Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de fitopatología
versão On-line ISSN 2007-8080versão impressa ISSN 0185-3309
Rev. mex. fitopatol vol.33 no.1 Texcoco 2015
Phytopatological notes
Association of a Potexvirus as a Causal Agent of Chlorotic Spots on Opuntia ficus-indica
1Postgrado en Fitosanidad, Colegio de Postgraduados-Campus Montecillo, km 36.5 Carretera México-Texcoco. Montecillo, Estado de México, C.P. 56230, México.
2Departamento de Parasitología Agrícola, Universidad Autónoma Chapingo, km 38.5 Carretera México-Texcoco. Chapingo, Estado de México, C.P. 56230, México.
3FES-Iztacala-UNAM. Unidad de Biotecnología y Prototipos. Avenida De los Barrios 1, Los Reyes Iztacala. Tlalnepantla, Estado de México, C.P. 54090, México.
The cactus pear (Opuntia ficus-indica) was recently introduced in Cuautepec de Hinojosa, Hidalgo. In this region, a virus was detected causing chlorotic halos and irregular spots on the cladodes. In severe infections, postharvest cladodes lose turgidity, which causes significant commercial losses. The virus was mechanically transmitted to six out of eleven indicator plants. In Chenopodium quinoa, it caused systemic infection showed as chlorotic veins and intervenal yellow spots. The virus was transmitted in 43 % of the inoculated cladodes, inducing chlorotic halos and irregular chlorotic spots at 7 and 25 days after inoculation, respectively. The electrophoretic analysis showed that the virus is an RNA single stranded genome, and flexible rod particles were observed using a transmission electron microscope. The RT-PCR diagnostic test indicated that the virus is a species of Potexvirus. In the cactus pear production units that were evaluated, the virus caused a 47-60 % and 51-79 % of incidence and severity, respectively, with an aggregated spatial pattern with strong row directionality, suggesting virus dispersion through pruning and harvesting.
Key words: cactus pear; cacti; RNA virus; flexible rod
El nopal verdura (Opuntia ficus-indica) se introdujo recientemente a Cuautepec de Hinojosa, Hidalgo. En esta región se detectó un virus ocasionando halos cloróticos y manchas irregulares en los cladodios. En infecciones severas los cladodios pierden turgencia en postcosecha, lo cual causa mermas comerciales significativas. El virus se transmitió mecánicamente en seis de 11 plantas indicadoras. En Chenopodium quinoa causó infección sistémica consistente en venas cloróticas y manchas amarillas intervenales. El virus se transmitió en 43 % de los cladodios inoculados induciendo halos cloróticos y manchas cloróticas irregulares a los 7 y 25 días después de la inoculación, respectivamente. El análisis electroforético mostró que el genoma del virus es de ARN de cadena sencilla, mientras que al microscopio electrónico de transmisión se observaron partículas virales en forma de varilla flexible. El diagnóstico por RT-PCR indicó que el virus corresponde a una especie del género Potexvirus. En las unidades de producción evaluadas, el virus causó una incidencia del 47-60 % y severidad del 51-79 %, con distribución espacial en agregados y con fuerte direccionalidad en sentido de los surcos, debido posiblemente al manejo de poda y cosecha.
Palabras clave: nopal verdura; cactáceas; virus ARN; varilla flexible
Mexico has several different species of cacti that are of alimentary, ecological and cultural importance. Of those that are edible, the cactus pearscactus (Opuntia spp.) are the most important. These species are produced as young cladodes for human consumption, beeing O. ficus-indica (L.) Miller the most important one. Currently, Mexico is the first producer and consumer of cactus pear in the world. In 2009, the country had 13,123.91 hectares (SIAP-SAGARPA, 2013), beeing the central zone the main region for production and consumption. In Cuautepec de Hinojosa, Hidalgo, where the Atlixco-Puebla variety is cultivated, plants were detected with yellow to whitish ringspots around the areoles. Cladodes with more severe symptoms showed a generalized yellow color, thinning and eventual wilt. The cladodes that are harvested for sale become progressively dark and within 24 hours could showed a copper-like color that reduced its commercialization. In Mexico, Venezuela and the United States of North America: Cactus virus X (Lastra et al., 1976), Saguaro cactus virus (Nelson and Tremaine, 1975), Sammons' Opuntia virus (Milbrath and Nelson, 1972), Cactus virus 2 (Brandes and Wetter, 1959), Zygocactus virus (Fauquet et al., 2005), and Tobacco mosaic virus are reported in some cacti (Giri and Chessin, 1975). In Cuautepec de Hinojosa, the symptoms on the cladodes of the cactus pear cause economic losses to the producer, which justifies the importance of generating knowledge regarding the etiology of this disease. According to this information, the objectives of this research were to determine the causal agent of the chlorotic spots on the cactus pear in Cuautepec de Hinojosa, Hidalgo, and to estimate the incidence and severity in commercial plants in this region.
