Introduction
Diplodus vulgarisis a demersal fish species found in the Mediterranean and western Black seas and along the eastern Atlantic coast (France to Senegal and Angola to South Africa), including the Madeira, Azores, and Canaries archipelagoes (Bauchot and Hureau 1990). This species is mostly found in shallow waters (less than 50.0 m depth) and prefers rocky and sandy bottoms, althoughD. vulgarisjuveniles generally prefer coastal lagoons (Monteiro 1989, Ribeiro et al. 2008, Altin et al. 2015a). In addition,D. vulgarisindividuals less than 12.0 cm total length (TL) have been found in coastal seagrass areas where ample food resources are available (Abecasis et al. 2009, Altin et al. 2015b).
Along the northwest Mediterranean and Portuguese coasts, the spawning season ofD. vulgaristakes place in October and November (Bauchot and Hureau 1986, Jug-Dujaković and Glamuzina 1988, Gonçalves and Erzini 2000, Hadj-Taieb et al. 2012, Mouine et al. 2012). In the central Mediterranean, the spawning season has been reported to take place from December to February (Hadj-Taieb et al. 2013). At the time of the first reproduction, the body length ofD. vulgarisis approximately 17.4 cm, which corresponds to an age of 4 years (Mouine et al. 2012).
Diplodus vulgarisis an economically important species that is extensively caught by artisanal and industrial fisheries. Along Turkish coasts, the most common fishing gears used to capture this species are gill nets and longlines. Growth data ofD. vulgarisare crucial for the development of proper management strategies, yet many biological aspects of this species remain unknown (Pauly 1983), with most age and growth studies focusing on adult individuals (Gordoa and Molí 1997, Gonçalves et al. 2003, Pajuelo and Lorenzo 2003, Mouine et al. 2010, Dulčić et al. 2011, Hadj-Taieb et al. 2013). Although validations of daily ring deposition (Vigliola 1997, Villanueva and Molí 1997), TL and otolith measurements (Altin and Ayyildiz 2018), spawning dates, and pelagic larval duration (PLD; Macpherson and Raventos 2006, Galarza et al. 2009, di Franco et al. 2013) have been reported, only one daily growth rate study has been conducted to date (Ayyildiz et al. 2015).
The primary objective of the current study was to evaluate the hatching period, PLD, daily growth rate, and mortality of young-of-the-year (YOY)D. vulgarisfrom the shallow waters of Gökçeada Island, Turkey, using otolith microstructure.
Materials and methods
This study was conducted in the shallow waters (0.00-2.00 m) of Gökçeada Island, Turkey, between June 2013 and June 2014 (Fig. 1). In total, 309 YOYD. vulgarisindividuals were collected using a 32.00-m (TL) beach seine (13.00-mm stretch mesh) with 15.00-m wing lengths. The cod-end measured 2.00 × 2.00 × 0.60 m, with a mesh size of 5.00 mm. An overdose of quinaldine was used to kill the collected fish, and the specimens were stored in a 70.00% alcohol solution.
