Introduction
The brown smooth-hound shark, Mustelus henlei (Gill 1863), is the second most caught species in the artisanal elasmobranch fishery on the west coast of Baja California Sur (BCS, Mexico) (Furlong-Estrada et al. 2017). Genetic studies have shown that there are probably 5 subpopulations of this species in Mexican waters, 3 in the Gulf of California and 2 off the west coast of the Baja California Peninsula (Sandoval-Castillo and Beheregaray 2015); however, only the reproductive biology of the stock in the northern Gulf of California region has been studied to date.
Despite the natural abundance and diversity of elasmobranchs on the Pacific coasts of Mexico and the low-tech fishing gear used by the small-scale elasmobranch fishery off northwestern Mexico, the risk of stock overexploitation remains a problem due to the low rate of stock renewal in elasmobranchs. This condition makes elasmobranchs vulnerable even at moderate levels of fishing mortality (Musick 1999).
In Mexico, several elasmobranch conservation strategies have been implemented. In 2006, the Official Mexican Standard 029 was published to establish guidelines for the use of elasmobranchs, banning finning and promoting the protection of several endangered species along the Mexican coasts (DOF 2007). To regulate fishing mortality and protect the recruitment and critical habitat of elasmobranchs, this standard stablished fishery closures, which have been implemented, since 2012, from 1 May to 31 July in the Pacific Ocean, from 1 May to 30 June in the Gulf of Mexico, and from 1 May to 30 June and 1 to 30 August in the Bank of Campeche (DOF 2007).
The resilience of a stock can be associated with the reproductive biology of the species; species with short reproductive cycles and high fecundity can support higher rates of exploitation (Walker 1998). To achieve adequate fishing management of M. henlei, it is necessary to know the reproductive parameters of all the stocks (Saïdi et al. 2009). Some reproductive parameters (fecundity, length at maturity, and gestation period) of M. henlei in the Gulf of California and on the coast of California have been described, with differences between both stocks (Yudin and Cailliet 1990, Pérez-Jiménez and Sosa-Nishizaki 2008). The variation of reproductive parameters between different stocks has also been reported for other Mustelus species, for example, Mustelus antarcticus in Australia (Walker 2007) and Mustelus manazo in Japan and Taiwan (Yamaguchi et al. 2000). These variations are attributed to differences in environmental conditions and must be considered in stock assessment and the development of management strategies.
The objective of this study is to present, for the first time, information on the reproductive biology of M. henlei off the west coast of BCS based on macroscopic and histological analyses and to compare it with existing information on the neighboring stocks. This information is expected to contribute to the development and improvement of the management measures recently established for the elasmobranch fishery in Mexico.
Materials and methods
Field sampling was performed from May 2008 to May 2016 at 4 artisanal fishing camps along the west coast of BCS (Mexico): Punta Lobos to the south, San Lazaro and San Ignacio in the central region, and Bahía Tortugas to the north (Fig. 1). Individuals were identified by examination of tooth and dorsal fin features following the guide by Compagno et al. (2005); M. henlei has high-cusped “pavement” teeth, and its trailing dorsal fin edges appear broadly frayed with dark margins of bare ceratotrichia.
Total length (TL) of each reported shark was measured, to the closest millimeter, from the tip of the snout to the end of the caudal fin, with the caudal fin extended. Sex was determined by the presence of claspers in male sharks, and clasper length was obtained by measuring, to the closest millimeter, the distance from the base to the tip of the claspers.
Males were considered mature if their claspers were completely calcified and articulate at the base with a 180º rotation (pers. obs., Hamlett 2005). Females were considered mature if they had oocytes measuring >6 mm and had completely developed oviducal glands or embryos or eggs in the uterus (pers. obs., Conrath and Musick 2002). The reproductive system of each organism was removed and fixed in 10% formaldehyde. Only reproductive systems with proper fixation were used for histological processing.
Student’s t tests were performed to check for significant differences in TL between sexes and between development stages (mature and immature). The composition and temporal variation of sizes were analyzed with frequency histograms. Sex ratio was compared with a 1:1 ratio via a χ-squared test.
Histological sections of ovaries and testicles were made to determine maturity stages and to describe oogenesis and spermatogenesis using the classification by Serra-Pereira et al. (2011). Histological sections of the oviducal glands and seminal vesicles were made to determine the possibility of semen storage. The Mallory staining technique was applied for the ovaries because it helped identify collagen and reticulin, and the periodic acid-Schiff technique was applied in the case of oviducal glands to identify polysaccharides, mucopolysaccharides, mucoproteins, glycoprotein, and mucins. Finally, the Feulgen technique was used to identify deoxyribonucleic acid of spermatozoa in the testicles and the seminal vesicles (Pearse 1968, Humason 1979).
