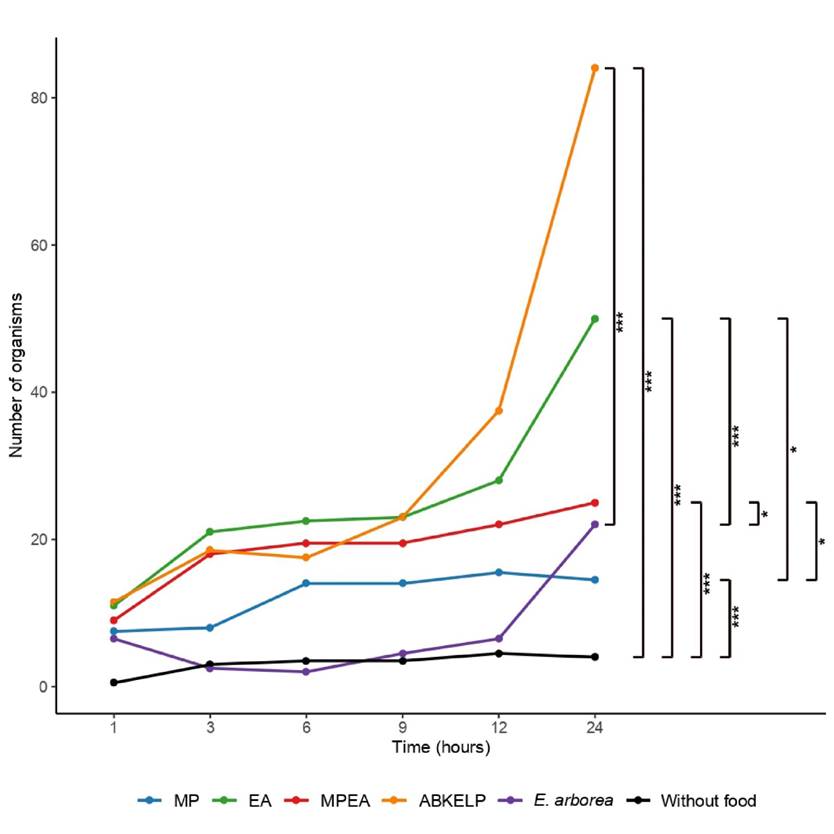
The attractability of fresh E. arborea was significantly lower compared to that of the EA (p < 0.01), MPEA (p < 0.05), and ABKELP (p < 0.01) diets (Fig. 4). With the exception of fresh E. arborea, the control treatment (i.e., structure without food) was significantly different from those of the other diet treatments (p < 0.05). The attractability of the MP diet was also significantly lower when compared to those of the EA and MPEA diets (p < 0.05).
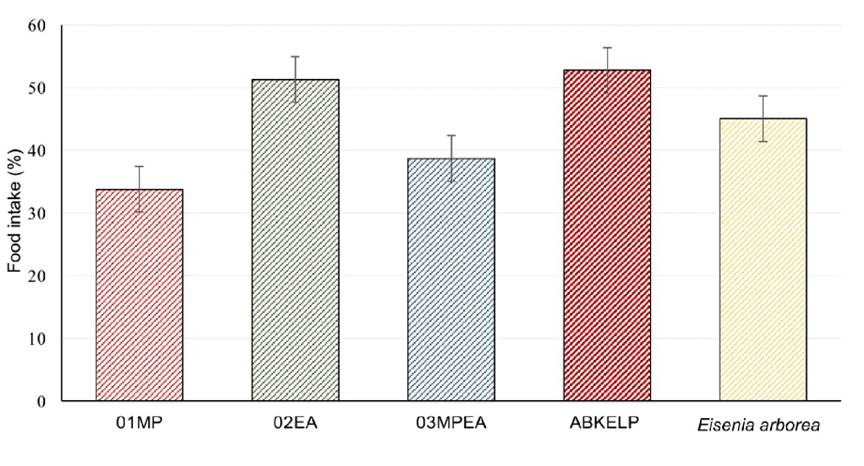
The most consumed diets (> 50%) were the ABKELP and EA diets. The abalone consumed more than 7.5 g of these diets. The control treatment of fresh E. arborea showed an intermediate food intake value of 45%, which was equivalent to 6.8 g of food consumed. The lowest intake value was recorded for the MP and MPEA (< 40%) diets, with both diets showing consumption values lower than 6 g (Fig. 5). However, the median difference test indicated that no significant differences were present among treatments (p < 0.05).
DISCUSSION
Toughness is critical to elaborating suitable food for abalone. These organisms feed by browsing, and their ability to ingest foods depends on their radula type (i.e., rhipidoglossan in H. fulgens) (Fitzgeral, 2008). In their natural environments, abalone feed on algae stipes and thus tend to consume foods that are not overly rigid (Fitzgeral, 2008; Currie et al., 2016). In this study, the ABKELP control diet exhibited the highest toughness (> 700 g cm-2) when compared to those of the formulated diets (MP, EA, and MPEA). This toughness value may be considered appropriate for juvenile abalone based on the results reported by McShane et al. (1994) with H. rubra. Moreover, an appropriate interval of toughness for the juvenile stage of H. fulgens has been found to be between 500-600 g cm-2 (Rivero & Viana, 1996), which seems to favor attraction and consumption. This could explain why the attractability in this study was highest after 24 h when both the formulated and the control diets were softer in texture.
