INTRODUCTION
Given the multiple environmental and anthropogenic stressors, the current worldwide abalone production has dramatically changed from fishing to farming. Wild aba lone fisheries landings have decreased significantly in the past decades, from 20,000 metric tons (mt) in the 1970s to about 6,500 mt in 2016/2017 (Cook 2019), whereas pro duction of farmed abalone has increased from 50 mt in the 1970s to 160,987 mt in 2016/2017 (Cook 2016, 2019). Aquaculture has served as a promising tool to reduce the impact of fisheries and has been highlighted as a poten tial solution to food availability and security in the United Nations 2030 Agenda (UN-DESA 2018). However, there is a paucity of scientific information on ensuring a robust and sustainable aquaculture regime in the face of a changing climate. Rising sea surface temperatures and the increased frequency and severity of storms are stressors to maricul ture worldwide (Barange and Perry 2009) and particularly detrimental to developing abalone aquaculture industries in countries like Mexico.
Wild abalone populations have declined in Mexico and recovery is now uncertain owing to climate change impacts. Although the worldwide trend is an increase in abalone aqua culture, in Mexico the wild abalone fisheries still produce 10 times more than the abalone aquaculture industry (300 vs. 23.5 mt·y-1, respectively; Cook 2019). The abalone fishery in Mexico takes place on the Pacific coast of the Baja California Peninsula, where it continues to be economically important despite the fact that landings in recent years are only at 5% of the historical maximum (Morales-Bojórquez et al. 2008; Guzmán-Del Próo et al. 2013, 2017). Fishing in the north eastern Pacific, including Baja California, has deteriorated because of a combination of multiple stressors, such as over fishing, diseases, extreme warming events, and hypoxia (Lafferty and Kuris 1993, Karpov et al. 2000, Micheli et al. 2012, Ben-Horin et al. 2016, Boch et al. 2018, Lonhart et al. 2019). For example, in northern California, wild abalone populations have been affected by extreme warming events combined with other stressors, which caused mass mor talities of red abalone leading to the closure of the fishery in 2018 (Rogers-Bennett and Catton 2019). It is crucial to increase aquaculture production, as it has been shown around the world to support the local fishery both ecologically and economically.
Additional conservation and management actions are needed for the recovery of natural populations and sustain able fisheries in the northern portion of the Baja California Peninsula. Shifts in oceanic conditions have resulted in land ings of less than 100 mt from 2012 up until 2017 (DOF 2018). The Mexican government highlights that aquaculture is a potential strategy to increase abalone production (DOF 2018). Therefore, an opportunity exists to develop abalone aquaculture programs in Mexico that might help support conservation programs.
In Ensenada, Baja California, red abalone maricul ture (ocean-based efforts) has been carried out in the past with different techniques and macroalgae diets. In 1990, Searcy-Bernal and Salas-Garza (1990) conducted the first experimental mariculture project to grow red abalone, Haliotis rufescens, with a diet of giant kelp, Macrocystis pyrifera, and feather boa kelp, Egregia menziesii, in floating cages in Todos Santos Bay. At about the same time, a commercial red abalone mariculture com pany called “Abulones Cultivados” was established near Todos Santos Island, using cages and M. pyrifera as the main feed. However, the company moved to land-based facilities (Searcy-Bernal et al. 2010) and as of 2020 is still operating in the area of Ejido Erendira. More recently, Zertuche-González et al. (2014) evaluated the growth performance of red aba lone in an integrated multi-trophic aquaculture farm for one year in San Quintin Bay. Their study demonstrated the fea sibility of growing red abalone juveniles at sea with a diet of Eisenia arborea. These studies have laid the basis for the development of abalone mariculture in the region, but it is important to continue experiments to cope with environ mental changes associated with climate change.
Since the early mariculture efforts in Baja California, the understanding of local oceanographic and circulation patterns, along with mariculture technology, has improved. Furthermore, the ongoing declines of wild abalone have altered the attitudes and perceptions of local fishing coop eratives towards aquaculture as a potential solution. Efforts to enhance the wild populations are now supported by those economically linked to abalone fisheries. Mariculture could promote production of abalone for outplanting in local reefs and promote conservation aquaculture to restore natural populations (Froehlich et al. 2017, 2018). Nevertheless, the changing climate generates new challenges that are particu larly affecting the availability of macroalgae, a fundamental resource for the abalone aquaculture industry.