Length at maturity (L50) for each sex and length at maternity
(Lm50) were calculated using the logistic equation
The length and width of testicles and oviducal glands were measured to the nearest millimeter after fixation. The width of both organs was taken by the longest transverse section. The relationship between TL and the length of the testicles and width of the oviducal glands was analyzed to determine the onset at which these organs increase in size; in the case of oviducal glands, this relationship was analyzed for each of the development stages (immature, mature, and pregnant).
The number of embryos in each pregnant female was quantified to estimate average fecundity. Size at birth was calculated at the midpoint between the maximum size of embryos and the minimum size of free-living organisms with open umbilical scars. Birth season and gestation period were determined by the size of the embryos, maximum diameter of oocytes, and presence of newborns throughout the year.
Results
A total of 1,469 M. henlei individuals (715 males and 754 females) were analyzed. The sex ratio (1.05F:1.00M) was not significantly different from 1:1 (χ2 = 1.03, P > 0.05) (Table 1). Mustelus henlei was recorded in landings from February to November each year (except from May to July after 2012, because of the fishing closure period established for Mexican Pacific waters), with seasonal variations between the different sampling zones; it was common in the southern region during the first months of the year, in the central region during the middle part of the year, and in the northern region at the end of the year.
Table 1 Sex ratio (F:M) for Mustelus henlei at 4 artisanal fishing camps in Baja California Sur.
| Fishing camp | Females | Males | Sex ratio |
| Punta lobos | 374 | 310 | 1.2:1.0 |
| San Lázaro | 138 | 330 | 0.4:1.0 |
| San Ignacio | 22 | 47 | 0.4:1.0 |
| Bahía tortugas | 220 | 28 | 7.8:1.0 |
The length of individuals varied between 450 and 1,530 mm TL (average = 753 mm, SD = 111). Females were larger (1,530 mm TL) than males (1,160 mm TL), though no significant differences were found between the average TL of both sexes (Student’s t0.05, 1,469 = -14.93) (Fig. 2). Around 11% of measured sharks surpassed the maximum TL reported in previous studies.

Figure 2 Distribution of length by sex for Mustelus henlei caught in artisanal fisheries off the west coast of Baja California Sur, Mexico, from May 2008 to May 2016. GC = maximum reported size for the population in the northern Gulf of California region, C = maximum reported size for the California population.
The gonads of 685 organisms (401 males and 284 females) were analyzed to identify maturity stages by examination of macroscopic characteristics and histological analysis. All immature male specimens of M. henlei analyzed in the present study had no articulate (rotation) claspers, but all mature individuals had calcified, rotating claspers. The rotation of claspers alone was not considered evidence of maturity unless claspers were fully calcified. Immature males and females measured between 450 and 810 mm TL. Mature males and females measured more than 610 mm TL and accounted for 85.8% of all measured specimens. No significant differences were found between the sizes of both sexes by maturity stage (Student’s t0.05, 98 = -0.59 for immature specimens and Student’s t0.05, -13.89 = -0.59 for mature specimens). Pregnant females larger than 650 mm TL were recorded in April, May, August, and November and represented 41% of total reported females (Fig. 3). Estimated L50 for males (635 mm TL, SD = 6.03) was slightly lower than that for females (657 mm TL, SD = 7.43). Estimated Lm50 for the northwestern coast of BCS (where most pregnant females were caught) was 670 mm TL (SD = 9.8) (Fig. 4), slightly larger than the female L50, as expected.

Figure 3 Seasonal variation of maturity stages for Mustelus henlei off the west coast of Baja California Sur, Mexico. (a) Maturity stages for males and (b) maturity stages for females. The numbers above the bars represent monthly sample size.

Figure 4 Length at maturity for female and male Mustelus henlei off the west coast of Baja California Sur (BCS) and length at maternity for individuals off the northwestern coast of BCS. Ogives are given for length at maternity (- - -), males length at maturity (- - -), and female length at maturity (--).
The female reproductive system includes only one functional ovary. Histological analysis of immature female gonads revealed the first phases of oogenesis, with small oocytes (average diameter = 2 mm) defined by the follicular epithelium and a poorly differentiated granular layer (Fig 5a). Mature females had large oocytes (diameter range = 6-12 mm, average = 9 mm), with the follicular epithelium differentiated by 4 layers (from the inside to the outside): pellucid zone, granular layer, basal surface, and the theca cells (Fig. 5b). Maximum oocyte diameter was recorded in April (Fig. 6).