Three units for the production of cactus pear were examined, each 102 x 46 m, located in Puerta de Yolo in Cuautepec de Hinojosa, Hidalgo. The plants were sown in furrows with 35 cm between plants and 60 cm between rows, and were fertilized with cattle manure. The producer included intensive pruning as part of the management of the plantation and harvest. In fall of 2009, 50 young cladodes were collected from 50 cactus plants located in these units; 25 had chlorotic spots around the areoles (Figure 1A) and the rest were asymptomatic. The samples were transported to the greenhouse and to the laboratory of Plant pathogenic virus at the Postgraduate College. In order to discard the presence of fungi and phytopathogenic bacteria, the symptomatic cladodes were directly observed through a stereo microscope and were dissected in 1 cm2 fragments that were spread on PDA and King B media. For each medium, 40 fragments of cladodes were placed and, distributed on four Petri dishes. This analysis was repeated twice, and due to they were obtained negative results for these microorganisms, the double-stranded RNA (dsRNA) was extracted in order to determine the presence of a possible virus. The extraction was done independently from the epidermal tissue of four symptomatic and four asymptomatic (control), following the protocol of Dodds et al., (1987).

Figure 1 Cladodes of cactus pear in the field with chlorotic ringspots around the areoles or between them (A). Symptoms on indicator plants inoculated with sap of cladodes of cactus pear with chlorotic ringspots (B). Necrotic local injuries on Nicotiana tabacum var. Xanthi (B1). Chlorotic local injuries on Chenopodium quinoa (B2). Chlorotic local injuries on N. benthamiana (B3).
The partial purification of the possible virus was performed in according to the protocol proposed by Lastra et al., (1976). Furthermore, the partial purification of the virus and the maceration of the epidermis that covered the chlorotic spots of the sick cladodes were independently placed on formvar coated copper 400 mesh grids. They were contrasted with 2 % uranyl acetate and observed under a JEOL transmission electron microscope, models JEM-1200 EXII and JEM-100 CX II of the Central de Instrumentación de Microscopía de la Escuela Nacional de Ciencias Biológicas del IPN and the Facultad de Estudios Superiores Unidad Iztacala de la UNAM, respectively.
The transmission of the possible virus was performed through mechanical inoculation on three groups of eleven indicator plants (Nicotiana tabacum cv. Xanthi, N. rustica, N. glutinosa, N. occidentalis, N. benthamiana, N. clevelandii, Capsicum annuum, Chenopodium quinoa, Ch. amaranticolor, Datura stramonium y Solanum lycopersicum). The epidermis of symptomatic cladodes collected in Cuautepec de Hinojosa was used as a source of inoculum. The plants were kept in greenhouse at 25-30 ºC with relative humidity of 70 ± 5 % and 12 daylight-hours; they were examined during 15 days in order to record the appearance of symptoms. Mecanical transmission to cactus plants was also done mechanically on seven asymptomatic cladodes of different plants. The epidermis of symptomatic cladodes was used as a source of inoculum, which was macerated in phosphate buffer + DIECA 0.01M pH 7.2. 600 mesh carborundum was sprinkled over a surface of approximately 5 cm2 on the upper portion of the cladode; the inoculum was taken with a cotton swab and was rubbed over the surface. As a negative control, seven asymptomatic plants were inoculated with a cotton swab previously moistened with the buffer mentioned above. All the plants were kept in greenhouse for 45 days and were observed every 72 hours in order to record the appearance of symptoms. The plants used in this phase were obtained from Tlalnepantla, Morelos; a region where no viral symptoms have been observed, in order to have natural growth conditions.
