Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.14 spe 29 Texcoco sep./oct. 2023 Epub 17-Nov-2023
https://doi.org/10.29312/remexca.v14i29.3546
Research notes
Population parameters of Chrysoperla carnea under laboratory conditions in Puebla
1Campus Puebla-Colegio de Postgraduados-Laboratorio de Control Biológico. Carretera Federal México-Puebla km 125.5, Santiago Momoxpan, Puebla, México. CP. 72760. Tel. 222.2851443, ext. 2200.
2Benemérita Universidad Autónoma de Puebla-Instituto de Ciencias-Centro de Agroecología. Edificio VAL1, Carretera a San Baltazar Tetela km 1.7, San Pedro Zacachimalpa, Puebla, México. CP. 72960. Tel. 222 2295500, ext. 1302. (marthaa.diazrivas@viep.com.mx; miguel.aragons@correo.buap.mx).
Chrysoperla carnea Stephens (Neuroptera: Chrysopidae) is a predator used in biological pest control, so establishing laboratory breeding methods is essential for the use of these organisms in the field. The objective of this work was to obtain the biological and population parameters of this species under laboratory conditions. The experiment was carried out in the Laboratory of Biological Control of the College of Postgraduates, Puebla Campus in 2021; with a population collected in the vegetable-growing area in Los Reyes de Juárez, Puebla. Following a standardized breeding protocol and after the population was adapted to the laboratory conditions, bioassays were performed to obtain the population parameters, individualizing 50 eggs in Petri dishes, and observing the development until the adult stage. Three couples of adults were formed and followed until death. The preoviposition period was 4.6 days, average longevity of 53 days, on average a female laid a total of 1 289 eggs, 25.56 eggs per day, and a hatching of 88.35%. The average development from egg to adult was 24.19 days with cumulative survival of 32% and a sex ratio of 0.6, the rm value was 0.18014. The knowledge generated in this work has been very useful to program the breeding of this insect, obtain egg production on certain dates, and for people who would like to engage in the breeding of this predator.
Keywords: biological control; green lacewings; insect breeding; predators
Chrysoperla carnea Stephens (Neuroptera: Chrysopidae) es un depredador utilizado en el control biológico de plagas, por lo que establecer métodos de reproducción en laboratorio es fundamental para el uso de estos organismos en campo. El objetivo de este trabajo fue obtener los parámetros biológicos y poblacionales de esta especie en condiciones de laboratorio. El experimento se realizó en el laboratorio de Control Biológico del Colegio de Posgraduados, Campus Puebla en el año 2021; con una población colectada en la zona productora de hortalizas en Los Reyes de Juárez, Puebla. Siguiendo un protocolo de cría estandarizado y después de que la población fue adaptada a las condiciones de laboratorio, se realizaron bioensayos para obtener los parámetros poblacionales, individualizando 50 huevos en cajas petri observando el desarrollo hasta la etapa adulta. Se formaron tres parejas de adultos y se les dio seguimiento hasta la muerte. El periodo de preoviposición fue de 4.6 días, y longevidad media de 53 días, en promedio una hembra puso en total 1 289 huevos, 25.56 huevos por día, y eclosión de 88.35%. El desarrollo promedio de huevo a adulto fue de 24.19 días con supervivencia acumulada de 32% y una ratio sexual de 0.6, el valor de rm fue 0.18014. El conocimiento generado en este trabajo ha sido de gran utilidad para programar la cría de este insecto, obtener la producción de huevos en fechas determinadas y para las personas que quisieran dedicarse a la cría de este depredador.
Palabras clave: control biológico; cría de insectos; crisopas; depredadores
The production of entomophagous organisms is carried out throughout the Mexican Republic and the organisms that are most distributed are Chrysoperla carnea Stephens (Neuroptera: Chrysopidae) and Trichogramma pretiosum Rirely, 1879 (Hymenoptera: Trichogrammatidae) (Tamez et al., 2001). C. carnea is a cosmopolitan species, widely distributed in a wide variety of climates and vegetation. This species is frequently found in fruit trees, alfalfa and vegetable crops, and natural vegetation. Green lacewings larvae are predators of aphids, whiteflies, pacific mealybugs, thrips, and eggs of various insects (Van Driesche et al., 2007). It is important to know the basic aspects of the biology and population behavior of this predator under laboratory conditions, to obtain quality individuals with good performance in the field and to make more precise planning of their management and reproduction in the laboratory.