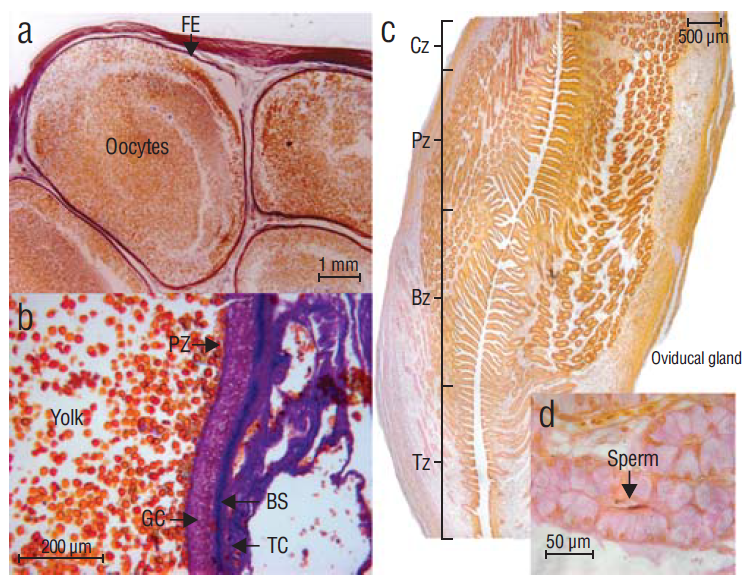
Figure 5 Histological analysis of Mustelus henlei female gonads. (a) Immature oocytes delimited by undifferentiated follicular epithelium, (b) mature oocyte showing differentiation of follicular epithelium, (c) oviducal gland showing 4 zones, and (d) acini in the terminal zone showing sperm storage. FE = follicular epithelium, PZ = pellucid zone, GC = granular layer, BS = basal surface, TC = theca cells, Cz = club zone, Pz = papillary zone, Bz = baffle zone, Tz = terminal zone.

Figure 6 Seasonal variation of embryo length and oocyte diameter for Mustelus henlei females off the west coast of Baja California Sur. Values indicate mean ± standard deviation. The numbers next to the symbols indicate the monthly sample size. The solid arrow indicates the month when embryos were largest in size and the dashed arrow the month when embryos were already visible in the uterus.
Oviducal glands of immature females measured 3-8 mm long and <10 mm wide, whereas oviducal glands of mature females were 10-15 mm long and 10-20 mm wide. No relation was found between oviducal gland width and TL for any development stage (immature, mature, and pregnant; r2 = 0.27, 0.07, and 0.008, respectively; P < 0.05). Four characteristic zones were identified in the histological sections of the oviducal glands: club zone, papillary zone, baffle zone, and terminal zone (Hamlett et al. 2002, Hamlett 2005); these sections had a simple ciliated columnar epithelium with acini formed by ciliated cells and secreting cells. The presence of semen was detected (April) in the terminal zone, forming conglomerates at the center of the acini (Fig. 5c, d).
The number of embryos per female ranged between 1 and 20 (average = 9, SD = 5.021, n = 41). There was a linear relation between the number of embryos and the size of females (r2 = 0.57, n = 41; P > 0.05). The embryonic sex ratio (0.76F:1.00M) was not significantly different from 1:1 (χ2 = 0.92, P > 0.05), as was the case for the free-living organisms. The first embryonic stages (15-25 mm TL) were observed in females caught in the northern region in August, when 95% of captured females were pregnant; however, maximum embryo size (255 mm TL; average = 198.3 mm TL, SD = 60.3) was recorded in the southern region in April. Size at birth was estimated to be 352 mm TL and gestation period was 10-11 months, which probably started before August, when embryos were already visible in the uterus, and lasted until May-June; newborn individuals were found in August (Fig. 6).
Both testicles were functional. Testicles of immature males measured <50 mm long and 10 mm wide on average, whereas testicles of mature males were 65 mm long and 14 mm wide on average. The first phases of spermatogenesis, including the development of the germinal epithelium in spermatic cysts and spermatogonias, were detected in immature individuals (≤510 mm TL) (Fig. 7a). Completely developed sperm cells were found in testicles of mature males (>630 mm TL) (Fig. 7b), and semen storage was found in the seminal vesicle as spermatozeugmata (Fig. 7c, d). Sperm cells in adult individuals were fully developed and grouped into organized clusters moving to the periphery of the spermatocytes associated with Sertoli cells, ready to be expelled from the testicle, through the epididymis, for storage in the seminal vesicle.