The availability of macroalgae, the main food for abalone, is crucial for the development of the aquaculture industry. In the northeastern Pacific, abalone farms in Mexico and the USA typically use giant kelp, M. pyrifera, harvested from the wild as feed (Evans and Langdon 2000, Garcia-Esquivel and Felbeck 2009). However, in the last 2 decades extreme events such as ENSO and marine heatwaves have substan tially affected giant kelp populations in Baja California (Ladah et al. 1999; Arafeh-Dalmau et al. 2019, 2020; Cavanaugh et al. 2019; Beas-Luna et al. 2020). Because of the recent variability in giant kelp biomass availability, it is important to assess other macroalgae as alternative food sources for abalone mariculture. For example, the southern sea palm kelp, E. arborea, is capable of surviving in rela tively warmer waters (>20 ºC) with low nutrients, where other kelps cannot survive (Hernández-Carmona et al. 2000, 2001; Zertuche-González et al. 2014). Therefore, E. arborea or other kelp species, such as Pelagophycus porra, may potentially provide a food supply to aquaculture operations during times of low giant kelp biomass. Extreme events not only affect macroalgae availability but also present chal lenges to abalone physiology and mariculture structures.
Increased frequency and intensity of storms and heat wave events can negatively affect shallow mariculture sys tems. An option to potentially ameliorate the detrimental effects of these issues is to move the cages deeper in the water column to avoid warm surficial waters and to minimize storm-induced damage via intense wave action and surge. As the frequency, duration, and intensity of marine heat waves increases in the coming decades (Oliver et al. 2018), finding depth refuges from warm water and alternatives to giant kelp resources is of utmost importance for the future of abalone production. To test the potential for abalone mariculture in San Jeronimo Island, Baja California, Mexico, under current conditions we explored the feasibility of growing red aba lone in cages attached to a vertical long-line system. Specif ically, we tested for the effects of depth (surface and bottom at 5 m) and 3 different brown macroalgal diets on the growth and survival of red abalone juveniles.
MATERIALS AND METHODS
Experimental study area
This study was carried out at San Jeronimo Island (1.3 km long and 500 m wide), located in the Ensenada Municipality in the state of Baja California, Mexico (Fig. 1; 29º47′34.9″ N, 115º47′31.9″ W). The marine resources of this area are under the jurisdiction of the fishing cooperative “Sociedad Cooperativa de Producción Pesquera Ensenada, S.C.L.”, which participated in this study. Juvenile red abalone were provided by the commercial aquaculture farm “Abulones Cultivados”, which is 250 km north of the study area. Juve nile abalone (ca. 30 ± 1 mm) were transported from the aqua culture farm to San Jeronimo Island in coolers with a wet sponge base. After arriving, 2,100 juvenile abalone were immediately transferred to the experimental cages and fed ad libitum fresh M. pyrifera for a month prior to initiating the experiment. Average sea surface temperature during this period was 14.98 ± 0.74 ºC.
Experimental design
An experimental mariculture system was designed to assess the effect of different rearing conditions (depth and diet) on the growth and survival of abalone. The outplanting site was a maximum of 5 m deep, had a sandy bottom, and was located on the protected southeast region of the island. The experimental mariculture system consisted of cages attached to a line at 2 depths (Fig. 2). Two anchors (iron pipes 7.5 cm in diameter and 1.5 m long) were buried in the seabed at an angle of approximately 45º. Buoys (200-L drums with pressurized air) were attached to the anchors and distributed along the 100-m long float line (polypropylene rope). Cages consisted of plastic-coated metal mesh (0.80 × 0.80 × 0.36 m), which was then wrapped with 0.5-cm plastic “Vexar” mesh. Eight cages were attached to the float line, 4 suspended near the surface and 4 on the sandy seafloor (5 m depth) weighted with rocks in each corner of the cage. Each cage contained 3 Australian baskets (0.75 × 0.25 × 0.20 m) stacked on one another (Fig. 2). Additionally, to increase the available surface for the abalone, Australian baskets were each modified at the center with a plastic plate with holes, allowing abalone to move throughout the basket. One HOBO U22 Temp Pro V2 temperature sensor was installed in each cage, and temperature (ºC) was recorded every half hour throughout the experiment.

Figure 2 Design of the experimental long-line system. Depth = 5 m; A, metal cage; B, Australian baskets; S, surface treatment; Bo, bottom treatment. Diets: Mp, Macrocystis pyrifera; Ea, Eisenia arborea; Pp/Mp, 50% Pelagophycus porra/50% Macrocystis pyrifera. Filled ovals represent buoys along the float line.