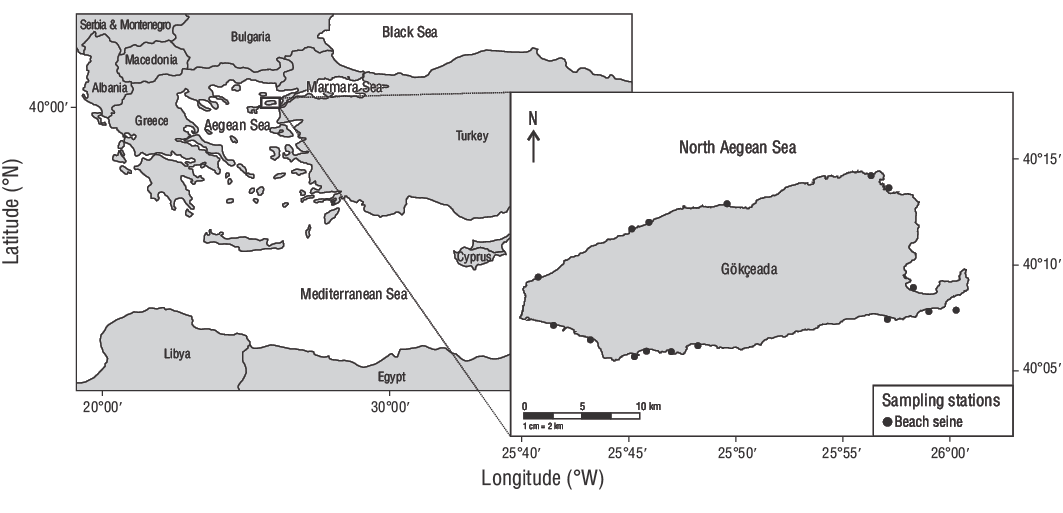
Figure 1. Sampling stations employed in this study. A beach seine was used to collect common two-banded seabream (Diplodus vulgaris) individuals from the shallow waters of Gökçeada Island, Turkey.
TheD. vulgarisspecimens were measured for TL (nearest 1.00 mm) and total weight (W, nearest 0.01 g). The relationship betweenWand TL was determined using the following power function:
whereais the regression constant andbis the regression coefficient. The regression parameters (a,b) and the coefficient of determination (r 2) were calculated for all individuals.
Sagittal otoliths were extracted from the specimens and stored in Eppendorf tubes. In the preliminary examination, 79 otoliths were excluded from the age determination analysis due to fractures. Thus, 230 sagittal otoliths, which covered all length classes, were embedded in thermoplastic cement on glass slides. Abrasive papers (12.00, 9.00, and 3.00 mm) were used to sand each sample. Afterwards, each sample was polished with a polishing cloth and alumina paste (0.30 mm) until the daily growth rings were recognizable from the otolith core to its edge (Secor et al. 1991, Jones 1992). The number of otolith rings for each sample was determined by blind counts performed by 2 independent readers who had no knowledge of the specimen. A light microscope was used to count daily growth rings (Fig. 2). Estimations of the precision of the daily growth ring counts of the readers were detected using the coefficient of variation (CV) of Chang (1982) and average percentage error of Beamish and Fournier (1981).
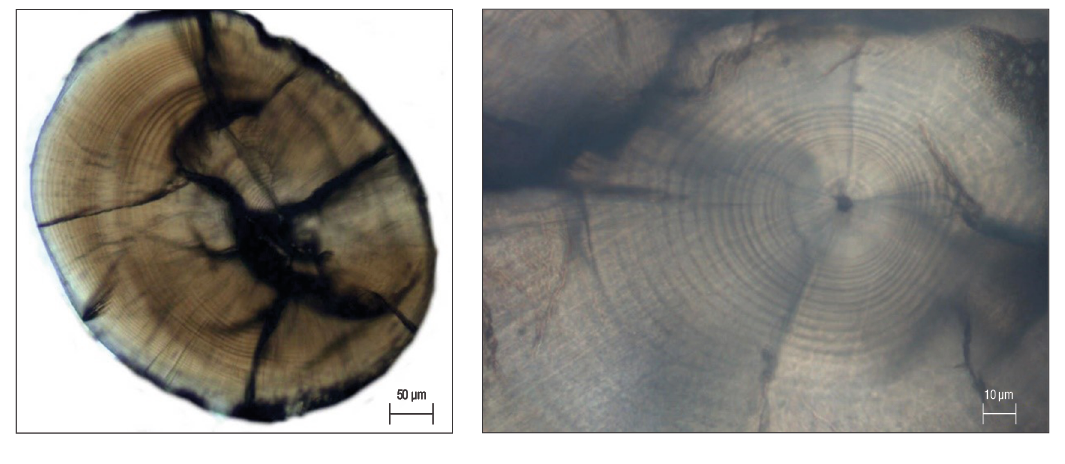
Figure 2. Images of the growth rings seen in a polished sagittal otolith (17 mm total length) from a 54-day-old young-of-the-yearDiplodus vulgarisspecimen.