The extraction of RNA was done with the epidermis of symptomatic and asymptomatic cladodes with the commercial product Trizol(c) following the manufacturer's instructions, and with the silica method. The RT-PCR was done with primers for Tobacco mosaic virus (TMV), Carnation mottle virus (CarMV) and for the Potexvirus and Tobamovirus genera. For TMV, the RT-PCR was done in a single step with the specific primers TMVCPF and TMVCPR (Ortega et. al, 2007) that amplify a fragment of 240 pb. The fragment of the protein cover of the CarMV (1.05 kb) was amplified with the CarMV and CarMV/F (Singh et al., 2005). For the Potexvirus genus (Van der Vlugt et al., 2000) used the POTEX1RC, POTEX2RC. The PTEX1RC / POTEX5RC and POTEX2RC / pairs POTEX5RC amplify a fragment of 735 and 584 pb, respectively, corresponding to the viral replicase. For any member of the Tobamovirus genus, the primers used were TMV5vAccl / TMV3vc and TMVcp5/primers TMVcp3, which amplify a fragment of 360-419, and 300 pb, respectively. For each pair of primers, the RT-PCR was done in a single step.
In order to evaluate the distribution of the incidence of symptoms in the production units, the number of plants with irregular or circular chlorotic spots and the presence of chlorotic rings around the areoles were determined. The first unit was divided in three sections of five furrows each (located at the entrance, middle and end of the greenhouse, respectively) in order to evaluate 50 plants/furrow/section (250 plants total). In each of the other two units, 20 continuous plants were evaluated in five furrows (100 plants total). The severity of the disease was estimated quantifying the number of symptomatic cladodes with regard to the number of total cladodes per plant, considering that the induced damages are of a systemic type, and under the supposition that greater number of infected cladodes implied a greater period of infection. The data obtained was analyzed the using Surfer(r) program ver. 8.0 in order to generate dispersion maps of severity with geostatistic interpolation.
The cladodes of cactus pear with symptoms associated to virus showed ringspots around and between the areoles (Figure 1A), and 48 hours after having been collected they turned aqueous and brown. The general phytosanitary analysis of the symptomatic cladodes did not show signs of phtypathogenic fungi or bacteria; however, the double-stranded RNA was isolated, which indicated the presence of a virus with genomic RNA. In some members of the Cactaceae family the: Cactus virus X, Zygocactus virus, Cactus virus 2, Saguaro cactus virus, Sammon's Opuntia virus and Tobacco mosaic virus, whose genome is also RNA. However, all of these viruses have been reported discarded as possible causes for the observed symptoms due to the fact that the symptoms showed in the range of the host plants (Table 1), and the form and size of the viral particles (Figure 2, Table 2) do not coincide with what has been reported so far (De la Torre et al., 2007; Hausbeck and Gildow, 1991; Lastra et al., 1976; Nelson and Tremaine, 1975).
Table 1 Symptoms on indicator plants inoculated with the virus isolated from cladodes of cactus pear with chlorotic ringspots. Cuautepec de Hinojosa, Hidalgo.

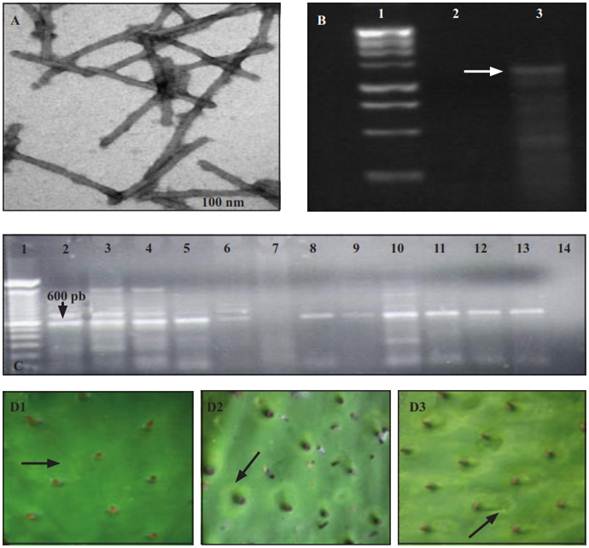
Figure 2 Viral particulates found in the partial purification obtained from cladodes of cactus pear with putative virus symptoms (A). Bands of double-stranded RNA obtained from the cactus pear (B). Line 1: molecular marker of 1Kb; line 2: asymptomatic cladodes; line 3: cladodes with chlorotic ringspots around the areoles. Products of RT-PCR amplification for the genus Potexvirus (C). Line 1: molecular marker 1 Kb (Plus Invitrogen); line 2-6 and 8-12: cladodes of cactus pear with chlorotic ringspots; line 13: foliar tissue of orchid infected with Potexvirus; line 14: negative control; line 7: without sample. Chlorotic ringspots in growths of cladodes inoculated with infected sap at 7 (D1), 14 (D2) and 25 (D3) days after inoculation.
