Study contribution
In this study, we found that the endocrine system and metabolic status in male cane toads play important roles in the immune competence against parasites during the reproductive season. The approach taken in this study could be a useful model to better understand the adaptive strategies of invasive species. Moreover, it could help design future studies on amphibian species that are vulnerable or endangered, enabling more informed management decisions and guiding last resort interventions.
Introduction
The adaptation to change is not a simple task. Multiple factors interact during the process and these connections also vary over time. Particularly, interactions between the endocrine system, the immune system and energy expenditure are critical in contributing to the maintenance of homeostasis during stressful periods in vertebrates.1 Among the hormones released by the endocrine system, glucocorticoids (GCs) and androgens appear to have a direct effect on the ecology and evolution of host-parasite interactions in vertebrate males.2 Studies of the effect of environmental events and survival outcomes have reported that GCs vary with environ- mental change and can be used as survival predictors.3-5 They also affect the animal’s behavior during migration,6-9 dispersion,10 and foraging.11 For instan- ce, androgens such as testosterone enhance sexual behavior in male toads, which is energetically costly12,13 and increases their vulnerability to parasites.14-16
An important consideration is that parasitism applies an obligate energetic cost to the host. Therefore, the host needs to balance the cost of moving to new territories against the cost of controlling their parasite levels or keeping their parasite levels in check. Studies have shown that the incidence of parasites in colonizers introduced in new areas is lower than that of local residents.17-19 These findings can be attributed to host density, as well as the parasite biological cycle.17 However, as mentioned above, the physiological strategies employed by the host must be considered as these can influence the immune, metabolic, and behavioral outcomes.
Both GCs and androgens can vary seasonally and can affect energy trade off with the immune response.2 Still, prolonged exposure to high levels of GCs and androgens decreases immune competence against parasites, leaving individuals more susceptible to infestations.5,20
Previous studies have demonstrated a link between the endocrine system, energy balance, and immune function in rodents,21,22 marsupials,23,24 and reptiles.25,26 In amphibians, however, researchers have focused on simple associations between parasite levels and endocrine, immune, or energetic factors,27-29 while little is known on how the interactions between these factors affect immune competence to parasites.30,31 Therefore, we hypothesized that there is a relationship between endocrine responses, energy status, and immune competence to parasites in cane toads from different areas. To investigate this hypothesis, we simultaneously evaluated lungworm parasite (Rhabdias spp.) infestation, immune competence, levels of plasma corticosterone and testosterone, and energy status in three different populations of cane toad (Rhinella marina) from different areas and with suspected differences in parasite intensities.
Materials and methods
Ethical statement
All procedures followed the National Health and Medical Research Council Guidelines for Animal Experimentation and were approved by The University of Queensland Animal Ethics Committee (SBMS/437/09/URG/GOVTMEX/HSF/ CFOC).
Animals
The cane toad (Rhinella marina) is the anuran with the widest known world distribution. It is native to America but was introduced in Australia and several Pacific and Caribbean islands.32 It is an opportunistic species and a dietary generalist 33 that equips it very well to invade new habitat. However, smaller sub-adult cane toads from Australia were found lagging behind the migration front. These animals were found to carry parasitic lungworm larvae identified as Rhabdias pseudosphaerocephala, a South American species.34
Field work
During the wet season of 2010-2011, we captured a total of ninety adult male cane toads at night (9-11 pm). Animals originated from two populations in Brisbane, Queensland, Australia (QL, n = 57), and Kununurra, Western Australia (WA, n = 18) and a third population from the Catemaco region, Veracruz, Mexico (MX, n = 15). The animals were transported to a field laboratory located near the collection site. Six animals from each site were chosen randomly and kept for a standardized immune challenge. The remaining animals were subjected to blood sampling to determine plasma levels of testosterone (T), corticosterone (B) and free fatty acids (FFA).
Sample collection
The animals selected for drawing blood were weighed and anesthetized intra-muscularly with a dose of 200 mg/kg body weight (BW) of ketamine combined with 0.2 mg/kg BW of diazepam.35 Under anesthesia, blood (500 µL) was collected via cardiac puncture with heparin-treated syringes, placed into Eppendorf tubes and centrifuged at 1 300 ×g for 5 min to separate plasma, which was then removed and stored at -20 ºC for later analysis. All animals were euthanized with an intra-cardiac overdose of 100 mg/kg of pentobarbital. After death, snout-vent length was determined, a complete post-mortem examination was carried out for each animal to rate body fat size and lungworm count, while the spleen was removed and preserved for examination.
