Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de fitopatología
versão On-line ISSN 2007-8080versão impressa ISSN 0185-3309
Rev. mex. fitopatol vol.37 no.1 Texcoco Jan. 2019 Epub 21-Ago-2020
https://doi.org/10.18781/r.mex.fit.1810-3
Phytopathological report
First report of Fusarium wilt on Citrus sinensis var. Valencia in the Yaqui Valley, Mexico
1 Campo Experimental Norman E. Borlaug, INIFAP, Norman E. Borlaug Km 12, C.P.85000, Ciudad Obregón, Sonora, México,
2 Instituto Tecnológico del Valle del Guadiana, Carretera Durango - México Km 22.5, C.P. 34371, Ejido Villa Montemorelos, Durango,
3 CONACYT-Instituto Tecnológico de Sonora, 5 de febrero 818 Sur, Colonia Centro, C.P.85000, Ciudad Obregón, Sonora, México.
Death of orange trees (Citrus sinensis L. Osberck) var. “Valencia” was observed in the Yaqui Valley, Mexico, in August 2017. In order to identify the causal agent, leaves, branches, and roots from symptomatic trees were collected. Seven fungal isolates were obtained from brown roots, encoded FS1 to FS7. Macro and microscopic characteristics typical of the genus Fusarium were observed in all obtained single-spored isolates, such as: abundant white cottony mycelium and a purple or yellow undersurface on PDA, curved macroconidia widest in the middle of their length, and oval, reniform, elongated oval to obovoid with a truncate base, and septated microconidia. The sequencing of the Internal Transcribed Spacer (ITS) region showed high coverage and similarity (>99%) to sequences of the Fusarium solani species complex (FSSC). The inoculation of 1-year-old orange trees with each obtained Fusarium isolate caused browning of the root vascular system and death of these trees, confirming that those isolates were the causal agents of Fusarium wilt observed in orange tress in commercial fields in the Yaqui Valley. This is the first report of Fusarium wilt on citrus in that region.
Key words: Fungi; Koch’s postulates; ITS
Muerte de naranjos (Citrus sinensis L. Osberck) var. “Valencia” fue observada en el Valle del Yaqui, México, en agosto de 2017. Para identificar el agente causal, se recolectaron hojas, ramas y raíces de árboles sintomáticos. Siete aislados fúngicos fueron obtenidos de raíces presentando oscurecimiento del sistema vascular, llamados FS1 a FS7. Las características macro y microscópicas típicas del género Fusarium fueron observadas en todos los aislados mono-espóricos obtenidos, tales como: abundante micelio algodonoso de color blanco y un anverso de color púrpura o amarillo en PDA, macroconidias curvas más ancha en la parte central de su longitud, y ovaladas, reniformes, alargadas, de ovaladas a obovoides con una base truncada, y microconidias septadas. La secuenciación de la región de Espaciadores Internos Transcritos (ITS) mostró una alta cobertura y similitud (> 99%) con secuencias del Complejo de Especies de Fusarium solani (CEFS). La inoculación de naranjos de 1 año de edad con cada uno de los aislados de Fusarium obtenidos provocó el oscurecimiento del sistema vascular de la raíz y la muerte de estos árboles, lo que confirma que estos aislado fueron los agentes causales del marchitamiento por Fusarium observados en árboles de naranja en campos comerciales del Valle del Yaqui. Este es el primer reporte de marchitamiento por Fusarium en cítricos en dicha región.