Table 2 Type and size of a viral particle in a partial purified obtained from cactus pear cladodes with clhorotic ringspots. Cuatepec de Hinojosa, Hidalgo.
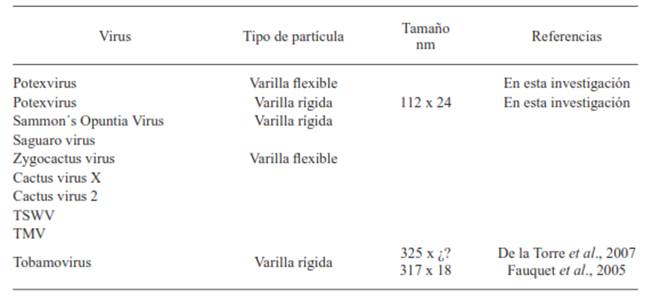
In the epidermis of symptomatic cladodes, viral particles were observed in a flexible rod form, and in the partial purification they look like fragmented rods of a rigid type (Figure 2A). These particles had an average size of 112 x 24 nm, which differ from what was reported by Fauquet et al. (2005) and De la Torre et al. (2007) (Table 2), therefore it was discarded that the rigid fractions in the cactus pear corresponded to any species of Tobamovirus, genus that was found in prickly pear cacti (De la Torre et al., 2007). The flexible rods in the cladodes of did not correspond to those of the Cactus virus X, Zygocactus virus (Alphaflexiviridae family) nor to Cactus virus 2 (Betaflexiviridae family), given that their size is greater than that of the observed particles.
The mechanical transmission of the causal agent occurred in three out of seven inoculated cladodes. No control cladode showed symptoms. Seven days after inoculation (dai), the first symptoms appeared on all the shoots emerged from the inoculated cladodes. The symptoms were chlorotic rings and spots found mainly on the mid-part of the shoots (Figure 2D). At 14 dai, the chlorosis was more evident and the number and size of the chlorotic spots increased, mainly circling the areoles (Figure 2D). At 25 dai, the rings became chlorotic spots, circular and irregular due to their coalescence (Figure 2 D). These symptoms contrast with those reported by De la Torre et al. (2007), who observed chlorotic spots of 0.5-1cm uniformly distributed outside the areolar zone on the cladodes of the prickly pear cacti (O. amyclaea). Of the viruses reported in cacti, only in the Cactus virus X - Nopalea cochenillifera interaction (Lastra et al., 1976), the symptoms showed are similar to to those observed in this work. In wild cacti, this virus induces simple or concentric yellowish ringspots of 2-4 cm surrounding the areoles. According to identification by RT-PCR, the symptomatic cladodes were infected with a species of the genus Potexvirus (Figure 2D). This result is congruent with the transmission electron microscopy analysis, where flexible viral particles were observed, so the symptoms associated to cladodes of cactus pear could correspond to some specie of the genus Potexvirus, except Cactus virus X and Zygocactus, whose particles are larger than those isolated from the cactus cladodes; furthermore, the induced symptoms by Zygocactus virus on C. quinoa are different to those found in this work.
The incidence of diseased plants and the average severity was of 47-60 and 51-79 %, respectively, which suggest a chronic infection and the vegetative transmission of the causal agent. In Taiwan, the Cactus virus X caused on the pitahaya (Hylocereus undatus) an incidence of 90, 50 and 60-70% in Pingtung, Kinman and in other areas, respectively (Liao et al., 2003). The spatial distribution pattern of diseased plants was added with directionality of the furrows (Figure 3). The aggregates were 2-5 plants implicating possible dissemination by means of pruning and harvest tools, which are carried out using a non-desinfested knife. This possibility agrees with the results of the mechanical transmission (43%), therefore the management of this virus must be preventive through the use of healthy material and desinfested tools. Due to the Mexican plateau is the most important cactus pear production region, it is necessary to determine the species of the Potexvirus that was found and to carry out epidemiological and management studies. Given that the Mexican plateau is the main region that produces cactus pear, it is necessary to determine the species found in the genus Potexvirus and carry out epidemiological and management studies.