Life and reproduction tables are useful tools that help us understand the dynamics of a group of organisms and ensure population management (Cano-Vazquez, 2001). With them, some aspects of the biology of the insect, such as development time, fertility, and survival, are known with certainty and they allow the calculation of parameters characteristic of the species, such as the net reproduction rate (Ro), the generation time (T) and the intrinsic rate of natural increase (rm) (Flores-Pérez et al., 2004). The objective of this work was to obtain the biological and population parameters of this species, under laboratory conditions, through the use of life tables.
The experiment was conducted in the Laboratory of Biological Control of the College of Postgraduates, Puebla Campus in 2021. The conditions of the breeding chamber were: temperature of 24 °C, relative humidity of 52%, and photophase of 16:8 light:dark, with white LED light.
The eggs of C. carnea were obtained from the maintenance brood of said laboratory. The experiment began with 50 eggs less than 24 h old oviposited on filter paper, the eggs were individualized with entomological tweezers and placed in Petri dishes of 4.5 cm in diameter by 1.5 cm in height. The emerged larvae were kept in the same boxes and fed daily during their development with eggs of Sitotroga cerealella (Oliver 1789) (Lepidoptera: Gelechiidae). Daily observations were made using a Motic MSZ-168 stereo microscope. Each day the exuvia was sought as a reference to measure the time of instar change, and its survival was recorded.
Once the adults emerged, on the same date, three couples were formed, which were individualized in plastic boxes of 12 cm in diameter by 7.5 cm in height, with modified lids for aeration. The boxes were equipped with a drinker and cotton gauze that served as a substrate for oviposition. The couples were fed every third day using a tongue depressor with an artificial diet based on wheat germ, brewer’s yeast, fructose, honey, condensed milk, egg, and water (Vogth et al., 2000). The preoviposition time was recorded.
Oviposition was assessed every third day by changing the gauze and counting the eggs placed on the boxes. The viability was assessed using gauzes of 24 h of each couple, which were placed in plastic boxes of 12 cm in diameter by 7.5 cm in height, on a base of Bond paper folded as an accordion and with eggs of S. cerealella as food; hatching, fertile eggs (with embryo) and infertile eggs (green) were counted. The development time of each stage and instar was counted; the proportion of males to females was also calculated. The life and reproduction tables were constructed according to the methodology described by Vera-Graziano et al. (1997).
The estimated population parameters were: fertility (mx), Ro, T, and rm. Likewise, the rm 2.0 program (Taberner et al., 1993) was applied to the previously calculated data, considering a sex ratio of 0.6, according to the proportion of males and females observed in the development of the bioassay itself. Data analysis was carried out using the Bootstrap technique, performing 500 repetitions, as suggested by Meyer et al. (1987). Based on the life tables, we estimated: survival by age (lx) and mx, as well as the following parameters: Ro, T, rm, finite rate of increase (λ), and doubling time of population (DT).
Table 1 presents the population parameters of C. carnea females reared under laboratory conditions, where it is observed that the preoviposition time was less than four days, a female can lay more than 1 200 eggs throughout her life, and of those eggs, 80% can hatch, on the other hand, a female can live more than 50 days approximately. Of the eggs laid by these adults, it was estimated that 60% were female, and 40% male, they kept a ratio of 3:2.
Table 1 Biological parameters (mean ±SD) of C. carnea females kept under laboratory conditions.
| Parameter | Mean |
|---|---|
| Preoviposition period (days) | 3.67 ±0.67 |
| Fecundity (eggs/female) | 1289 ±448.17 |
| Egg hatching | 1031.2 ±358.53 |
| Female longevity (days) | 53 ±14.01 |
| Sex ratio | 0.6 |
These results are superior to those obtained by Aragón-Sánchez et al. (2020), where these authors evaluated the effect that the application of plant extracts has on the population parameters of C. carnea, highlighting that the females came to present a preoviposition period greater than five days and a number of eggs less than 400, as well as a longevity lower than that obtained in this work, with an average of less than 40 days.
This may be related to the fact that the eggs of S. cerealella used by these authors, with which this population of C. carnea was fed, were treated by immersion in water, contrary to this research, where they were offered eggs that were not treated.
Table 2 shows the development time of the immature stages of C. carnea, these organisms can complete their development in 24 days, the stage of development that took the longest was the larval one (11.34 days on average) and it was observed that the instar of shortest duration was the second. Males pupated and emerged earlier than females, so the average development time of females was slightly longer (24 days) than that of males (23 days).