Parasite intensity
To assess the presence of lungworm parasites, dissection and organ removal was performed after morphological measurements were completed. Lungs were removed and nematodes were quantified by directly counting the number of visible parasites (intensity) in the lungs, bronchi, and trachea.36 We did not conduct morphological or molecular identification of the parasite found in the lung. However, previous studies have identified this parasite as part of the Rhabdiasidae family in cane toads from Australia34 and Mexico.37
Immune competence against parasites
Immune competence is the ability of an individual to resist and combat pathogens and parasites through the response of their immune system. This feature can be assessed by measuring biological responses against disease, such as the efficiency of innate or adaptive immune responses.21 The cell-mediated immune competence and lymphoid organ development were the two strategies that we chose to assess immune competence in the cane toads under study.
Immune responses to antigens
The immune responses to antigens were determined using a standardized immune challenge that measures the level of swelling caused by a single intra-dermal injection of phytohaemagglutinin (PHA).38-40 The immune challenge was conducted during the first 12 h of capture, and the average of three consecutive blind measurements was taken to minimize the observer effect, as well as measurement error. Briefly, the first measurement of the thickness of the fourth phalanx of the hind limb was made where the interdigital webbing ends. After the measurement of the phalanx, 5-mg PHA was diluted in 5 mL of phosphate-buffered sterile saline solution (0.5 mM).
From this stock, 0.5 mL was injected subcutaneously using a 1 mL syringe, 27G × ½” needle. The injection was given dorsally at the same site where the first measurement was carried out. A contralateral injection of 0.5 mL of phosphate-buffered sterile saline solution (0.05 mM) served as a control. Measurements were repeated after 24 h. After injection, animals were placed in plastic containers and adapted to aquaria. An ambient temperature of 22-24 ºC with a light cycle of 12D:12L. The measurements were carried out with a digital vernier caliper, with an accuracy of ± 0.03 mm. The animals were subjected to a fasting period of 36 h during enclosure. The delayed hypersensitivity index (DHI) was calculated as previously described.41
Lymphoid organ development
Spleens were dissected out, cleared of connective tissue and fat, and then stored at 4 ºC in a solution of 4 % paraformaldehyde in phosphate buffer (0.05 mM) (PFA 4 %). Organ mass was determined by weighing it to the nearest mg using an analytical balance. To eliminate the effect of body size on spleen size, we calculated the index between spleen weight and body weight (spleen weight/[body weightspleen weight]) as described elsewhere.41
Hormone measurements
To assess the levels of corticosterone (B) and testosterone (T) in blood, we performed radioimmunoassays with 20 µL of plasma.42,43 The tracer used was tritiated corticosterone [corticosterone (1,2,6,7-3H) 2.59 TBq/mmol] and testosterone {testosterone, [1,2,6,7,-3H(N)]-3.96 TBq/mmol}. For this study, we used a polyclonal antibody against corticosterone developed by AbCam laboratories (Product Number: ab77798). The cross-reactions documented were 0.67 % to 11-dehydrocorticosterone, 1.5 % to deoxycorticosterone, and < 0.01 % to 18-OH-DOC, cortisone, cortisol, and aldosterone.
The testosterone antibody assay was conducted as previously reported.44 The cross reactions included 5-alpha dihydrotestosterone 11 %, androstenedione 3.5 %, 11 oxotestosterone 2.5 %, 5-alpha androstane 3 beta, 17 beta-diol 2.5 %, epitestostreone 2 %, DHEA 2 %, progesterone 1.5 %. Assay sensitivity was 10 pg.
For both steroid assays, radioactivity was counted in a liquid scintillation spectrometer. The plasma samples were measured in duplicates and the steroid concentrations were calculated from a standard curve, as described by Dudley.45 Data are expressed as ng/mL of plasma.
Indices of energetic status
Body condition, and free fatty acid (FFA) concentrations, were the two variables used as indicators of the energetic status in the cane toads captured from the different areas.