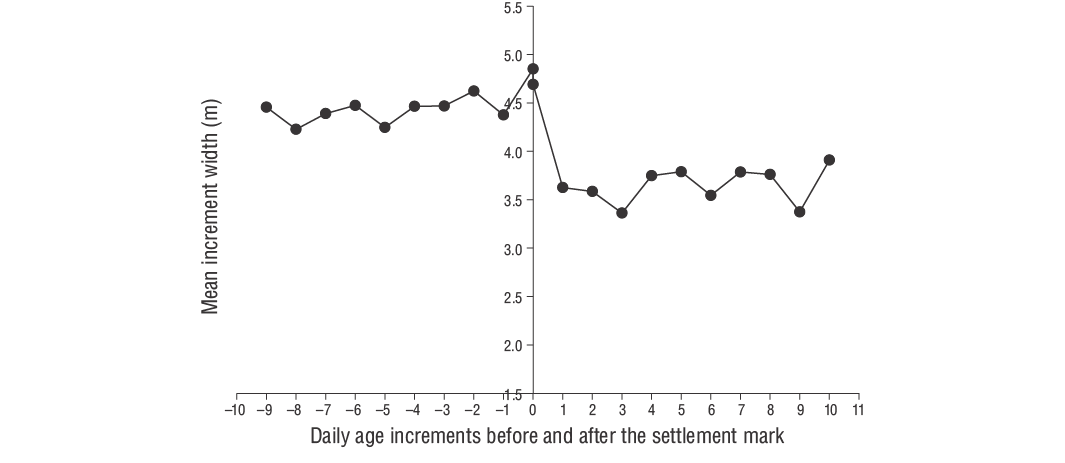
The relationship between fish age (days) and size (TL) was evaluated via linear regression with the mean growth rate (mm·d-1). The daily growth rates for each individual were estimated using the slope of the regression analysis of daily age and TL. The daily otolith growth (increment width) was measured using ImageJ (v.1.52a). According to Wilson and McCormick (1999), the settlement-mark can be classified into one of 3 categories (I, II, or III) and 2 subcategories (a, b). Type I is classified by a clear decrease in increment width across the settlement-mark. In Type Ia, the settlement transition concludes within one increment, whereas the settlement transition occurs over multiple increments in Type Ib (Raventós and Macpherson 2001).
By deducting the number of increments from the date of capture, hatch date distributions were computed (back-calculation). Using the length-age relationships, fish size was converted to age (day) in order to determine the instant mortality coefficient (Z). Fish abundance was grouped into 20-d intervals. The age-based catch curve method of Ricker (1975) was used to estimate the mortality coefficient. A linear regression analysis was conducted with the ln-transformed data set, and the slopes of the regression lines reflected Z. Daily mortality percentages (DM) were determined as follows:
QCapture imaging software (QImaging; Surrey, British Columbia, Canada) was used to measure otolith length (OL), width (OW), and radius (OR) to the nearest 0.001 mm. Differences between the measurements of the left and right otoliths were analyzed using a pairedt-test. The relationships between TL and morphometric otolith measurements were examined.
Results
A total of 309 YOYD. vulgarisspecimens (14.00 to 102.00 mm TL) were captured between June 2013 and June 2014 (Fig. 3). The parameters of the length-weight relationships are provided for all samples in Figure 4. Daily age estimates were successfully determined from the subsample consisting of 209 sagittal otoliths of YOYD. vulgaris(14.00 to 102.00 mm TL; Table 1). The minimum and maximum daily age estimates were 41 d (14.00 mm TL) and 339 d (102.00 mm TL). No significant difference in daily age estimates was detected between the right and left otoliths (pairedt-test:n= 30,P> 0.05). The average percentage error and CV were 5.40% and 6.90%, respectively, and only 21 otoliths were excluded as they were overly sanded or cracked. The average percentage error and CV obtained in this study are close to their accepted reference points. The dominant daily age groups were 41-59 and 60-79 d (14.00-27.00 mm TL; 47.80%). The mean daily growth rate ofD. vulgariswas 0.330 mm·d-1(Fig. 5).