Palabras clave: Hongo; Postulados de Koch; ITS
The citrus production in Mexico has increased as this crop is the highest value fruit crop in the international trade (http://www.fao.org), due to its use in the production of juice, marmalade, among others. However, citrus diseases, particularly those caused by fungi, cause economic losses throughout the world, and in some cases can reduce total production by 50% (Ochoa et al., 2007). In August 2017, symptoms such as chlorosis, necrosis, dieback, premature leaf drop, wilting, and then death (~ 60 ha y-1) of orange trees (Citrus sinensis L. Osberck) var. “Valencia” were observed in orange commercial fields in the Yaqui Valley, Sonora (26° 45’-27° 33’ N latitude and 109° 30’-110° 37’ W longitude), one of the most important agricultural regions in Mexico (Figure 1A). Then, leaves, branches, and roots from ten symptomatic orange trees were collected and placed into moist chambers at 4 °C for their transportation to laboratory, in order to identify the biological casual agents of those symptoms. The visual analysis of collected samples showed browning of the root vascular system (Figure 1B). Thus, fifty pieces of brown roots were surface sterilized using 1% sodium hypochlorite for 2 min, washed twice using sterile (121 °C and 15 psi for 15 min) distilled water, and cut into 1 cm pieces. Later, these root pieces were placed on Potato Dextrose Agar (PDA) (supplemented with 80 µg mL-1 nalidixic acid), and incubated for 5 days at 28±2 °C (12 h of darkness and 12 h of light) (Villa-Rodriguez et al., 2016). After the incubation period, each mycelial colony was isolated using the culture medium and growth conditions mentioned above, obtaining seven fungal isolates, which were encoded FS1 to FS7. These isolates were deposited in Colección de Microorganismos Edáficos y Endófitos Nativos- COLMENA (www.itson.edu.mx/colmena) (de los Santos-Villalobos et al., 2018).
Macro and microscopic characteristics (semipermanent preparations in lactic acid were made and examined with an light microscope) typical of the genus Fusarium were observed for the seven obtained single-spored isolates (Leslie and Summerell, 2006), i.e. 1) abundant white cottony mycelium and a purple or yellow undersurface on PDA, 2) curved macroconidia widest in the middle of their length, and 3) oval, reniform, elongated oval to obovoid with a truncate base, and septated microconidia (Figure 2). The molecular identification -at genus level- of these isolates was carried out by sequencing the Internal Transcribed Spacer (ITS) region. Firstly, 1 x 106 spores of each fungal isolate was separately inoculated in tubes containing 5 mL of Potato Dextrose Broth (PDB), and incubated for 3 days at 28 °C (24 h of darkness). At the end of the incubation period, cell suspension was centrifuged for 5 min at 2,500 Relative Centrifugal Force (RCF) to obtain fungal mycelium. The fungal genomic DNA was extracted from 500 mg of fresh mycelium, which was re-suspended in 500 µL extraction buffer (200 mmol Tris HCl pH 8.5, 250 mmol NaCl, 25 mmol EDTA, and 0.5% SDS) by stirring with a pipette tip. The slurry was mixed homogeneously with 350 µL phenol. Then, 150 µL chloroform were added and mixed, and the suspension was centrifuged for 1 h in an Eppendorf centrifuge (13,000 RCF). The upper aqueous phase was transferred into a sterile Eppendorf tube and mixed with about 0.54 volume isopropanol (250 µL), and centrifuged for 5 min in an Eppendorf centrifuge (13,000 RCF) for DNA precipitation (Valenzuela-Aragon et al., 2018). The obtained DNA was dried at room temperature and re-suspended in 30 µL Nuclease-Free Water (sterile). Then, the 50 µL PCR mixture contained 100 ng fungal genomic DNA as template, 0.2 µmol of each pair of primers [ITS1 (5´-TCCGTAGGTGAACCTGCGG-3´) and ITS4 (5’-TCCTCCGCTTATTGATATGC-3’) (White et al., 1990)], and 4 U MyTaq DNA polymerase (Bioline). The PCR conditions for the amplification of ITS region consisted of an initial denaturation step at 94 °C (3 min), 35 cycles of denaturation at 94 °C (30 s), followed by annealing at 55 °C (30 s), and extension at 72 °C (1 min), and a final step at 72 °C (10 min) (de los Santos-Villalobos et al., 2013). The PCR products were analyzed by electrophoresis in agarose/TAE gel (2%), and purified using ISOLATE II PCR and Gel Kit (Bioline), and then sequenced using Sanger platform (ABI 3730 XL, Applied Biosystem) by Genomic Services Laboratory (LANGEBIO, Irapuato, Guanajuato, Mexico). The obtained DNA sequences were edited and analyzed using the software FinchTV 1.4.0 by Geospiza, Seattle, WA; and BLAST (NCBI, www.ncbi.nlm.nih.gov), respectively. The obtained ITS sequences showed high coverage (>99%) and similarity (>99%) to Fusarium solani species complex (FSSC), which is a group currently estimated to contain at least 60 phylogenetically distinct species (Coleman, 2016). Thus, based on the ITS molecular identification, all seven studied isolates were affiliated to the genus Fusarium, where additional and more robust molecular phylogenetic relationships need to be carried out in order to classify those isolates at specie level. The obtained Fusarium sequences were deposited in the NCBI Genbank as: FS1 (MG262553), FS2 (MG262554), FS3 (MG262555), FS4 (MG262556), FS5 (MG262557), FS6 (MG262558), and FS7 (MG262559).