The 2,100 juvenile red abalone were randomly placed into the 24 modified Australian baskets. Each basket had between 87 and 104 abalone, with a density of 102-122 abalone per square meter, as recommended by Viera et al. (2014). Fif teen abalone per Australian basket (360 individuals, ~17% of the 2,100 animals) were tagged with shellfish tags (Floy Tag and Mfg.) and measured at the start of the experiment. It was not possible to tag more animals because of logistic and economic constraints. The mean initial shell length of these tagged abalone was 32 ± 3.33 mm (mean ± SD).
In addition to the effect of depth (surface vs. 5 m), the effect of macroalgal diet on abalone growth parameters was evaluated. Considering the most abundant brown mac roalgae around the mariculture area, red abalone were fed 1 of 3 different diets. The first diet tested was 100% giant kelp, M. pyrifera, the second diet was 100% southern palm kelp, E. arborea, and the third diet was a mix of 50% elk kelp, P. porra, and 50% M. pyrifera. All diets consisted of kelp blades. Of the 4 surface cages, 2 cages housed M. pyrifera diets, 1 cage the E. arborea diet, and 1 cage the mixed 50% P. porra/50% M. pyrifera diet. We repeated this design with the 4 bottom cages. Given the logistic constraints, only 8 cages could be used; therefore, only 1 diet per depth could be replicated, and we decided to do this for the 100% M. pyrifera diet, which is the most abundant and the standard feed in culture systems in northern Baja California Peninsula. The E. arborea blades were harvested by scuba diving; the canopy blades (upper 3 m) of M. pyrifera, by hand from a boat; and the P. porra blades, from algae that had drifted into the mari culture area. Abalone were fed ad libitum once per week, and the excess of macroalgae was removed weekly. The experi ment ran for 3 months (90 d), from March to June 2019.
Growth of red abalone
Shell length of the 360 tagged red abalone was measured at time zero and at days 47 and 90 using a digital caliper (Mitutoyo Absolute AOS CD 6”AX) with ±0.01 mm accu racy. Means for each cage were calculated from all the ani mals measured in the 3 baskets inside the cage (experimental unit). The mean for the M. pyrifera treatment was calculated from the 2 replicate cages. Data for the 3 baskets per cage were pooled, since it was not an objective of this study to assess the variability among baskets. These baskets were used to provide protection, larger culture surface, and even distribution of abalone and algae.
Mean shell length of tagged abalone was used to calculate the daily increment in shell length (DISL), monthly growth rate (MGR), and total growth (TG) as reported by Hopkins (1992). Survivorship was assessed weekly during feeding, and dead abalone were removed from the cages.
Statistical analysis
All statistical analyses were performed with STATISTICA v.12 software. To test for differences in abalone growth among treatments, we conducted a two-way analysis of vari ance (ANOVA) with water depth and diet as fixed factors. This design can be considered as an unbalanced factorial with replication (n = 2) for only the M. pyrifera diet in both depth treatments since, given the logistic constraints, it was not possible to include replicates for the 2 other diets. Statistical analysis of the main effects is possible, but the interaction term cannot be adequately evaluated. Results must there fore be used cautiously given the lack of replication (Aitkin 1978, Langsrud 2003). We did a posteriori power analysis to find the smallest sample size for the differences to be statis tically different between treatments with JMP V.14 software. Levene’s F-test was used to assess the homogeneity of vari ances (Brown and Forsythe 1974). Only the analysis for total abalone growth (mm) at the end of the trial is reported, since the analysis for the daily and monthly rates were similar.
RESULTS
Depth effect on red abalone growth and survival
When pooling diet means for each depth, abalone had a mean DISL of 93 ± 12 μm·d-1 in the surface cages and 82 ± 13 μm·d-1 in the bottom cages. On average, during the exper imental period, the abalone from the surface cages grew more than the abalone from the bottom cages, 8.33 ± 1.06 vs. 7.38 ± 1.13 mm TG, respectively, and 2.8 ± 0.4 vs. 2.5 ± 0.4 mm·month-1 MGR, respectively. These differences were not statistically significant (Fig. 3, Table 1; two-way ANOVA, F 1,4 = 3.066, P = 0.155), so we performed a posteriori power analysis to find the smallest sample size for the differences between the 2 depth treatments to be statistically signifi cant. If our experimental design would have had 3 or 4 repli cates per treatment, the difference in growth between depths might have been statistically significant (α = 0.05, σ = 0.77, δ = 0.47): N = 18, power = 0.69; and N = 24, power = 0.82, respectively. Growth was 84 ± 2 μm·d-1 during the first sam pling period (days 0-46) and 91 ± 12 μm·d-1 during the second period (days 47-90) (Fig. 3). Average abalone survival was 99% in surface cages and 95% in bottom cages.