Figure 7 Histological analysis of Mustelus henlei male gonads. (a) First phase of spermatogenesis, germinal epithelium; (b) advanced stage of spermatogenesis, spermatocytes; and (c) and (d) storage of sperm in the seminal vesicle forming spermatozeugmata.
Immature males had claspers that were <80 mm long and not calcified. Mature males had fully calcified claspers measuring 60-140 mm in length (average = 80 mm). There was a significant linear relationship between the length of the testicles and TL (r2 = 0.52, n = 54; P > 0.05); however, no significant relationship between the length of the claspers and TL was found with the linear model (r2 = 0.28, P < 0.05) or with the nonlinear approaches (Fig. 8).
Discussion
During the present study, a new maximum size (1,530 mm TL) was recorded for M. henlei, considerably larger than that reported for this species in the Gulf of California (905 mm TL, Pérez-Jiménez and Sosa-Nishizaki 2008), off California (1,000 mm TL, Yudin and Cailliet 1990) and off Costa Rica (665 mm TL, Clarke et al. 2014). Similar species in the study area, such as Mustelus californicus and Mustelus lunulatus, have conspicuous features, like different teeth, and never dark broadly frayed margins on the dorsal fin, like M. henlei, and we thus consider that the probability of species misidentification is low.
The difference in specimen sizes among different regions could be attributed to population differences, but it could also be attributed to the selectivity of fishing gear and to the fishing zone. Mustelus henlei is caught off the western coast of BCS (present study) using gillnets with a mesh size of 150 mm at a depth of approximately 100 m, and in the Gulf of California it is mainly caught with bottom-set longlines, trawl nets, and gillnets with smaller mesh size (100 mm) at depths between 80 and 100 m (Pérez-Jiménez and Sosa-Nishizaki 2008). Off California M. henlei is caught with gillnets, trammel nets, otter trawls, and hooks, but only in shallow waters not exceeding 38.1 m (Yudin and Cailliet 1990).
Female M. henlei in the study area showed larger sizes than males, similar to that reported for this species in the Gulf of California (Pérez-Jiménez and Sosa-Nishizaki 2008), off California (Yudin and Cailliet 1990), and off Costa Rica (Clarke et al. 2014) and for other mustelids, such as M. antarcticus (Walker 2007), Mustelus canis (Conrath and Musick 2002), Mustelus lenticulatus (Francis and Mace 1980), and M. manazo (Tanaka and Mizue 1979). This difference in size could be associated with a reproductive strategy, as females need a bigger size to keep embryos inside their bodies (Walmsley-Hart et al. 1999).
Seasonal variation in the abundance of M. henlei in landings along the western coast of BCS could be due to migratory behavior associated with reproductive or dietary factors (Campos et al. 2009). Migratory behavior associated with species reproduction has been suggested to ensure that embryos are exposed to favorable temperatures during their development (Francis 1988). In addition, the search for food to increase energy reserves for the nutrition of embryos during their development has been stated as an explanation for M. lenticulatus migration (Francis and Mace 1980, Francis 1988). The western coast of BCS is characterized by high productivity rates in different regions and seasons. Since prey are found in high-productivity areas, several elasmobranchs migrate along the peninsula following their prey (Block et al. 2011). However, the lack of telemetry studies limits knowledge on the causes for M. henlei migration.
In the present study we estimated L50 for M. henlei off the west coast of BCS for the first time using a logistic model. Maturity stages were determined and validated through histological analysis. Likewise, Lm50 was estimated for the first time for this species. Estimating Lm50 allowed us to validate the annual reproductive cycle of M. henlei in Bahía Tortugas. Though we could not validate this for the southern and central portions of the west coast of BCS, the presence of fully developed embryos and large oocytes (up to 12 mm) indicate that the reproductive cycle might not be different from what was found in Bahía Tortugas. We recommend sampling pregnant females in these areas all year around.