Body condition
Body condition was based on the residuals obtained from the linear regression between body weight (dependent) and snout vent length (independent) was used as a proxy for tissue mass.
Energy mobilized
To estimate the available energy mobilized, we conducted an assay to determine free fatty acid (FFA) concentrations in 10 µL plasma samples using the method of Laurell and Tibbling.46 Palmitic acid was used as the reference standard in the FFA assays, and data are expressed as mmol/dl. FFA was not evaluated in toads used in the immune response assays because the animals were fasted for more than 48 h and mobilization of FFA was expected.
Statistical analysis
All variables were normalized. Parasite intensity, body condition, testosterone levels, FFA levels, and the differences in toe thickness, 24 h after PHA injection, were logarithmic transformed (Log10+1).47 Corticosterone levels followed a Poisson distribution, consequently the square root (SQRT+⅜) was chosen to normalize data. The arcsine transformation was used to normalize the proportions of the spleen somatic index.48
We also assessed the association between lungworm intensity and all continuous variables by conducting simple linear regression analysis. Moreover, to identify the strongest associations, stepwise multiple linear square regression analysis was also conducted. The multiplicative dummy variable approach proposed by Gujarati51 was used to determine the equality of slopes between sets of coefficients between populations. Spearman’s correlation was used to determine direct associations between immune competence variables, hormone levels and indices of energetic status. Finally, a mediation analysis was performed to determine whether a variable might predict the interaction of parasite intensity (dependent) with immune competence, indices of energetic status, and hormone levels. Values with P < 0.05 were considered as statistically significant. Statistical analyses were conducted using SPSS software, version 19.
Results
The analysis of the counts of parasites from the different populations of toads showed differences in the intensity of lungworms between populations [F(2, 87) 69.51, P = 0.01]. Toads from WA had the lowest intensity of parasitism followed by QL population (P < 0.01). MX had the highest intensity (P < 0.01) (Table 1).
Table 1 Parasite intensity, immune competence, hormone levels, and indices of energetic status variables of the male cane toad populations under study1
| Region of origin of male cane toad populations | ||||
| Variables | Mexico | Queensland | Western Australia | P-value |
| Parasite intensity | 20.4 ± 10.4a | 12.8 ± 10.3b | 0.0 ± 0c | 0.01 |
| n | 15 | 57 | 18 | |
| Immune competence | ||||
| Spleen somatic index | 0.0008 ± 0.0004 | 0.0010 ± 0.0005 | 0.0007 ± 0.0004 | 0.08 |
| n | 15 | 18 | 18 | |
| DHI2 at 24 h | 0.16 ± 0.09b | 0.03 ± 0.02c | 0.22 ± 0.11a | 0.042 |
| n | 6 | 6 | 6 | |
| Hormone levels | ||||
| Testosterone (ng/mL plasma) | 4.3 ± 1.9a | 3 ± 1.3b | 1.7 ± 1c | 0.001 |
| 15 | 54 | 18 | ||
| Corticosterone (ng/mL plasma) | 4.7 ± 3.5a | 4.4 ± 3.8a | 1.6 ± 1.0b | 0.023 |
| n | 15 | 53 | 16 | |
| Indices of energetic status | ||||
| Body condition3 | -0.062 ± 0.013b | -0.002 ± 0.013c | 0.007 ± 0.012a | 0.049 |
| n | 15 | 57 | 18 | |
| FFA (mmol/dL) | 5.38 ± 1.41b | 7.01 ± 1.91a | 7.82 ± 1.17a | 0.043 |
| n | 10 | 42 | 9 | |
1Means and standard deviations of non-transformed data
2DHI: delayed hypersensitivity index.
3Body condition: calculation based on the residuals obtained from the linear regression between body weight (dependent) and snout vent length (independent). It was used as a proxy for tissue mass.
Results showed that a single intra-dermal injection of phytohaemagglutinin (PHA) could elicit an inflammatory response in the toe after 24 h in all toads [F (4, 108) 2.49; P = 0.05]. The strongest response was found in the WA toad population, followed by that from MX and QL [F(2, 15) 3.91, P = 0.042]. No significant differences were found in the spleen somatic index among the populations (P > 0.05) (Table 1).