Table 2 Average length in days (Mean ±SD) for the different stages and instars for the egg-adult period of Chrysoperla carnea fed with Sitotroga cerealella.
| Parameters | Egg | L1 | L2 | L3 | Pupa | Total (egg-adult) |
|---|---|---|---|---|---|---|
| Time of development (days | 4.13 ±0.34 | 3.93 ±1 | 3.41 ±0.67 | 4 ±0.69 | 9.06 ±0.44 | 24.19 ±0.44 |
These results agree with what was reported by Aragón et al. (2020), where C. carnea presented an egg development time of four days, and a larval development time of 10 days, similar to that obtained in this research. For the time of development of pupa, the results differ from those of these authors, since they report a time greater than 14 days, a time longer than that obtained in this research.
This increase in the time of development of pupa is attributed to the management of the population, emphasizing that a population maintained for breeding, without changes in diet, can decrease its development time from egg to adult, as was the case in this research, where the management that was given to the population causes that C. carnea culminates its immature development approximately in 24 days.
On the other hand, Giffoni (2007) estimates the development time of the immature instars of Chrysoperla externa (Hagen) (Neuroptera: Chrysopidae) when they are fed with different prey, highlighting that within the treatments used are the eggs of S. cerealella, where it obtained a development time for the egg stage of three days, for the larva and pupal stage a development time of seven days; it should be noted that this decrease in development time compared to this work is due to the change in temperature since this decrease probably occurred because the organisms were placed at 27 °C.
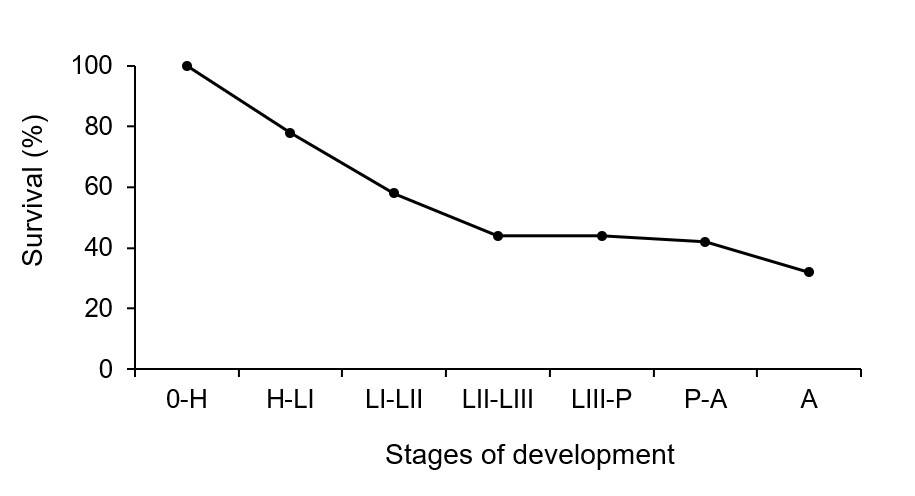
Regarding the life tables (Table 3), it was obtained that mortality occurred in almost all stages of development of C. carnea, with the exception of the passage of larval instar from two to three. The probability of death (dx) was higher in the egg stage (0.22) and in the larval instar LI (0.2). The mortality rate (qx) was higher in the larval instar L1 (0.26), followed by the larval instar LII (0.24) and the pupal stage (0.24). The probability of survival (px) was higher in the larval instars LII (1) and LIII (0.95). Life expectancy was similar from the egg stage to the larval instar LII but decreased in the larval instar LIII and pupa. The probability of survival (lx*100) from egg to emergence of adults was 32%.
Table 3 Specific life table for each stage of C. carnea fed with S. cerealella under laboratory conditions.