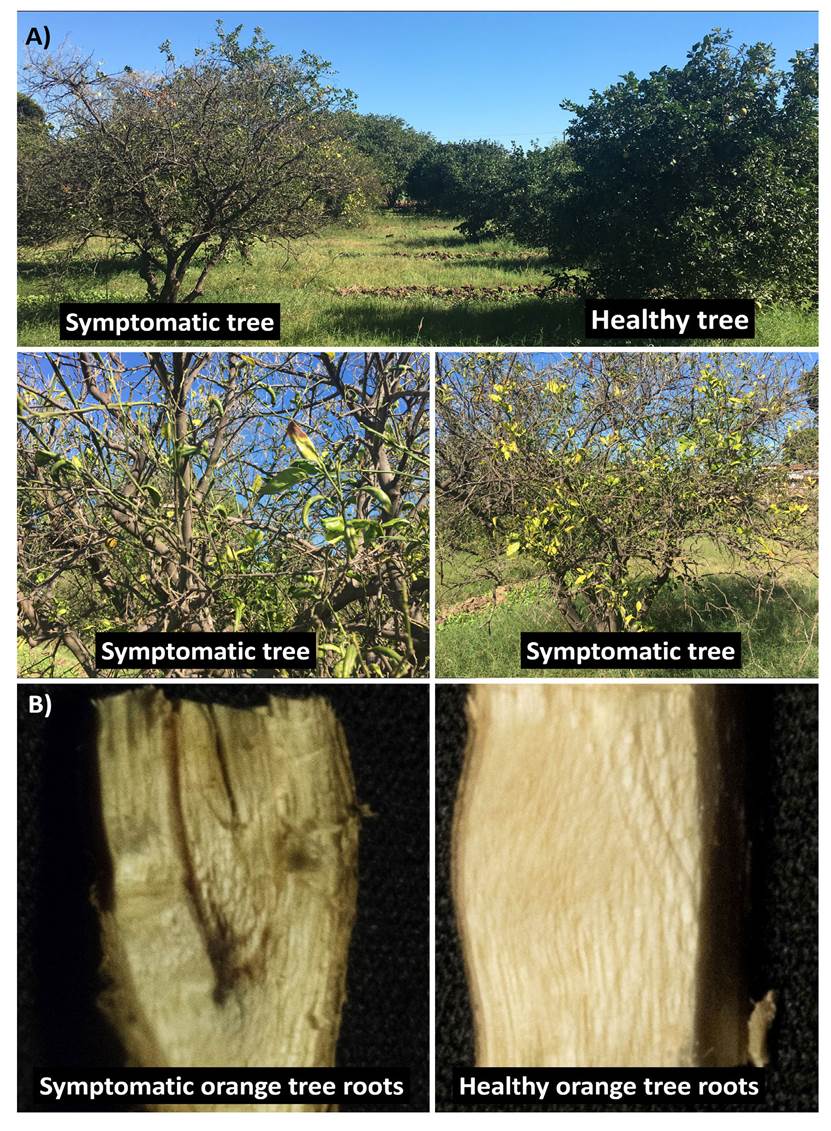
Figure 1 Symptoms of orange trees (Citrus sinensis L. Osberck) var. Valencia” in commercial fields in the Yaqui Valley, Sonora. A) Symptoms include: chlorosis, necrosis, dieback, premature leaf drop, and wilting vs. a healthy tree. B) Symptomatic orange tree root showing browning of the root vascular system vs. healthy root.