We also found differences in testosterone and corticosterone levels. Toads from WA had the lowest testosterone levels, followed by those from QL and MX, respectively [F(2, 84) 11.27, P = 0.001]. Likewise, the lowest corticosterone concentration was observed in animals from WA [F(2, 81) 3.98, P = 0.023], while there was no difference between toads from QL and MX (Table 1). Results for variables associated with the energetic status showed that animals from WA had the best body condition compared with the other two populations [F(2, 87) 3.130, P = 0.049]. However, no differences in FFA were found between toads from WA and QL, while those from MX had the lowest FFA levels [F(2, 58)3.32, P = 0.043] (Table 1).
Figure 1 shows the associations between parasite intensity and immune competence; hormone levels, and variables related to indices of energetic status. Linear regression analysis between parasite intensity and immune competence variables showed that only the DHI at 24 h had a negative association with the parasite intensity (β = -0.57, t[10] -2.23, P < 0.05) (Figure 1, A). Simple linear regression analysis did not reveal associations between spleen mass and lungworm intensity (P > 0.05) (Figure 1, B). In the case of hormone levels, corticosterone (β = 0.36, t[60] 2.98, P < 0.05) and testosterone (β = 0.62, t[66] 6.43, P < 0.01) exhibited positive associations with parasite intensity (Figure 1, C and D). Finally, the associations between indices of energetic status and parasite intensity show that body condition was not associated with parasite number (β = 0.33, t[70] 1.96, P > 0.05), whereas free fatty acids (FFA) showed a negative association with lungworm intensity (β = -0.55, t[54] -4.92, P < 0.01) (Figure 1, E, F).
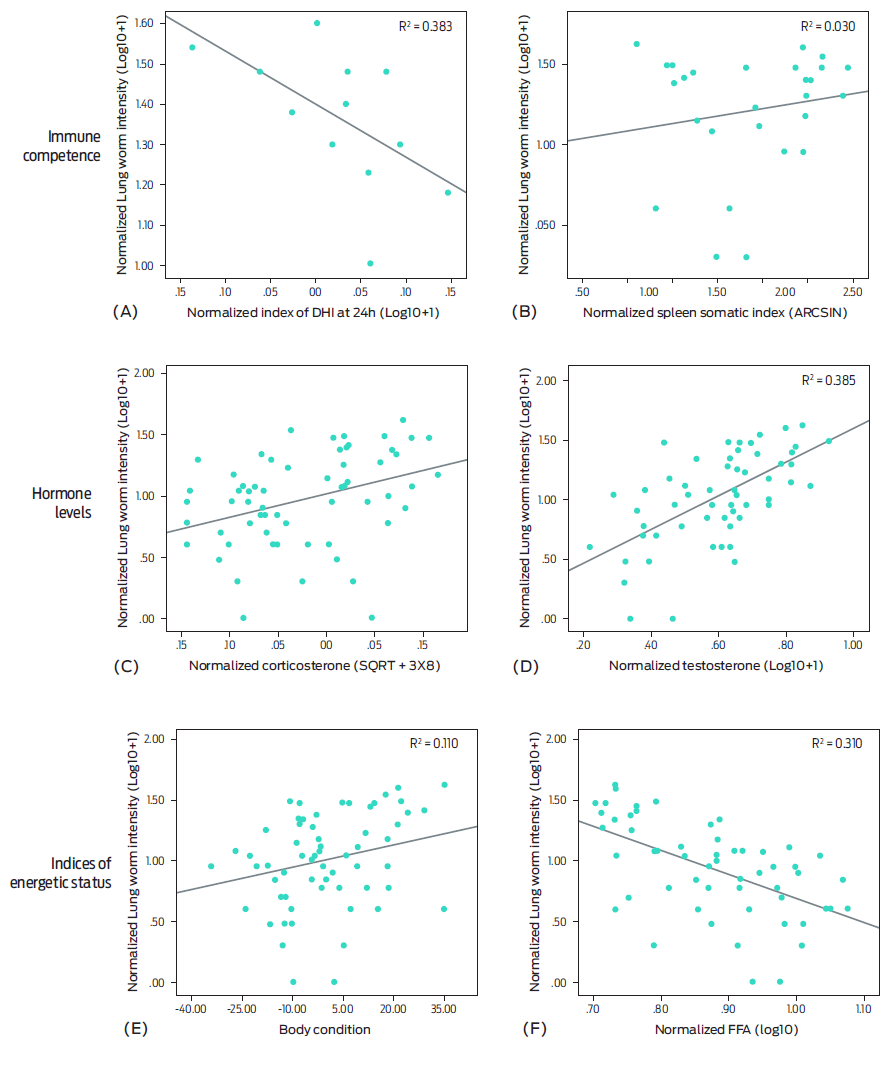
Figure 1 Results of linear regression analysis between parasite intensity and variables of immune competence: (A) DHI at 24 h: delayed hypersensitivity index, (B) spleen somatic index, hormone levels, (C) corticosterone, (D) testosterone, and indices of energetic status, (E) body condition, (F) FFA: free fatty acids of male cane toads.