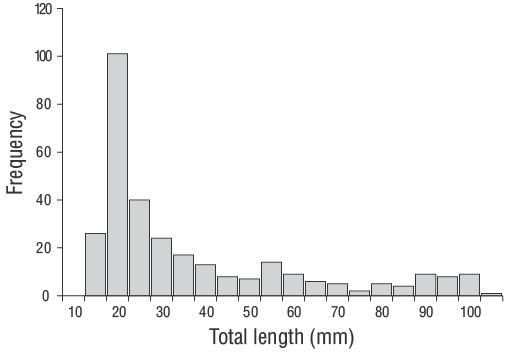
Figure 3. Length-frequency distribution of young-of-the-yearDiplodus vulgariscollected (n= 309) from the shallow waters of Gökçeada Island during the entire sampling period of this study (June 2013 to June 2014).
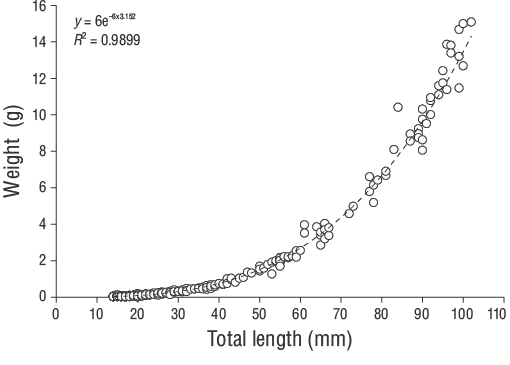
Figure 4. Length-weight relationships of young-of-the-yearDiplodus vulgariscollected from the shallow waters of Gökçeada Island.
Table 1. Age-length key for young-of-the-yearDiplodus vulgaris.N, total number of individuals per length/age group.
| Length groups (mm) | Daily age | N | ||||||||||||||
| 41-59 | 60-79 | 80-99 | 100-119 | 120-139 | 140-159 | 160-179 | 180-199 | 200-219 | 220-239 | 240-259 | 260-279 | 280-299 | 300-319 | 320-339 | ||
| 14-20 | 41 | 34 | 75 | |||||||||||||
| 21-27 | 1 | 23 | 14 | 38 | ||||||||||||
| 28-34 | 1 | 10 | 9 | 3 | 23 | |||||||||||
| 35-41 | 1 | 8 | 9 | 1 | 1 | 20 | ||||||||||
| 42-48 | 1 | 2 | 2 | 5 | ||||||||||||
| 49-55 | 2 | 3 | 3 | 8 | ||||||||||||
| 56-62 | 1 | 2 | 3 | 6 | ||||||||||||
| 63-69 | 2 | 2 | 4 | |||||||||||||
| 70-76 | 1 | 1 | 2 | |||||||||||||
| 77-83 | 3 | 4 | 7 | |||||||||||||
| 84-90 | 6 | 4 | 10 | |||||||||||||
| 91-97 | 1 | 6 | 7 | |||||||||||||
| 98-102 | 3 | 1 | 4 | |||||||||||||
| N | 42 | 58 | 25 | 18 | 16 | 7 | 7 | 5 | 3 | 3 | 4 | 7 | 10 | 3 | 1 | 209 |
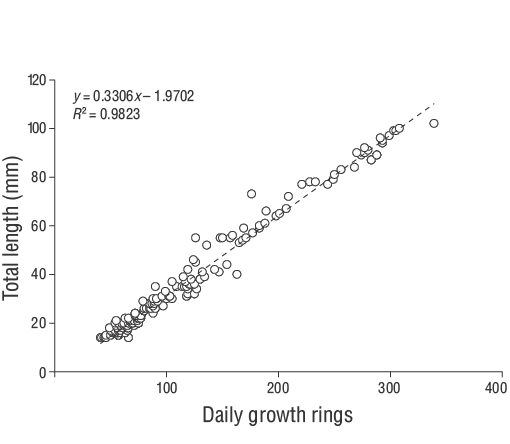
Figure 5. Age-length relationships estimated for young-of-the-yearDiplodus vulgariscollected from the shallow waters of Gökçeada Island from June 2013 to June 2014.
