Introduction
Spontaneous coronary dissection (SCAD) is an unusual cause of acute myocardial infarction. It is characterized by a separation of the coronary artery wall, not associated with trauma, iatrogenesis or atherosclerotic plaque.1 It occurs predominantly in young and middle-aged people without risk factors, manifesting clinically as acute coronary syndrome (ACS), arrhythmia, or sudden cardiac death.2
Some predisposing factors such as female gender, pregnancy, age < 50 years, hormonal treatment, connective tissue diseases (vascular Ehlers-Danlos syndrome, Marfan syndrome) and fibromuscular dysplasia have been identified. Related triggers are emotional and physical stress, intense isometric exercise, and weight lifting.3
The case of a patient with ST-elevation acute coronary syndrome (STEACS) and cardiac arrest secondary to spontaneous coronary dissection while performing physical activity is presented.
Case presentation
49-year-old male patient with no known history. While the patient was doing aerobic physical activity in the gym -running on a band- the patient presented an altered state of consciousness for which he was transferred to a health center where he entered cardiorespiratory arrest, with an initial rhythm of ventricular fibrillation. Advanced cardiopulmonary resuscitation was performed, with which he returned to sinus rhythm and was referred to a more complex center.
The patient was admitted hemodynamically stable and with a Glasgow coma scale of 8/15. A 12-lead electrocardiogram was taken showing ST-segment elevation in aVR and ST-segment depression in DI, DII, DIII, aVL and V2-V6, which was interpreted as a pattern suggestive of left main coronary artery involvement (Figure 1).

Figure 1: ST-segment elevation in the derived aVR and ST-segment depression in DI, DII, DIII, aVL and V2-V6. Source: Own creation.
Medical treatment was started with a single 300 mg loading dose of acetylsalicylic acid and, subsequently, 100 mg every 24 hours, a single 600 mg loading dose of clopidogrel, a single 40 mg loading dose of subcutaneous nadroparin every 24 hours, single 80 mg dose of atorvastatin and subsequently 40 mg every 24 hours, 50 mg metoprolol every 12 hours and amiodarone infused at 1 mg/min for 6 hours and subsequently at 0.5 mg/min due to the arrest rhythm presented. Coronary angiography was performed, showing type 2a spontaneous coronary dissection of the distal third of the circumflex artery, the second obtuse marginal artery (OM2) with TIMI 3 residual flow and extensive intramural hematoma (IMH) (Figure 2). His care was continued in the special care unit, where he remained stable and had no recurrence of arrhythmias. A control electrocardiogram was taken that showed the resolution of the electrocardiographic alterations (Figure 3). Transthoracic echocardiography reported a slightly reduced ejection fraction (50%) and akinesia with the remodeling of the basal segment of the inferior wall, without other relevant findings. Magnetic resonance angiography of abdominal vessels was performed without evidence of changes in intra-abdominal visceral arteries for fibromuscular dysplasia.