Multiple linear regression demonstrated that testosterone and FFA had the strongest association with parasite intensity (R2 = 0.5, P = 0.001). Testosterone alone accounted for 38 % of the variance and was the most meaningful variable [F(4,43) 10.3, R2 = 0.42,P = 0.001, slope ± SE = 1.08 ± 0.3, transformed values].
The Table 2 summarizes the Spearman’s correlation between variables related to immune competence, hormone levels, and indices of energetic status. Corticosterone was the only variable that correlated negatively with variables related to immune response (spleen somatic index) (P < 0.05). Both hormones had a negative effect on FFA (P < 0.01), while testosterone was negatively correlated with body condition (P < 0.01), as well as the spleen somatic index (P < 0.05).
Table 2 Pearson correlation coefficients (probability values) between variables related to immune competence, hormone levels, and indices of metabolic status in male cane toads
| SSI | DHI | T4 | B2 | FFA | BC | |
| Immune competence SSI: spleen somatic index(proportion) |
- | -0.31 (0.205) |
0.22 (0.123) |
-0.30 (0.038) |
0.38 (0.086) |
-0.29 (0.021) |
| DHI: delayed hypersensitivity index at 24 h | - | - | -0.35 (0.183) |
-0.34 (0.148) |
-0.03 (0.569) |
|
| Hormone levels | ||||||
| T4: testosterone (ng/mL plasma) | - | - | - | 0.20 (0.075) |
-0.42 (0.001) |
-0.39 (0.001) |
| B2: corticosterone (ng/mL plasma) | - | - | - | - | -0.47 (0.001) |
0.01 (0.955) |
| Indices of energetic status | ||||||
| FFA: free fatty acids (nmol/dL plasma) | - | - | - | - | - | 0.18 (0.637) |
| BC: body condition | - | - | - | - | - | - |
Finally, Table 3 summarizes the mediating role of variables associated between immune competence; indices of energetic status; and hormone levels, on parasite intensity (dependent). Among the variables included as mediators, only FFA was found to have a total mediating effect between hormone levels and parasite intensity in cane toads (P < 0.05). The inclusion of FFA simultaneously with corticosterone significantly reduced the P value and increased the R2 coefficient of model II, demonstrating that FFA acts as a mediator between corticosterone levels and parasite intensity.53 The effect of FFA in the association between testosterone and parasite intensity was not strong enough to reduce the P value. However, the R2 coefficient was higher in model II, indicating that FFA is partially mediating the effect of testosterone levels on parasite intensity. No other variable was found to have the same effect in the rest of the associations.
Table 3 Mediation analysis
| β | t | P | R2 | |
| Model I | ||||
| Corticosterone | 0.36 | 3.04 | 0.01 | 0.13 |
| Model II | ||||
| Corticosterone | 0.18 | 1.34 | 0.18 | 0.35 |
| FFA (m) | -0.48 | -3.59 | 0.01 | |
| Model I | ||||
| Testosterone | 0.61 | 6.39 | 0.01 | 0.38 |
| Model II | ||||
| Testosterone | 0.40 | 3.67 | 0.01 | 0.51 |
| FFA (m)1 | -0.45 | -4.11 | 0.01 |
1FFA: free fatty acids.
Model I shows values of the simple regression between lung worm intensity and independent variables. Model II shows values of the regression with the mediator (m) included.