| x* | nx* | Dx* | dx* | qx* | px* | Lx* | Tx* | ex* | lx* | Lx 100* |
|---|---|---|---|---|---|---|---|---|---|---|
| x* | nx* | Dx* | dx* | qx* | px* | Lx* | Tx* | ex* | lx* | Lx 100* |
| 0-H | 50 | 11 | 0.22 | 0.22 | 0.78 | 44.5 | 174 | 3.48 | 1 | 100 |
| H-LI | 39 | 10 | 0.2 | 0.26 | 0.74 | 34 | 129.5 | 3.32 | 0.78 | 78 |
| LI-LII | 29 | 7 | 0.14 | 0.24 | 0.76 | 25.5 | 95.5 | 3.29 | 0.58 | 58 |
| LII-LIII | 22 | 0 | 0 | 0 | 1 | 22 | 70 | 3.18 | 0.44 | 44 |
| LIII-P | 22 | 1 | 0.02 | 0.04 | 0.95 | 21.5 | 48 | 2.18 | 0.44 | 44 |
| P-A | 21 | 5 | 0.1 | 0.24 | 0.76 | 18.5 | 26.5 | 1.26 | 0.42 | 42 |
| A | 16 | 0 | 0.32 | 0 | 1 | 8 | 8 | 0.5 | 0.32 | 32 |
*Stages and instars (x); number of individuals (nx); number of dead individuals (Dx); probability of death (dx); mortality rate (qx); probability of survival (px); average number of living individuals (Lx); cumulative sum of Lx to obtain values expressed in number of individuals per units of time (TX); life expectancy (ex); probability of survival from egg (lx) and probability of survival from egg in percentage (lx*100).
Since this organism is polyphagous, it was observed that it can be bred under laboratory conditions, with other types of prey, such as Aspidiotus hedericola Leonardi (Hemiptera: Diaspididae), where similar values were contemplated in the specific life table for each stage to those obtained in this work (Ozbesnili and Ozsisli, 2013).
The survival curve of C. carnea was constructed using the values of the probability of survival in percentage from egg to adult (Figure 1), where it is observed how mortality begins from the early stages of development of the organisms, becoming established when they pass to instar L3, maintaining this mortality during the end of the larval and pupal phase, finally, a slight decrease in the population when moving to the adult stage.
These results coincide with another neuropteran Sympherobius barberi Banks (Neuroptera: Hemerobiidae), predator of the cochineal Dactylopius opuntiae Cockerell (Hemiptera: Dactylopiidae), where under laboratory conditions, a curve similar to that presented for C. carnea in this research can be observed, where mortality remains once predators pass to larval development stage LIII until the adult stage (Pacheco-Rueda et al., 2011).
Figure 2 shows the fertility curve of C. carnea, which shows the average number of eggs produced by each female throughout her life. It was observed that the most fertile period occurred in the fourth and seventh weeks and that it decreased from the eighth week. It was estimated that the average number of eggs per female per day was 25.
The parameters of the life table are condensed in Table 4, observing that the value of the intrinsic rate of increase rm was 0.18014 females obtained per each female, which indicates an increase in the population after several generations maintained under these conditions, in the same Table 4, the rest of the values obtained from the life table are presented. This rm value obtained was higher than that reported by Aragón et al. (2020) for the predator C. carnea, where they establish a calculation of 0.1190; also, a decrease in the rest of the values of the life table parameters compared to what was obtained in this research.
Table 4 Parameters of the life table of C. carnea kept under laboratory conditions.
| Parameter | Mean |
|---|---|
| Intrinsic rate of increase (rm) (95% confidence interval for rm) | 0.18014 (0.1582-0.202) |
| Net reproduction rate (Ro) | 415.3094 |
| Mean duration of generation (T) (days) | 33.4685 |
| Finite rate of increase (λ) | 1.1973 |
| Doubling time (TD) (days) | 3.8478 |
In addition to the good management that must be given to the breeding, it must also be considered that the food used to reproduce populations of C. carnea in the laboratory was eggs of S. cerealella, this food has also been used for the reproduction of other lacewings such as Chrysoperla defreitasi (Brooks, 1994), where lower mortality in larvae and shorter development cycles of this predator were observed (Biagioni and Freitas). Likewise, the use of artificial diets has also been evaluated for the reproduction of C. carnea under laboratory conditions, having results greater than 80% survival in larvae and pupae of this predator, when they are raised with a diet based on water, sugar, yeast, and honey (Murtaza et al., 2020).
Conclusions
The population parameters estimated in this work suggest that the breeding of this predator carried out under the afore mentioned conditions of temperature, humidity, photoperiod, feeding, and management is productive, although mortality was also observed in all stages of development, with the probability of death in the stage of egg development and first larval instar standing out.