According to Wilson and McCormick (1999), YOYD. vulgarisindividuals exhibit Type Ia settlement-marks (Fig. 6). The duration of the pelagic larval phase of YOYD. vulgarisindividuals was 29 d. The hatching period spanned November to April, although higher hatching rates were observed in December and January (Fig. 7). The Z value of YOYD. vulgariswas 0.0195, which corresponds to a daily mortality rate of approximately 1.95% (Fig. 8). Otolith morphometric measurements of OL, OW, and OR ranged from 0.514 to 7.331 mm, 0.391-4.349 mm, and 0.247-3.551 mm, respectively (Table 2). The relationships between TL and OL, OW, and OR are described by linear equations in Table 3.
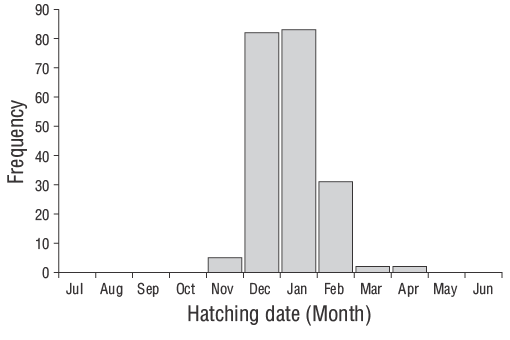
Figure 7. Hatching-date frequency distributions back-calculated by using daily age estimates and sampling dates of young-of-the-yearDiplodus vulgariscollected from the shallow waters of Gökçeada Island from June 2013 to June 2014.
Table 2. Otolith morphometric measurements of young-of-the-yearDiplodus vulgarisaccording to the length groups. Min., minimum value; Max., maximum value; SD, standard deviation.
| Length group (mm) | Otolith length | Otolith width | Otolith radius | ||||||||||||
| Min. | Max. | Mean | SD | Min. | Max. | Mean | SD | Min. | Max. | Mean | SD | ||||
| 14-20 | 0.515 | 1.244 | 0.812 | 0.130 | 0.392 | 0.849 | 0.584 | 0.093 | 0.247 | 0.599 | 0.386 | 0.076 | |||
| 21-27 | 0.515 | 1.724 | 1.054 | 0.205 | 0.404 | 1.190 | 0.740 | 0.126 | 0.247 | 0.838 | 0.503 | 0.107 | |||
| 28-34 | 1.138 | 1.618 | 1.388 | 0.106 | 0.764 | 1.120 | 0.970 | 0.071 | 0.564 | 1.279 | 0.695 | 0.143 | |||
| 35-41 | 1.205 | 1.838 | 1.623 | 0.152 | 0.884 | 1.481 | 1.136 | 0.118 | 0.566 | 0.945 | 0.798 | 0.090 | |||
| 42-48 | 1.213 | 2.134 | 1.791 | 0.375 | 0.895 | 1.322 | 1.166 | 0.165 | 0.592 | 1.018 | 0.858 | 0.175 | |||
| 49-55 | 2.226 | 2.455 | 2.348 | 0.080 | 1.491 | 1.731 | 1.545 | 0.078 | 1.071 | 1.222 | 1.142 | 0.057 | |||
| 56-62 | 2.382 | 2.938 | 2.565 | 0.197 | 1.575 | 1.865 | 1.673 | 0.104 | 1.146 | 1.272 | 1.190 | 0.044 | |||
| 63-69 | 2.663 | 2.849 | 2.745 | 0.083 | 1.791 | 1.916 | 1.854 | 0.052 | 1.285 | 1.431 | 1.337 | 0.066 | |||
| 70-76 | 3.098 | 3.136 | 3.117 | 0.027 | 1.926 | 2.092 | 2.009 | 0.117 | 1.496 | 1.514 | 1.505 | 0.013 | |||
| 77-83 | 2.976 | 3.402 | 3.185 | 0.163 | 2.057 | 2.241 | 2.144 | 0.076 | 1.437 | 1.635 | 1.548 | 0.074 | |||
| 84-90 | 3.404 | 3.967 | 3.602 | 0.178 | 2.173 | 2.529 | 2.382 | 0.117 | 1.644 | 1.914 | 1.735 | 0.084 | |||
| 91-97 | 3.243 | 4.021 | 3.776 | 0.239 | 1.983 | 2.621 | 2.447 | 0.203 | 1.487 | 1.932 | 1.804 | 0.138 | |||
| 98-104 | 3.860 | 4.206 | 4.033 | 0.141 | 2.518 | 2.789 | 2.643 | 0.112 | 1.778 | 1.950 | 1.902 | 0.083 | |||