Discussion
Geographic, environmental factors and host biology are important factors determining parasite infections.54,55 Changes in these factors might lead to differences in parasite incidence and abundance between populations of the same species. However, in invasive species, parasite intensity and pathogen diversity are lower than those in their native distribution,17,56,57 which is consistent with the findings from this study. The WA population at the front line of invasion had the lowest intensity of lungworm parasites compared with the established populations (MX, QL). These results agree with previous studies documenting lower loads of parasites in dispersing animals from Australian cane toad populations.58
Lungworm intensity was not the only variable different in the WA cane toads. These animals also had the lowest levels of B and T, the strongest response to the PHA, and the best body condition. Our observation of a strong immune response in dispersing animals, compared with established populations, contrasts with previous reports of bacterial infections in cane toad populations invading the Northern Australian territory.59,60 Similarly, Brown and Shilton40 reported a blunted immune response in cane toads colonizing territories in the same region.40 These differences between studies might be associated with the period of collection, as well as a possible effect of favorable environmental factors that might have resulted in a more robust immune system in our surveyed cane toad populations.
The animals in this study were collected during the wet season that corresponds to the reproductive season in this species. Males face important challenges in their physiological and immune responses during the reproductive season.61 Folstad and Karter62 proposed that high testosterone levels have a cost in enhancing secondary sexual characteristics, as it reduces immune competence against parasites.62 However, there is no agreement in this respect. While several studies have reported an increase in parasite burdens and immune suppression in males with high levels of testosterone,63,64 others have observed no change in either cellular or humoral immune responses.65-68
These disparities between studies can be attributed to additional physiological responses, environmental, and behavioral factors influencing the outcome between the immune-endocrine associations. For instance, an increase in testosterone levels has been positively associated with an increase in glucocorticoids, which are potent immune suppressors.1,69,70 Moreover, energy reserves71,72 and the environment5,73 also influence the outcome between the immune-endocrine associations. Experimental evidence also shows that testosterone stimulates the dispersing behavior during the reproductive season, which is energetically costly in amphibian males.74,75 The roaming of cane toad into new areas, might increase the probability of infection either from other males or females carrying high parasite loads, or entering areas having a high prevalence of parasites in the soil and water.76
This study found the existence of direct associations and interrelationships between levels of B and T with parasite intensity, immune competence variables, and energy status variables in male cane toads. Among the variables associated with immune competence, only DHI 24 h showed a negative association with parasite intensity. The estimation of immune response by using the level of swelling elicited by PHA has been reported to be a reliable measure of cell-mediated immune competence in birds,38,39,41,67 and amphibians.40,77,78 A strong response to PHA has previously been associated with a reduction in susceptibility to parasites.79 PHA antigen activates CD8 and CD2 in skin immune cells.80 CD8 is expressed in amphibian skin and has been associated with allograph rejection.81 Moreover, while corticosterone inhibits CD8+T cells, testosterone has a stimulatory effect.
2 Few studies deal with the association between the immune response elicited by PHA and hormone levels. However, a weak cellular response to PHA has been reported in amphibians exposed to environmental stressors82 and pollutants.78
In this study, neither corticosterone nor testosterone was found to influence the DHI response in male cane toads. This lack of association was also observed in a zebra finch study, after PHA administration in birds,66 which might be attributed to the cell division stage during the experimental period. It has been observed that glucocorticoids induce cell death during the G1 phase, in lymphocyte stimulated in vitro with PHA.83 The same effect has been reported in vivo with other cell lines.84 In any case, the differences between cane toad populations observed here clearly show an environmental effect on the immune competence of these animals.
Energy also had an important effect on parasite numbers in male cane toads. The linear regression analysis did not reveal an association between body condition and parasite intensity, however FFA had a negative association with parasites. This result, in conjunction with the reduction of parasite number in animals with high ranks of energy reserves, is consistent with the assumption that energy reserves and nutritional status impact parasite susceptibility.28,29,85,86
Unlike the direct catabolic action of glucocorticoids in energy reserves, the effect of testosterone is indirect.72 Courtship and territorial behavior are associated with high metabolic rates in amphibians.87,88 This might explain the partial mediation effect of FFA between the association of T and parasites and the total mediation effect between B and parasites. The mediating effect of FFA upon parasites is explained by the negative effect of corticosterone in FFA. Glucocorticoids in amphibians are released in response to stressful conditions by mobilizing energy and stimulating hepatic gluconeogenesis 72,89,90.