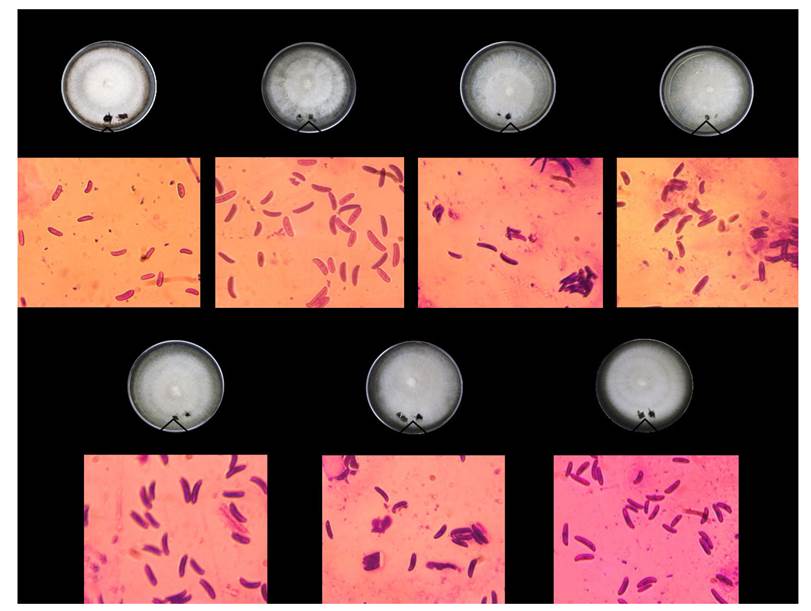
Figure 2 Morphological characterization of associated agents isolated from symptomatic roots. Macroscopic characteristics, abundant white cottony mycelium and a dark-purple undersurface on PDA. Microscopic characteristics, curved macroconidia widest in the middle of their length.
Pathogenicity assays were conducted separately for each studied Fusarium isolate under greenhouse conditions (13 h of darkness at 14 °C, 2 h of light at 18 °C, 7 h of light at 25 °C, and 2 h of light at 18 °C; and 80% of relative humidity). Thus, five 1-year-old orange trees var. “Valencia” having damaged (small cuts were made using a sterile scalpel) roots were inoculated with a conidial suspension (1x104 conidia mL-1 and 50 mL plant-1), a negative control with sterile distilled water sprayed on the plant wound. After 30 days, browning of the root vascular system of 100% of inoculated trees was observed (control plants were asymptomatic and no Fusarium colony was recovered), causing death of them, from which each Fusarium isolate was recovered and characterized as described above, confirming 1) the high virulence of those isolates, and 2) their role as the causal agents of the observed symptoms and death of orange trees in commercial fields in the Yaqui Valley.
Based on morphological observations, partial molecular characterization, and the fulfilment of Koch’s postulates, we identified that studied isolates (belonging to the FSSC) were the causal agent of wilt on orange trees var. “Valencia” in the Yaqui Valley. Similar findings have been reported in California (United States), identifying to Fusarium solani and Phytopstora spp. as the causal agent of dark decay in the bark of large scaffold roots and the lower crown of the trunk, and chlorosis in leaves, and wilting on lemon and orange trees (Adesemoye et al., 2011). In addition, Fogliata et al. (2013) reported the presence of Fusarium rot on lemon trees, identifying Fusarium oxysporum as the causal agent. Recently, Sandoval-Denis et al. (2018) established the Fusarium diversity associated to citrus trees in Europe, by using morphological and molecular multi-locus analysis. The most commonly isolated species were F. sarcochroum, F. oxysporum and Fusarium solani. They also proposed a new fully-supported lineage, phylogenetically and morphologically divergent, which is named F. citricola species complex (FCCSC).
Although the citrus wilt had been observed previously in Mexico, there are only two cases reported in the US National Fungus Collection (https://nt.ars-grin.gov/fungaldatabases/). Thus, to our knowledge, this is the first report of Fusarium wilt on the orange trees in the Yaqui Valley, causing browning of the root vascular system and death of trees (~ 60 ha y-1). Since Fusarium is a virulent phytopathogen, probably the intensive use of tillage in order to disaggregate compacted clayed soil in the Yaqui Valley´s orange commercial fields damaged the orange trees root system, which in combination with soil and climatic (temperature and humidity) conditions, was the major driving forces for the Fusarium wilt outbreak.