Stability is a physical property that must be considered when formulating abalone feed. This is because abalones feed slowly, and thus food must remain as stable as possible after being immersed in water to avoid losing its nutritional properties and negatively affecting water quality (Britz et al., 1994; Kinkerdale et al., 2010; Ruff et al., 2014; Ansary et al., 2018). Based on the suggestions by Fitzgerald et al. (2008) and Ruff et al. (2014), stability results higher than 80% are acceptable. In this study, the stability values of the formulated diets (MP, EA, and MPEA) were above 81%, although lower than that of ABKELP (> 95%). It is important to highlight that the formulated diets (MP, EA, and MPEA) were manually prepared in the laboratory in contrast to the ABKELP® control feed. Additional factors, such as density, binder type, particle size, and the production process, directly influence stability as has been described by Fleming et al. (1996), Sales & Jensens (2004), and Ruff et al. (2014). In this study, the stability values of the formulated diets were acceptable for H. fulgens. If these diets were to be industrially produced, their stability would likely increase (Ruff et al., 2014).
Attractability is the attraction that food generates toward an organism through the release of chemical compounds that are detected by sense organs (Britz et al., 1994). When optimizing abalone cultivation, it is important to develop diets that contain attractive ingredients that stimulate food intake, which improves weight gain, and that favor abalone quality (especially of the foot) (Bansemer et al., 2016; Denti et al., 2016). When compared with the attraction to fresh E. arborea (the algae used by cooperatives in northern Baja California Sur), the juvenile H. fulgens in this study showed higher attraction at 24 h to the control food ABKELP, followed by their attraction to the EA and MPEA diets. The lower attractability recorded for fresh E. arborea may be due to the formulated diets (MP, EA, MPEA, and ABKELP) containing fish and soybean meal and other ingredients (e.g., oils, amino acids, and by-products) that are known to stimulate attraction (O’Mahoney et al., 2014; Bansemer et al., 2016). Our formulated diets contained hydrolyzed algae. Hydrolyzation degrades proteins (amino acids) and carbohydrates (monosaccharides, disaccharides, and oligosaccharides), which makes assimilation more efficient (Cruz-Suárez et al., 2000). The hydrolyzed algae functioned as an attractant and were easily detected by the abalone due to the low molecular weights of the compounds that were released into the water column (Cruz-Suárez et al., 2000; Viana, 2002; Hou et al., 2017). Despite the presence of hydrolysates in the formulated diets, attractability was higher with the ABKELP diet, which may be due to the use of compounds (ingredients not reported by the manufacturer) that favor attraction. Fleming et al. (1996) indicated that information related to diet formulations and the ingredients used to favor attraction can be extensive, although it may not be reported in some cases.
It is important to consider the food selectivity of abalone. For example, species, such as H. discus, H. laevigata, and H. rufescens, have shown specific attractions to diets composed of certain groups of macroalgae that depend on their geographical distributions (Cornwall et al., 2009; Sakata, 2013). This affinity can influence the attraction of abalones to formulated diets (Sakata, 2013). This was observed in this study among the formulated diets given that the attractability of H. fulgens was higher for diets that contained E. arborea (EA and MPEA). On the other hand, Aviles & Shepherd (1996) indicated that H. fulgens can feed on E. arborea or M. pyrifera depending on the season and geographic distribution. However, given that E. arborea has a higher tolerance to unfavorable weather conditions than M. pyrifera, it is consumed more than M. pyrifera in some areas of the Baja California peninsula (Zertuche-González et al., 2014). This could explain the low attractability that H. fulgens juveniles exhibited towards the diets containing M. pyrifera (MP) compared to the diet consisting entirely of E. arborea (EA). On the other hand, since the algal composition of ALBKELP is not specified, we are unable to explain why it exhibited higher attractability than our formulated diets.
The food intake values of the formulated diets compared to those of the commercial and negative controls were not significantly different. This may be because the MP, EA, and MPEA diets were composed of the algae that H. fulgens usually consumes (E. arborea grows in the same area in which the study was conducted). Although it is not usual for abalone to consume M. pyrifera in the area of La Bocana, it has been observed that consumption can occur due to massive upwellings from the northern coast of La Bocana (Mazariegos-Villarreal et al., 2012; Vega-García et al., 2016). A similar situation could explain the food intake of the ABKELP control diet, as it is produced in Baja California where M. pyrifera and E. arborea are also present, and thus these species are likely important components of this product.
The formulated MP, EA, and MPEA diets presented toughness values of 600-630 g cm-2 and stability values > 80%. These values, coupled with the physicochemical characteristics, render the diets appropriate for abalone culture conditions. The EA, MPEA, and ABKELP control diets were more attractive to the abalone than fresh E. aborea. Despite this, the food intake values of the formulated diets and controls were not significantly different.
With the evaluation of the physicochemical and acceptability characteristics (attractability and food intake) of the formulated diets, we can conclude that the use of hydrolyzed algae in diets for H. fulgens is acceptable and may constitute a viable alternative for feeding under culture conditions. Even so, it is necessary to evaluate the effects of these compounds on the growth, digestive physiology, immunostimulant activity, nutrition, and overall health status of cultured abalone. This information will greatly contribute to improving the feeding of cultured abalone.











 nova página do texto(beta)
nova página do texto(beta)