Figure 3 Red abalone, Haliotis rufescens, mean growth in shell length during 90 d of experiments in 2 depth treatments (two-way analysis of variance, F1,4 = 3.066, P = 0.155): surface and bottom. Vertical bars denote standard error.
Table 1 Statistical information of the two-way analysis of variance performed with the data of total growth in shell length of red abalone under diet and depth treatments during the 90-d experiment. SS, sum of squares; MS, mean square.
| SS | d.f. | MS | F | P | |
| Diet | 4.832 | 2 | 2.412 | 4.120 | 0.107 |
| Depth | 1.798 | 1 | 1.798 | 3.066 | 0.155 |
| Error | 2.345 | 4 | 0.586 |
Diet effect on red abalone growth and survival
Red abalone fed a 100% M. pyrifera diet had a DISL of 88 ± 10 µm·d-1, a TG of 7.94 ± 0.88 mm, a mean MGR of 2.6 ± 0.3 mm·month-1, and 99% survival. With the 100% E. arborea diet, DISL was 99 ± 7 µm·d-1, TG was 8.86 ± 0.62 mm, mean MGR was 3.0 ± 0.2 mm·month-1, and survival was 98%. Abalone fed a mixed diet of 50% M. pyrifera/50% P. porra had a DISL of 74 ± 13 µm·d-1, TG of 6.68 ± 1.19 mm, mean MGR of 2.2 ± 0.4 mm·month-1, and 94% survival. Mean DISL and MGR rates for each cage are presented in Table 2. We did not find statistically signif- icant differences in the growth parameters among diet treat- ments (Fig. 4, Table 1; two-way ANOVA, F2,4 = 4.120, P = 0.107). We also carried out a power analysis to find the least significant sample size for the difference between the 3 diet treatments to be statistically significant. If our experimental design had had 2 or 3 replicates per treatment, the differ- ence in growth between diets might have been statistically significant (α = 0.05, σ = 0.77, δ = 0.78): N = 12, power = 0.74; and N =18, power = 0.94, respectively. Mean growth across all treatments was 84 ± 15 µm·d-1 during the first sampling period (days 0-46) and 90 ± 9 µm·d-1 during the second period (days 47-90) (Fig. 4).
Table 2 Total mean growth in shell length of red abalone fed 3 different macroalgal diets in the surface and bottom treatments. DISL, daily increase in shell length; MGR, monthly growth rate.
| Cage | Diet | Depth | DISL (µm·d-1) | MGR (mm·month-1) | Total growth (mm) |
| 1 | 100% Macrocystis pyrifera | Surface | 81 | 2.4 | 7.31 |
| 2 | 100% Eisenia arborea | Surface | 103 | 3.1 | 9.30 |
| 3 | 50% Macrocystis
pyrifera, 50% Pelagophycus porra |
Surface | 84 | 2.5 | 7.52 |
| 4 | 100% Macrocystis pyrifera | Surface | 102 | 3.1 | 9.19 |
| 5 | 100% Macrocystis pyrifera | Bottom | 81 | 2.4 | 7.31 |
| 6 | 100% Eisenia arborea | Bottom | 94 | 2.8 | 8.43 |
| 7 | 50% Macrocystis
pyrifera, 50% Pelagophycus porra |
Bottom | 65 | 1.9 | 5.84 |
| 8 | 100% Macrocystis pyrifera | Bottom | 88 | 2.7 | 7.95 |
| Average | 87 | 2.6 | 7.90 | ||
| SD | 13 | 0.4 | 1.10 | ||
| Min | 65 | 1.9 | 5.80 | ||
| Max | 103 | 3.1 | 9.30 |

Figure 4 Red abalone, Haliotis rufescens, mean growth in shell length during 90 d of experiments fed with 3 different macroalgal diets (two-way analysis of variance, F2,4 = 4.120, P = 0.107): Macrocystis pyrifera diet, Eisenia arborea diet, 50% M. pyrifera/50% Pelagophycus porra mixed diet. Vertical bars denote standard error.