Previous studies on elasmobranch reproduction determined maturity stages according to the macroscopic examination of reproductive organs and the external characteristics of organisms (Yudin and Cailliet 1990, Pérez-Jiménez and Sosa-Nishizaki 2008, Clarke et al. 2014). In the present study the histological analysis was used for the first time as a complementary tool to increase knowledge on the reproduction of M. henlei and to validate macroscopic observations. Both males and females off the west coast of BCS reach maturity at a larger size than do the stocks from the Gulf of California (Pérez-Jiménez and Sosa-Nishizaki 2008), California (Yudin and Cailliet 1990), and Costa Rica (Clarke et al. 2014) (Table 2). However, this comparison should be taken with caution, as different criteria were used to determine maturity. Yudin and Cailliet (1990) used the relationship between clasper length and TL for males and the presence of embryos and oocytes >10 mm in diameter for females. Pérez-Jiménez and Sosa-Nishizaki (2008), on the other hand, used the length and condition of claspers (free rotation) for males and oocytes diameter, size of oviducal gland, and uterus width (>8.1 mm, >11 mm, and >12 mm, respectively) for females. Clarke et al. (2014) used the length and condition of claspers (calcification) for males and the presence of oocytes in the ovary and the development of the oviducal gland for females. In the present study, we used the histological analysis of testicles and the length and condition of claspers (free rotation and complete calcification) for males and the presence of embryos, presence of oocytes >6 mm in diameter, and histological analysis of ovaries and oviducal glands for females.
Table 2 Comparison of reproductive parameters for Mustelus henlei populations in the eastern Pacific. TL = total length.
| Reproductive parameter | California (Yudin and Cailliet 1990) |
Gulf of California (Pérez-Jiménez and Sosa-Nishizaki 2008) |
Costa Rica (Clarke et al. 2014) |
West coast of Baja California Sur (present study) |
| Reproductive cycle | - | Annual | - | Annual |
| Length at maturity for males (mm TL) | 510-630 | 550-560 | 372 | 635 |
| Length at maturity for females (mm TL) | 520-660 | 570-660 | 397 | 658 |
| Length at birth (mm TL) | 190-300 | 280 | - | 352 |
| Fecundity | 1-10 | 1-21 | 1-12 | 4-20 |
| Pregnancy period (months) | 10-11 | 10 | - | 10-11 |
| Parturition period | - | January-April | - | May-June |
Differences in fecundity were also observed in comparison with the California, Gulf of California, and Costa Rica stocks (Yudin and Cailliet 1990, Pérez-Jiménez and Sosa-Nishizaki 2008, Clarke et al. 2014) (Table 1). Differences in reproductive parameters (e.g., length at maturity and fecundity) can be attributed to variations in water temperature at each study area. In the northern Gulf of California region, sea surface temperature varied from 18 to 28 ºC (Escalante et al. 2013) and off the California coast it varied from 18 to 20 ºC (Nezlin et al. 2004); these temperatures are considerably different from sea surface temperature off the west coast of BCS (12-30 ºC) (Sicard-González et al. 2012).
The number of embryos that a female can produce is highly variable among elasmobranchs. The relationship between the number of embryos and TL, such as that found for M. henlei in the present study, has been reported for some species, for example M. antarcticus (Walker 2007), M. lunulatus, and M. californicus (Pérez-Jiménez and Sosa-Nishizaki 2010). This relationship implies that larger females can have larger litter sizes (Clarke et al. 2014), which could explain a relation between the capacity of the population to increase in size and the number of larger females.
The breeding period in the study area was estimated to occur from May to June, according to the April records of embryos with sizes close to the reported size at birth (28 mm TL) for the northern Gulf of California region, though breeding in the northern gulf region had been estimated to occur from January to April (Pérez-Jiménez and Sosa-Nishizaki 2008). This difference can be attributed to environmental conditions as well. The association between breeding period and temperature variation has also been reported for M. antarcticus (Walker 2007) and M. manazo (Yamaguchi et al. 2000).
Many mustelids have annual reproductive cycles, and such is the case for M. antarcticus off the southwestern coast of Australia (Walker 2007), M. lenticulatus off New Zealand (Francis and Mace 1980), M. canis in the northwestern Atlantic Ocean (Conrath and Musick 2002), and M. manazo off Japan (Yamaguchi et al. 2000). Considering that maximum follicle size and fully developed embryos occurred simultaneously in April, M. henlei could too show a continuous annual reproductive cycle off the west coast of BCS. However, some populations have been shown to present different periodicity in relation to environmental conditions (Walker 2007).
The reproductive system of elasmobranchs has undergone evolutionary modifications and/or adaptations (Hamlett 2005). Some species, like Squalus acanthias, Squalus brevirostris, Notorynchus masculatus, and Carcharhinus anguineus, have 2 functional ovaries (Lutton et al. 2005). Other species, like M. henlei, have only 1 functional ovary, where oogenesis and the synthesis and secretion of hormones occur (Lutton et al. 2005). This condition has also been observed in other Mustelus species (Carrier et al. 2004), such as M. antarcticus (Walker 2007), M. lunulatus, and M. californicus (pers. obs.).