Table 3. Parameters of the linear relationship of otolith length, width, and radius with total length for young-of-the-yearDiplodus vulgariscollected from the shallow waters of Gökçeada Island. The number of individuals (n),y-intercept (a), slope of the regression line (b), and coefficient of determination (r 2) are shown.
| Otolith morphometry | n | a | b | r 2 | P |
| Otolith length | 209 | -5.8329 | 27.143 | 0.967 | <0.010 |
| Otolith width | 209 | -9.2516 | 43.013 | 0.957 | <0.010 |
| Otolith radius | 209 | -6.3141 | 57.930 | 0.921 | <0.010 |
Discussion
The hatching period of the juvenileD. vulgarisoccurred from November to April in the shallow waters of Gökçeada Island. The maximum hatching frequencies were observed in winter (December and January) when the water temperature was relatively low. Temporal and spatial variations in hydrographic conditions directly affect reproduction, spawning, fish availability, and the distributions of different species as well as the abundance of their larvae (Isari et al. 2008). Wootton (1990) reported that temperature is the crucial environmental factor controlling reproduction events. Indeed, when evaluating the reproductive characteristics of sparid species, Pajuelo et al. (2006) stated that reproduction is associated with nutrient availability and water temperature. However, the spawning periods of fish species are known to vary from year to year depending on environmental and biological factors (Gonçalves and Erzini 2000, Morato et al. 2003, Pajuelo et al. 2006).
Previous studies have reported winter spawning ofD. vulgarisalong the Portuguese coast (Gonçalves and Erzini 2000) and central Mediterranean (Jug-Dujaković and Glamuzina 1988, Mouine et al. 2012). Di Franco et al. (2013) also stated that spawning occurred from 20 October to 14 February in the Adriatic Sea. In the shallow waters of Çanakkale, Turkey, Ayyildiz et al. (2015) stated that the highest hatching frequencies ofD. vulgariswere observed in January. In addition, the spawning season for this species in the Gulf of Gabes has been reported to occur in winter (Hadj-Taieb et al. 2013). Thus, similar results have been reported for different regions by other researchers.
PLD, which is defined as the time larvae spend growing in the water column as part of the plankton, can be determined in fish using daily increment marks on otoliths (Macpherson and Raventos 2006). The number of increments from the otolith core to the settlement-mark can be used to determine PLD. In this study, all specimens exhibited settlement-marks, which are essential time markers needed to determine PLD and recruitment patterns (Wilson and McCormick 1999). The daily age determinations of YOYD. vulgariswere successfully performed with sagittal otoliths by counting increments (see methods).