Bibliografía
Aragón-Sánchez, M.; Serratos-Tejeda, C.; Huerta-de la Peña, A.; Aragón-García, A.; Pérez-Torrez, B. and Pineda, S. G. 2020. Effect by ingestion of stracts of Argemone mexicana L. on biological parameters and capability of Chrysoperla carnea (Stephens) to increase in a laboratory. Southwestern Entomologist. 45(2):405-414. [ Links ]
Biagioni, A. y Freitas, S. 2001. Efeito de diferentes dietas sobre o desenvolvimento pós-embrionário de Chrysoperla defreitasi Brooks (Neuroptera: Chrysopidae). Neotropical Entomology. 30(2):333-336. [ Links ]
Cano-Vázquez, E. 2001. Cría de Trichogramma pretiosum, Sitotroga cerealella y Chrysoperla externa. Manejo integrado de plagas. Avances en el fomento de productos fitosanitarios no-sintéticos. Costa Rica. 60:93-96. [ Links ]
Flores-Pérez, L.; Bautista-Martínez, N.; Vera-Graciano, J.; Valdez-Carrasco, J. y Angulo, O. A. 2004. Ciclo de vida y tasas de superviviencia y reproducción de Copitarsia incommoda Walker (Lepidoptera: Noctuidae) en tres cultivares de Brassica oleracea L. Agrociencia. México, DF. 38(5):517-523. [ Links ]
Giffoni, J.; Valera, N.; Díaz, F. y Vázquez, C. 2007. Ciclo biológico de Crhysoperla externa (Hagen) (Neuroptera: Crhysopidae) alimentada con diferentes presas. Bioagro. 19(2):109-113. [ Links ]
Meyer, J. S.; Ingersoll, C. G. and McDonald, L. L. 1987. Sensitivity analysis of population growth rates estimated from cladoceran chronic toxicity test. Eviron. Toxicol. Chem. 6(2):115-126. [ Links ]
Murtaza, G.; Ramzan, M.; Sulta, Y.; Saleem, F.; Rafique, M. A.; Sajid, S. and Jamil, M. 2020. Effect of different artificial diets on biological parameters of female Chrysoperla carnea under laboratory conditions. J. Sci. Agric. 4:50-54. [ Links ]
Ozbesnili, E. and Ozsilsi T. 2013. Biological features and stage specific lifetable of Chrysoperla carnea Stephens (Neuroptera: Chrysopidae) on Aspidiotus hedericola Leonardi (Hemiptera: Diaspidisae). Türkiye Biyolojik Mücadele Dergisi. 4(1):3-9. [ Links ]
Pacheco-Rueda, I.; Lomelí-Flores, R.; Rodríguez-Leyva, E. y Ramírez-Delgado, M. 2011Ciclo de vida y parámetros poblacionales de Sympheronius barberi Banks (Neuroptera: Hemerobidae) criado con Dactylopius opuntiae Cockerell (Hinenoptera: Dactylopiidae). Acta Zoológica Mexicana. 27(2):325-340. [ Links ]
Tamez-Guerra, P.; Galán-Wong, L. J.; Medrano-Roldán, H. M.; García-Gutiérrez, C.; Rodríguez-Padilla, C.; Gómez-Flores, R. A. y Tamez-Guerra, R. S. 2001. Bioinsecticidas: su empleo, producción y comercialización en México. Ciencia UANL. 2(4):143-152. [ Links ]
Van Driesche, R. G.; Hoddle, M. S. y Center, T. D. 2007. Control de plagas y malezas por enemigas naturales. Forest Health Technology Enterprise Team. USA. 8-48 pp. [ Links ]
Vera-Graziano, J.; Pinto, V. M. y López, J. C. 1997. Ecología de poblaciones de insectos. Universidad Autónoma Chapingo (UACH). Texcoco, Estado de México. 33-49 pp. [ Links ]
Taberner, A.; Castañera, P.; Silvestre, E. and Bopazo, J. 1993. Estimation of the intrinsec rate of natural increase and its error by both algebraic and resampling approaches. Computer Appl. Bio. 9(5):535-540. [ Links ]
Vogt H. F.; Bigler, K.; Brown, M.; Candolfi, F.; Kemmeter, C.; Kühner, M.; Moll, A.; Travis, A.; Ufer, E.; Viñuela, M. and Waldburger, W. A. 2000. Laboratory method to test effects of plant protection products on larvae of Chrysoperla carnea (Neuroptera:Chryrsopidae). In: Guidelines to evaluate side effects of plant protection products to non-target arthropods. Int. Organization for biological and integrated control of noxious animals and plants, West Palearctic Regional Sec. (IOBC/WPRS) Ed. Dijon, France. 27-44 pp. [ Links ]
Received: July 01, 2023; Accepted: August 01, 2023











 texto en
texto en