Oogenesis in elasmobranchs has been recorded to occur at an early age and oogonia are only observed during embryonic development (McMillan 2007, Serra-Pereira et al. 2011), so early stages of oogenesis can occur in immature females (Serra-Pereira et al. 2011). This was corroborated with the histological images of the first stages of development of the previtelogenic follicles found in immature M. henlei females showing early oogenesis.
In males, testicle development is marked by cell differentiation and growth, which are associated with changes in the diameter and composition of spermatic cysts (Maruska et al. 1996). Immature males showed the first stages of spermatogenesis: germinal epithelium, cells not well-organized, and/or primary spermatogonia. Primary spermatogonia were in active multiplication by mitosis and were aligned in the basal membrane, showing heterochromatin granules in the nucleosome and cytoplasm with limited presence of organelles (where mitochondria predominate). The nuclei of Sertoli cells were aligned toward the lumen of spermatic cysts.
In the present study we confirmed that semen storage occurs in the oviducal glands of M. henlei. This phenomenon has been reported for other mustelids, such as M. antarcticus, which has a short storage period between postpartum and the following ovulation (Walker 2007). Other sharks in which semen storage has been known to occur are Iago omanensis (Hamlett et al. 2002), Prionace glauca, Lamna nasus, Carcharhinus obscurus, Carcharhinus plumbeus, Galeocerdo cuvier, Sphyrna lewini, and Sphyrna tiburo (Pratt 1993).
Fertilization in elasmobranchs is internal and occurs in the oviducal gland, where semen can be stored for days, months, or years in the terminal zone as dense packages or in a loosely packed form depending on the species (Hamlett et al. 2005). This allows females to ensure fertilization and control the moment of fecundation while considering the optimum environmental conditions for the survival of the offspring (Maruzka et al. 1996). This strategy is used mainly by highly migratory species or those that present strong segregation by sex (Pratt 1993).
Sperm cells were found in acini on the periphery of the terminal zone of the oviducal gland and were associated with the mucous membrane of ciliated cells. Pratt (1993) determined that the storage period depends on the position of sperm inside the oviducal glands. The activity of acini in the terminal zone is associated with the storage, transport, and preservation (nutrition) of sperm cells inside the oviducal gland until the moment of fertilization (Hamlett et al. 2002). Because sperm were loosely packed in tubules, with heads away from the lumen, we can state that the period of semen storage in M. henlei is short.
Semen storage in males occurs in the seminal vesicles (Pratt and Tanaka 1994). In M. henlei storage of sperm cells occurred as spermatozeugmata, which are organized masses of free semen that are attached to a cohesive matrix that is ovoid or spherical in shape (Pratt and Tanaka 1994). This type of aggregation has been reported for other shark species too, such as S. lewini, P. glauca, and Carcharhinus falciformis (Pratt and Tanaka 1994). Semen packaging could be a highly efficient strategy to transfer semen to females, with lower losses during copulation compared with semen in liquid form (Matthews 1950).
The reproductive parameters estimated for M. henlei in the present study (relatively high fecundity, annual reproductive cycle, and early maturation) allow us to suggest that, like other mustelids, this species has relatively high biological productivity among elasmobranchs (Walker 2005b). We also defined differences in some reproductive parameters between M. henlei and other stocks in Mexican and United States waters, highlighting the need for regionalizing protective or fishing management measures for this species by taking into account the reproductive aspects of each stock.
The restrictions implemented in 2012 to the Mexican elasmobranch fishery, including the yearly closed fishing season from 1 May to 31 July (DOF 2007), apparently protect M. henlei off the west coast of BCS during the reproductive period. However, the present study provides new information on the reproductive biology of M. henlei for different regions along the west coast of BCS, which could contribute to improve fishery management measures for the sustainable use of this resource. Our results show that the northwestern coast of BCS is an important reproduction area, where up to 95% of M. henlei catches in August are pregnant females; therefore, an extension of the closed fishing season can be suggested for this area. On the other hand, on the southwestern coast of BCS, females with embryos nearly ready for birth can be found in April, and it would be important to consider this for the protection of this species as well. These results show the importance of the regionalization of conservation and management measures based on our new findings on the reproductive biology of M. henlei, considering the spatial and temporal distribution of this species.











 nueva página del texto (beta)
nueva página del texto (beta)