Figure 2: Type 2a spontaneous coronary dissection of the distal third of the circumflex artery and the second obtuse marginal artery. Source: Own creation.
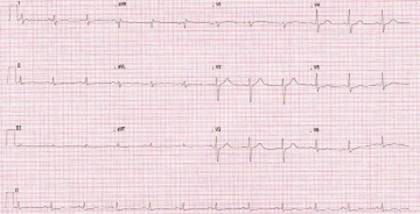
Figure 3: Control electrocardiogram was taken two days after coronary angiography. Source: Own creation.
In-hospital cardiac rehabilitation was started, and on the fifth day, the patient was discharged with 100 mg acetylsalicylic acid every 24 hours, 75 mg clopidogrel every 24 hours, 50 mg metoprolol succinate every 24 hours, 20 mg omeprazole every 24 hours, 200 mg amiodarone every 24 hours, cardiac rehabilitation for 24 sessions.
Discussion
The first record of SCAD is from 1931 by Dr Harold C. Pretty, who described it in the autopsy of a 42-year-old woman with sudden death.4 It is defined as a non-traumatic and non-iatrogenic separation of the coronary artery wall that can occur between the intima and media or between the media and the adventitia, creating a false lumen with an intramural hematoma decreases blood flow and generates ischemia.5 Its worldwide incidence varies from 0.1 to 0.28%, but a series of recent cases show a prevalence between 0.1 and 0.24%.6,7
One of its main manifestations is acute myocardial infarction, which occurs in more than 90% of patients, of which 20-50% are STEACS, and less than 5% have cardiogenic shock and ventricular arrhythmias.8
The cause of SCAD is unknown, but predisposing conditions such as fibromuscular dysplasia, peripartum period, connective tissue diseases such as Marfan syndrome, vascular Ehlers-Danlos syndrome, Loeys-Dietz syndrome, systemic inflammatory diseases such as systemic lupus erythematosus, Crohn’s disease, sarcoidosis, and polyarteritis nodosa. However, the prevalence of inflammatory disorders in patients with SCAD is low, with reports accounting for less than 5% of cases.8-10
Likewise, precipitating factors have been associated, such as activities involving the Valsalva´s maneuver, labor, drug abuse such as cocaine, hormonal therapy, emotional stress and physical stress such as intense exercise.11
The test of choice for diagnosis is coronary angiography. This is used to detect the dissection site and define its characteristics and severity. It also allows identifying other anatomical alterations and performing percutaneous coronary intervention, if necessary. According to the angiographic appearance, a defined classification has been established as the presence of a tear of the intima with a false lumen (type 1), presence of intramural hematoma with diffuse narrowing > 20 mm that recovers its caliber distal to the lesion (type 2a) or without recovering its size up to the distal coronary artery (type 2b), focal stenosis (type 3), abrupt occlusion without a lesion proximal to it (type 4). Type 2 dissection is the most common one reported and is observed in 67% of cases, followed by type 1 in 29.1% and type 3 in 3.4%.12
Regarding treatment, although there is no strong consensus in favor, it can be concluded according to several series that percutaneous coronary intervention (PCI) for SCAD is associated with worse short-and long-term results than those presented with PCI for atherosclerotic lesions.13,14 Technically, PCI can be a challenge in these patients since long lesions may require the use of multiple stents, coronary wires can enter the false lumen and cause occlusion of the vessel or being in the presence of tortuous coronary arteries can be prone to iatrogenic injury. Likewise, when faced with a hematoma, there is the possibility of expansion resulting in the loss of distal permeability or retrograde extension to more proximal vessels.15-17 These factors contribute to the success rates of PCI ranging between 47 and 72% in cohort studies. Therefore it is reserved for patients with high-risk characteristics such as involvement of multiple proximal vessels, main left coronary artery involvement or anterior descending artery ostium.17-19
In addition to the above, with medical management, a high probability of spontaneous cure is defined as restoration of blood flow and a decrease in the severity of the stenosis (evaluated angiographically), which ranges between 70 and 97% of patients cases.8 Said medical management is based on the administration of beta-blockers and antiplatelet agents. Beta-blockers reduce shear stress and significantly reduce the risk of recurrence, which is why they are recommended in all patients, especially those with high blood pressure. Regarding antiplatelet therapy, there is still controversy over the best strategy to follow due to the lack of solid clinical studies. According to recent reviews, dual antiplatelet management is suggested in patients undergoing PCI with an estimated duration of up to 12 months, but this recommendation is based on expert recommendations. If this strategy is chosen, the use of acetylsalicylic acid and clopidogrel is recommended.19 Randomized clinical trials are currently underway to determine the best treatment method. With regard to statins, some studies have shown that they increase the risk of recurrence of SCAD, and their pathophysiology is not mediated by atherosclerotic plaque rupture, so their use is not recommended unless the patient meets another indication for it.20
There is no clarity on which patients should be provided with medical management; however, it has been proposed that candidates should have a TIMI flow > two and hemodynamic stability with hospital surveillance between 5-7 days, since the extension of the intramural hematoma, or IMH, can occur in the 5-10% of cases.21 In our case, we present a type 2a SCAD of the distal third of the circumflex artery and the second obtuse marginal artery (OM2) that generates long tubular stenosis, in some segments of 90%, with residual TIMI three flow and extensive intramural hematoma. Our report agrees with that found in the literature, where the main precipitant in men is physical activity, with studies showing a presentation of 44% vs 2.8% in women.11
Likewise, our patient presented compromise in the distal third of the circumflex artery, which coincides with several reports where only 10% affect the proximal coronary artery.12 In the case of our patient, it presented with sudden death and ventricular arrhythmia; this is an unusual presentation that occurs in less than 5% of cases, as a precipitating event it was associated with aerobic exercise with only two other case reports in the literature.22,23
Conclusions
SCAD should be considered in young patients and in the absence of cardiovascular risk factors that present with suspected ACS. It is necessary to identify the predisposing factors and conditions that have been described so far. Regarding its management, a conservative approach is preferred, and revascularization is reserved for those patients with high-risk characteristics. Appropriate recognition of this entity will allow better treatment for patients, thus avoiding the recurrence of new major cardiovascular events.











 nueva página del texto (beta)
nueva página del texto (beta)