Temperature
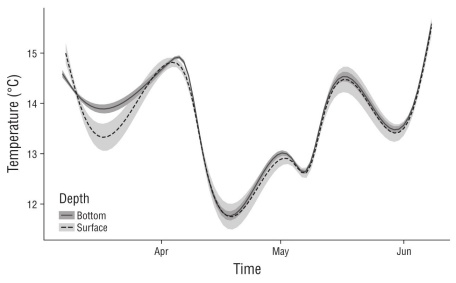
For the surface treatment, mean temperature was 13.69 ± 0.92 ºC (max = 18.62, min = 12.01) and for the bottom treatment, mean temperature was 14.01 ± 0.94 ºC (max = 17.85, min = 12.01) (Fig. 5). Using daily means, there were no statistically significant differences in temperature between the surface and the bottom (ANOVA, F1,270 = 0.88, P = 0.41).
DISCUSSION
To test the potential for red abalone, H. rufescens, mariculture at San Jeronimo Island, Baja California, Mexico, we explored the feasibility of growing red abalone in cages attached to a long-line system and assessed the effects of depth and diet on their growth. The present study describes the broad importance of developing experiments and analyses to provide alternative ways to enhance production and inform management of complex systems responding to complex climate change effects such as extreme marine heatwaves. Our results indicate that red abalone mariculture is feasible at San Jeronimo Island and that the use of varying culture depths and the use of diets with alternative algal species may be effective strategies to cope with the effects of marine heatwaves and storm surges. The power analyses showed that appropriate replication would have found slight differences between diets and depths and would have also allowed us to test for interactions between the variables. Therefore, given the lack of balanced treatment replication, due to logistic constraints, the results from this small-scale local experiment should be taken cautiously.
Depth effect on red abalone growth and survival
We tested the effect of depth on the growth and survival of juvenile red abalone, as climatic changes such as sea surface warming and storms will likely affect growth and sur vivorship for future mariculture projects. There were no statistically significant differences in growth between depth treatments (Fig. 3, Table 1). However, as the power analysis showed, if we had had 3 or 4 replicates per treatment, we might have found statistically significant differences on growth with depth, with slightly higher growth in the surface cages, although probably not important from the aquaculture perspective, since cages would be lowered to the bottom only for short-term periods during storms. Temperature was similar between the surface water and at 5 m depth (Fig. 5), so the trend of slightly greater growth at the surface may have been related to other variables such as dissolved oxygen, pH, light availability, currents, or turbidity (Morash and Alter 2016). We also noticed more sediment in the bottom cages, and this may have contributed to the trend of slightly lower growth rate and survival observed at this depth. However, we did not measure sedimentation rates, and their effects on abalone growth and survival in cage culture systems have not yet been studied to our knowledge. Our results indicate that during a severe storm in the area, it would be possible de macroalgas diferentes (análisis de varianza de 2 vías, F2,4 =to lower the cages to protect the caging system from damage due to storm-generated waves and surge without negatively affecting abalone growth rates.
Diet effect on red abalone growth and survival
In our experiment, we found that red abalone tended to grow better with the E. arborea diet (99 µm·d-1, 3 mm·month-1) than with the M. pyrifera diet (88 µm·d-1, 2.6 mm·month-1) or the 50% P. porra/50% M. pyrifera mixed diet (75 µm·d-1, 2.2 mm·month-1), although these dif- ferences were not significant (Fig. 4). However, as the power analysis showed, if we had been able to have 2 or 3 repli- cates per treatment, we would have found statistical differ- ences on growth between diets, with slightly better growth with the E. arborea diet. Our results support the study by Zertuche-Gonzalez et al. (2014), who found similar growth rates in red abalone (similar size, 20 mm) fed M. pyrifera (2.5 mm·month-1) and E. arborea (2.2 mm·month-1) in the same region. These slight differences in MGRs could be related to seed quality, temperature regime during culture, and the species-specific thermal preferences and optimum for growth. Díaz et al. (2000) found that juvenile red aba- lone (46-59 mm) acclimated to 17 ºC preferred tempera- tures of 18.8 ºC and that optimum temperature for growth was 18.4 ºC. Steinarsson and Imsland (2003) examined the growth of abalone acclimated to 15 ºC when exposed to tem- peratures ranging from 11 to 22 ºC and reported optimal temperature to be 16.5 ºC for 21 mm red abalone and 17.2 ºC for 25-66 mm red abalone. Moreover, macroalgae morphotypes and proximal composition can vary between sites in relation to oceanographic conditions such as currents, water motion, temperature, light, and nutrients (Roberson and Coyer 2004, Demes et al. 2009, Landa-Cansigno et al. 2017).