Figure 3 Spatial distribution map of the severity of the disease associated with chlorotic spots on cactus pear in Cuautepec de Hinojosa, Hidalgo.
Conclusions
In this work, a new virus is reported affecting the Cactaceae family in Mexico. The irregular and circular chlorotic rings and spots symptoms found on O. ficus-indica were related to a virus of a simple RNA chain with particles of a flexible rod type. Based on the RT-PCR results, it was confirmed that the virus belongs to the Potexvirus genus. The incidence of the disease was of 47-60%, with aggregated distribution and a strong tendency to row direction possibly due to management and harvest effects.
Acknowledgements
To M.C. Ma. Esther Sánchez Espíndola, of the Central de Instrumentación de Microscopía de la ENCB-IPN, for her aid in the observation and photographs using the transmission electron microscope (TEM). To the technician Jesús Espinoza of the FES-Iztacala UNAM, for the photographs in the TEM. To Fermín Cerón Téllez, for allowing this study in the production units of cactus pear. To the CONACYT for providing the postgraduate grant.
REFERENCES
Brandes J, Wetter C. 1959. Classification of elongated plant viruses on the basis of particle morphology. Virology 8: 99. [ Links ]
De la Torre AR, Salazar SM y Ruiz MR. 2007. Ocurrencia de un Tobamovirus asociado con manchas anulares amarillas en nopal tunero en México. Agrociencia 41:763-773. [ Links ]
Dodds J, Morris T and Jordan R. 1987. Plant viral double stranded RNA. Annual Review of Phytopathology 22:151-168. [ Links ]
Fauquet CM, Mayo MA, Maniloff J, Desselberger U and Ball LA. 2005. Virus taxonomy Eighth Report of the International Committee on Taxonomy of Virus. Academic Press, San Diego, CA. 1259 p. [ Links ]
Giri L and Chessin M. 1975. A severe strain of Tobacco mosaic virus from cactus. Phytopathology 65: 824-825. [ Links ]
Hausbeck MK and Gildow FE. 1991. First report of Tomato spotted wilt virus on thanksgiving cactus. Plant Disease 75: 215. [ Links ]
Lastra JR, Gaskin D. and Uzcategui RC. 1976. Virus X del cactus en Venezuela. Agronomía Tropical 26: 303-310. [ Links ]
Liao JY, Chang CA, Yan CR, Chen YC, and Deng TC. 2003. Detection and incidence of Cactus virus X on pitaya in Taiwan. Plant Pathology Bulletin 12: 225-234. [ Links ]
Milbrath MG and Nelson MR. 1972. Isolation and characterization of a virus from saguaro cactus. Phytopathology 62:739-742. [ Links ]
Nelson MR and Tremaine JH. 1975. Physiochemical and serological properties of virus from saguaro cactus. Virology 65: 309-319. [ Links ]
Ortega E, Zambrano K, Carballo O, Romano M. and Edgloris M. 2007. Caracterización de un Tobamovirus aislado de plantas de Canavalia (Canavalia ensiformis L.) Aragua, Venezuela. INCI. 32:202-205. [ Links ]
SIAP-SAGARPA. 2013. Anuario estadístico de la producción agrícola. Servicio de información agroalimentaria y pesquera. http://www.siap.gob.mx/index (consulta, junio del 2015). [ Links ]
Singh HP, Hallan V, Raikhy G, Kulshrestha S, Sharma ML, Ram R, Garg ID and Zaidi AA. 2005. Characterization of an Indian isolate of Carnation mottle virus infecting carnations. Current Science 88: 25. [ Links ]
Van Der Vlugt RAA, Stijger CM, Verhoeven JThJ and Lesemann DE. 2000. First report of Pepino mosaic virus on tomato. Plant Disease 84: 103. [ Links ]
Received: July 04, 2013; Accepted: October 15, 2014











 texto em
texto em 