Studies on the effect of GCs in FFA are scarce in amphibians. However, studies in mammals have demonstrated that GCs have an important effect on lipid FFA metabolism.91 Energy requirements in amphibians heavily rely on their fat reserves,72 which contribute as much as 75-95 % of the energy fuel in this species.92 Moreover, FFA, as a metabolic substrate, gives higher energy yields than glucose.
93 Harri94 observed a marked seasonal variation in frogs’ plasma FFA with a reduction in concentration during summer and an increase during spring and autumn. This reduction is related to depletion in fat reserves during the reproductive season when glucocorticoids and androgens are elevated and have an important function in energy utilization.72 This physiological pattern might explain why we observed FFA was negatively correlated with corticosterone and testosterone, and mediated the effect of corticosterone on parasite numbers.
In summary, this study further confirms that the endocrine system and metabolic status are central to adjusting the host-parasite relationship in cane toads during the reproductive season. During this period, the levels of corticosterone increase and, consequently, the immune competence and metabolic status are affected, increasing parasite intensity. Additionally, high testosterone levels have an indirect effect on parasite intensity as this hormone stimulates energetically costly behaviors that can also increase the probability of exposure and vulnerability of toads to parasites, resulting in an increased parasite intensity. These findings are in agreement with those of several studies reporting the existence of an energetic trade-off during the reproductive season between reproductive function and immune competence against parasites.95-98
This study can also explain why introduced populations often have reduced parasite loads.56 Our findings suggest that introduced species might be exposed to a selective pressure, not only by the environment or parasites, which might be carried by them or found in their new environment, but also by their physiological responses. Therefore, only those that have physiological strategies able to reduce the metabolic cost of immune function and energy during dispersion activities, might be able to succeed in their expansion progression.
The approach taken in this research could be a useful model for similar studies on amphibian species that are vulnerable or endangered, enabling more informed management decisions to be taken and guiding last resort interventions. Moreover, it could help better understand the adaptive strategies of invasive species. Further research in this area will improve our understanding of the ecophysiology of populations and diseases in amphibians. Moreover, it will provide further insight into their relationship with factors affecting the long-term survival of species.
Acknowledgments
The authors thank the logistical and technical support provided during the field work in Mexico by Dr. Marta C. Romano, from The Center for Research and Advanced Studies (CINVESTAV), the department of Ethology, Wildlife, and Laboratory Animals, Faculty of Veterinary Medicine, National Autonomous University of Mexico (UNAM), and the Biological Station “Los Tuxtlas” from the Biology Institute of UNAM. We are also grateful for the support provided by The Kimberly Toad Busters during the field work in Western Australia, and Prof. Robert B. Ashman, from the School of Dentistry, The University of Queensland, who provided valuable advice on the assessment of immune competence. We also thank Dr. Jose L. Pablos, from the department of Genetics and Statistics, Faculty of Veterinary Medicine of UNAM, for his advice on the statistical analysis.
Conflicts of interest
The authors have no conflicts of interest to declare regarding this publication.
Author contributions
Conceptualization: SE Hernandez, C Sernia, VH Reynoso, AJ Bradley Data curation: SE Hernandez, VH Reynoso. Formal analysis: SE Hernandez, JL Pablos Funding acquisition: SE Hernandez, AJ Bradley.
Investigation: SE Hernández
Methodology: SE Hernández
Project administration: SE Hernandez, C Sernia, VH Reynoso, AJ Bradley Resources: SE Hernandez, C Sernia, VH Reynoso, AJ Bradley.
Software: SE Hernández, JL Pablos. Supervision: C Sernia, VH Reynoso, AJ Bradley Validation: SE Hernández C Sernia, AJ Bradley.
Visualization: SE Hernández
Writing-original draft: SE Hernández
Writing-review and editing: C Sernia, AJ Bradley











 nueva página del texto (beta)
nueva página del texto (beta)