It is much easier and cheaper for fishers to obtain M. pyrifera than E. arborea. The latter does not create a floating canopy and requires scuba to harvest, whereas the canopy of M. pyrifera is easily harvested from the surface by hand from a boat. With future predictions of reduced wild populations of M. pyrifera in Baja California due to warmer temperatures or other environmental disturbances (Beas-Luna et al. 2020), our results suggest, as previous studies, that E. arborea is a suitable alternative food source for red abalone aquaculture.
Growth rates in red abalone fed M. pyrifera have shown to range from 34 µm·d-1 in organisms 8 mm shell length (Trevelyan et al. 1998) to 73 µm·d-1 in organisms 20-60 mm shell length (Zertuche-González et al. 2014). The 88-µm·d-1 growth rate observed in red abalone (32 mm shell length) fed with the M. pyrifera diet in this study is promising. Our findings suggest that San Jeronimo Island has high potential for red abalone mariculture based on an M. pyrifera diet alone, since currently it is the most abundant macroalga in the region. We examined alternatives to M. pyrifera algal diets that could be used for abalone in Baja California during extreme temperature events (Ladah y Zertuche 1999, Cavanaugh et al. 2019). Some authors recommend using red algae to boost red abalone growth; for example, Evans and Langdon (2000) obtained 124 µm·d-1 in juvenile H. rufescens fed dulse Palmaria mollis. In contrast, Leighton (1966) demonstrated that H. rufescens had a distinct preference for brown algae, particularly M. pyrifera and E. menziesii. Other studies have used mixtures of brown algae; for example, Searcy-Bernal and Salas-Garza (1990) obtained a growth rate of 74 µm·d-1 in H. rufescens fed both M. pyrifera and E. men- ziesii. Abalone growth rates on natural diets are reported to range from 0.8 µm·d-1 for Haliotis iris (20 mm shell length) fed Ulva lactuca (Stuart and Brown 1994) to 139 µm·d-1 for Haliotis discuss hannai (24-34 mm shell length) fed Eisenia bicyclis (Uki et al. 1986). More studies are needed to under- stand the effect of mixed algae diets and their economic via- bility in mariculture systems. Also, in order to develop an optimum mariculture system in a particular region, future research should consider that feeding rates of abalone depend on body size, type of food, density, and temperature (Capinpin et al. 1999, Nelson et al. 2002).
Temperature
Temperature is the main variable that controls the rates of most metabolic processes in abalone (Rogers-Bennett et al. 2010, Morash and Alter 2016). Outside their optimum range, individuals adjust basic physiological functions to maintain basal metabolic demands (Medina-Romo et al. 2010). In red abalone, preferential temperature increases during development until 30 mm shell length and then declines as the individual grows (Steinarsson and Imsland 2003). The preferential temperature of H. rufescens is 18 ºC (Leighton 1974, Díaz et al. 2000). At 25 ºC H. rufescens begins to present detachment symptoms, and the max- imum critical temperature is 27.5 ºC (Díaz et al. 2006). In this study, the mean temperature of the surface treatment was 13.69 ± 0.92 ºC (max = 18.62, min = 12.01) and for the bottom treatment it was 14.01 ± 0.94 ºC (max = 17.85, min =12.01). Temperature and H. rufescens growth and sur- vival data indicate that San Jeronimo Island has high poten- tial for red abalone mariculture, at least during the months over which this experiment was conducted. It is critical to have a robust understanding of the effect of temperature on abalone metabolism and growth (Morash and Alter 2016) to predict the future effects of rising ocean temperature on abalone farming.
Future directions
It is of utmost importance to develop sustainable mariculture in Mexico, as it will diversify the activities of fishing communities, produce valuable products, and help restore and conserve marine species. For example, abalone aqua- culture and mariculture projects have been very successful in several Asian countries. Currently, 155,939 mt of abalone are produced in China, South Korea, and Japan, equivalent to 95% of the world abalone production (Cook 2019). Valuable insights from these cases can be applied to future efforts within Mexico. For example, in these countries, aquaculture is co-managed between academia, private initiatives, local communities, and government agencies (Lee 2019). Conservation aquaculture projects in Baja California have the potential to enhance the resiliency of coastal communities to the impacts of climate change. Although the small-scale mariculture system used in this experiment was successful at San Jeronimo Island, more research should be conducted to determine its upscaling potential and feasibility at other locations.











 nueva página del texto (beta)
nueva página del texto (beta)