We determined that otolith growth (OL, OW, and OR) in YOYD. vulgarisis proportional to the TL and age of the fish. Indeed, this ratio exhibited linear relationships between OL, OW, and OR and TL and daily age. We also found that YOYD. vulgarisexhibit Type Ia settlement-marks according to the criteria of Wilson and McCormick (1999). The otolith settlement-marks of slow-growing species, such as sparids, can be determined by a rapid decrease in the daily growth increment widths (Vigliola et al. 2000, Ayyildiz and Altin 2021). In this study, we estimated the PLD forD. vulgaristo be 29 d.
Macpherson and Raventos (2006) determined a weak yet noticeable and positive relationship between PLD and the distribution range. In the Mediterranean, the PLD ofD. vulgarisdiffers among geographic areas. In Marseille, France, Vigliola et al. (2000) found thatD. vulgarishad settled in the demersal environment 25 d after hatching. In addition, Galarza et al. (2009) reported that the PLD ofD. vulgarisranged between 29 and 58 d in the western Mediterranean. The settlement times of YOYD. vulgarismay also differ by region in the Mediterranean Sea. For example, García-Rubies and Macpherson (1995) reported 2 apparent peaks for YOYD. vulgarisat the beginning of November and March/April in the northwest Mediterranean that reflect a strategy of intermittent recruitment. However, Vigliola et al. (2000) determined thatD. vulgarissettled in autumn and winter in Marseille, France. These findings further support the idea of Vigliola et al. (2000) thatDiplodusspecies with winter settlement periods (e.g.,Diplodus puntazzoandD. vulgaris) show longer PLD times than species that settle in warmer months (e.g.,Diplodus sargus; García-Rubies and Macpherson 1995, Macpherson 1998, Vigliola 1998).
The growth rates of fish during early life stages affect survival success, and larval mortality and growth are closely linked (Jones 1986, Vigliola 1997). In general, faster larval growth rates are associated with higher survivorship in the plankton, thus faster growth may result in high recruitment (Sim-Smith et al. 2012). Monteiro (1989) found that the daily growth rate of YOYD. vulgariswas 0.240 mm·d-1in the Ria Formosa lagoon. In another study conducted by Ayyildiz et al. (2015), the mean somatic growth rate of juvenileD. vulgariswas found to be 0.273 mm·d-1in the shallow waters of Çanakkale, Turkey. From the results of experimental age verification studies conducted in the Mediterranean, the daily growth rates were found to be 0.150 and 0.200 mm·d-1(Vigliola 1997). In this study, the average daily growth rate of juvenileD. vulgariswas 0.330 mm·d-1, which is higher than what has been reported in other studies. This may be due to the differences in nutrient availability among regions given that diet is an essential determinant of the growth rate in fishes (Jones 1986).
As previously mentioned, faster growth is associated with higher survival (Sogard 1997, Ayyildiz et al. 2014, Bouchoucha et al. 2018, Ayyildiz and Altin 2020). Indeed, fast-growing fish have been shown to progress through early life stages in relatively short periods of time while showing low mortality rates (Takahashi and Watanabe 2004). In this study, the daily mortality of YOYD. vulgariswas 1.93%. It is noteworthy that the mortality rate ofD. vulgarisin this study was lower than that of YOYDiplodus annularis(23.00%) from the same region (Altin et al. 2016). A similar study conducted in the southwest coast of France reported that the average mortality rate ofD. vulgarisjuveniles ranged from 71.40% to 76.50% in the 4 months following settlement (Planes et al. 2009).
In the north Aegean Sea, an area with nutrient-rich resources, we observed that YOYD. vulgarisspawn in winter. Although PLD is not a strong indicator of range size (Macpherson and Raventos 2006), a relatively short PLD was observed in this area compared to what has been reported for other areas of the Mediterranean. Finally, relatively low mortality and high growth rates were detected for juvenileD. vulgarisin the shallow waters of Gökçeada Island. Overall, these shallow waters constitute a suitable habitat for juvenile fish.











 texto en
texto en