Acknowledgements
The authors acknowledge support by Technological Institute of Sonora (ITSON) through Project PROFAPI 2018_0012. In addition, we express our thanks to Luis Abraham Chaparro, Bernardo Flores, and Levi Flores for molecular and microbiological support.
REFERENCES
Adesemoye A, Eskalen A, Faber B, O’Connell N. 2011. Current knowledge on Fusarium dry root rot of citrus. Citrograph 2(7):29-33. http://www.citrusresearch.org/citrograph/nov-dec_citrograph/#more-3226 [ Links ]
Coleman JJ. 2016. The Fusarium solani species complex: ubiquitous pathogens of agricultural importance. Mol Plant Pathol 17(2):146-58. https://10.1111/mpp.12289. [ Links ]
de los Santos-Villalobos S, Guzmán-Ortiz DA, Gómez-Lim MA, Délano-Frier JP, de-Folter S, Peña-Cabriales JJ. 2013. Potential use of Trichoderma asperellum (Samuels, Liechfeldt et Nirenberg) T8a as a biological control agent against anthracnose in mango (Mangifera indica L.). Biological Control 64(1):37-44. https://doi.org/10.1016/j.biocontrol.2012.10.006 [ Links ]
de los Santos-Villalobos S, Parra-Cota FI, Herrera-Sepúlveda A, Valenzuela-Aragon B, Estrada-Mora JC. 2018. Colección de microorganismos edáficos y endófitos nativos para contribuir a la seguridad alimentaria nacional. Revista Mexicana de Ciencias Agrícolas, 9: 191-202. https://doi.org/10.29312/remexca.v9i1.858 [ Links ]
Fogliata GM, Martínez CV, Acosta ME, Muñoz ML, Ploper LD. 2013. First report of Fusarium rot caused by Fusarium oxysporum on lemon in Tucumán, Argentina. Plant Disease. 97(7):989. https://apsjournals.apsnet.org/doi/10.1094/PDIS-01-12-0069-PDN [ Links ]
Leslie JF and Summerell BA. 2006. The Fusarium Laboratory Manual. Blackwell Publishing, Hoboken. https://www.wiley.com/en-us/The+Fusarium+Laboratory+Manual-p-9780813819198 [ Links ]
Ochoa JL, Hernández-Montiel LG, Latisnere-Barragán H, León de La Luz JL, Larralde-Corona CP. 2007. Aislamiento e identificación de hongos patógenos de naranja Citrus sinensis L. Osbeck cultivada en Baja California Sur, México. Ciencia y Tecnologia Alimentaria, 5(5): 352-359. http://www.redalyc.org/html/724/72450505/ [ Links ]
Sandoval-Denis M, Guarnaccia V, Polizzi G, Crous PW. 2018. Symptomatic Citrus trees reveal a new pathogenic lineage in Fusarium and two new Neocosmospora species. Persoonia. 40:1-25. https://doi.org/10.3767/persoonia.2018.40.01 [ Links ]
Valenzuela-Aragon B, Parra-Cota FI, Santoyo G, Arellano-Wattenbarger GL, de los Santos-Villalobos S. 2018. Plant-assisted selection: a promising alternative for in vivo identification of wheat (Triticum turgidum L. subsp. Durum) growth promoting bacteria. Plant and Soil. In press. https://doi.org/10.1007/s11104-018-03901-1 [ Links ]
Villa-Rodríguez E, Lugo-Enríquez C, de los Santos-Villalobos S, Parra-Cota FI, Figueroa López P. 2016. First report of Cochliobolus sativus causing spot blotch on durum wheat (Triticum durum) in the Yaqui valley, México. Plant Disease, 100(11): 2329. https://doi.org/10.1094/PDIS-05-16-0634-PDN [ Links ]
White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J. (Eds.), PCR Protocols: A Guide to Methods and Applications. Academic Press, San Diego. 315-322. [ Links ]
Received: October 13, 2018; Accepted: December 22, 2018











 texto em
texto em 


