Introduction
Subclinical hypocalcemia (SH) occurs mainly during the first 48 hours postpartum in dairy cows, the frequency of its occurrence increases in cows with higher parity (Reinhardt et al., 2011). Some cows may suffer SH for a longer period (Salgado et al., 2014a, b). Cows with serum calcium (Ca) concentrations greater than 2.1 mmol/L also have a lower concentration of serum nonesterified fatty acids (NEFA), indicating that SH predisposes the animals to other energy-balance problems (Reinhartdt, 2011). Cows with SH, defined as a serum Ca level of less than 2.0 mmol/L in at least one sample within the first 3 days postpartum, presented higher concentrations of ß-hydroxybutyrate (BHBA) and NEFA postpartum, as well as a higher incidence of metritis, lower pregnancy rate and higher birth-conception interval (Martinez et al., 2012). Subclinical hypocalcemia, high concentrations of BHBA and NEFA and a deficiency in vitamin E, selenium and other minerals decreases the strength of the immune system, which is required for the adequate delivery of the placenta (Kimura et al., 2006). Retention of fetal membranes increases the risk of endometritis and metritis, which negatively affect various reproductive parameters (Curtis et al., 1983, Martinez et al., 2012).
Prostaglandin F2 alpha (PGF2α) plays an important role in the mechanism underlying the expulsion of the placenta after calving, cows that had retained the placenta had a lower concentration of PGF2α in the cotyledons compared with that of cows that did not retained the placenta (Leidl et al., 1980). In another study, it was found that the concentrations of PGF2α and prostaglandin E1 (PGE1) increased in the first hour postpartum in the cows that did not retain the placenta (Wischral et al., 2001). In contrast, ewes treated with inhibitors of prostaglandin-synthesis during the early postpartum period retained the placenta (Chassagne and Barnouin, 1993).
The mechanism by which prostaglandins prevent placental retention and improve uterine health has not been fully elucidated. It is believed that increased uterine contraction is a factor of the mechanism of action. However, it has also been observed that PGF2α increased the phagocytic activity of neutrophils in the uterine mucosa and increased the level of various cytokines that stimulate immune function (Lewis and Wulster-Radcliffe, 2006), but this mechanism is not well understood.
A syndrome in which PGE1 is over-produced has been described in children; the affected infants have hypercalciuria and increased levels of serum calcitriol and Ca (Welch, 1997). Studies in rats have shown that the administration of PGE2 increased the synthesis of calcitriol (Yamada et al., 1983). Furthermore, it has been demonstrated in rabbits, both in vitro and in vivo, that the administration of PGE1 also increased the synthesis of calcitriol (Velásquez-Forero et al., 2006). In cultures of chicken kidney cells, both PGE1 and PGF2α were observed to increase the rate of conversion of calcidiol to calcitriol (Treschel et. al., 1980).
A deficiency in the synthesis or activity of calcitriol has been described as one of the leading causes of hypocalcemia in dairy cows (Goff et al., 1989; Goff et al., 1991). To our knowledge, no studies have examined the effect of PGF2α treatment on calcitriol synthesis in cattle. It is likely that prostaglandin increases the concentration of serum calcitriol, which would have a positive effect on the serum Ca concentration in early postpartum dairy cows, which may partially explain the mechanism of action of PGF2α on reproductive parameters.
The objective of this study was to evaluate the effect of prostaglandin F2α administration during the early postpartum period on the levels of blood serum calcium, phosphorus, magnesium and the metabolites of vitamin D and on the reproductive parameters of dairy cows.
Materials and method
Animals and blood samples
This study was conducted on a commercial farm with 3200 Holstein cows, with an annual mean milk production of 10845 kg/cow, located near the city of Torreon, Coahuila, Mexico. The cows were maintained in the same pen before and after calving, and they received a total mixed ration twice daily and water ad libitum (Table 1).
Table 1 Ingredients and chemical composition of prepartum and postpartum diets

CP = crude protein, NDF = neutral detergent fiber, NEL = Net energy of lactation, DCAD = dietary cation-anion difference using the formula: (Na + K) - (Cl + 0.6 S) in milliequivalents per Kg of dry matter.
Eighty multiparous cows with a body condition score (BCS) of 3.25 to 3.5 at calving were selected and were randomly placed in one of two groups. Cows that presented dystocia were excluded from the study. The cows in Group 1 (n = 38) were administered the synthetic PGF2α analog, sodium cloprostenol, i.m. at a dose of 500 µg (in 2 mL) per animal at the first hour and at 48 h after calving. The dose and frequency used was based on previous work by Ortega et al. (2013). The cows in Group 2 (n = 42) did not receive any treatment. Blood samples for serum preparations were collected through a tail vein puncture in 7-mL vacuum tubes lacking anticoagulant (Monoject®, Argtech, Inc., Manfield, MA, USA) from ten cows in each group before the administration of PGF2α and at 3, 12, 24, 48 hours and 7 d after treatment. Blood samples were centrifuged at 1200 g for 10 min within 1 h after sampling. The serum samples were placed in plastic vials and stored at -20°C until analysis. The incidence of a retained placenta, metritis and endometritis and the number of open days were recorded. A retained placenta was defined as placenta that a cow did not eliminate during the first 24 h postpartum. Metritis was characterized as a fetid brown-red vaginal discharge during the first 20 d postpartum, with clinical signs of disease. Endometritis was defined as the presence of a purulent vaginal secretion between 10 and 50 d after calving, without clinical signs. All of the procedures were conducted according to the guidelines of the Institutional Ethical Committee on the Use and Care of Animals of the Facultad de Medicina Veterinaria y Zootecnia (FMVZ), Universidad Nacional Autónoma de México (UNAM).
Blood serum analyses
The serum concentrations of Ca, P and Mg were determined using a colorimetric method (Randox, Crumlin, UK) in a semi-automatic model Selectra Junior analyzer (Vital® Scientific, Spankeren, The Netherlands) in the section of Clinical Pathology of the Department of Pathology (FMVZ, UNAM). The calcidiol and calcitriol concentrations were assessed using radioimmunoassays after I125 labeling was performed (DiaSorin, Stillwater, MN, USA) at the "Federico Gómez" Children's Hospital in Mexico City.
Statistical analysis
The levels of serum Ca, P, Mg, calcidiol and calcitriol were assessed using an analysis of variance for a completely randomized design with repeated measurements after verification of the homogeneity of variance and normality (SPSS software version 10.0 for Windows). The effect of treatment was the between-subjects factor, and the time of sampling was the within-subjects interaction, The Bonferroni adjustment was used post hoc to test for differences between means. The cases of subclinical hypocalcemia, retention of the placenta, metritis, and endometritis were assessed using a test for the homogeneity of proportions and a one-tailed Fisher exact test. The number of open days was evaluated using a Kaplan-Meier survival curve and the differences between the curves were evaluated using a Log Rank test (Medcalc software, MedCalc Statistical Software version 13.2.0). Effects were considered significant at P < 0.05.
Results
Figure 1 shows serum concentration for Ca, P, and Mg throughout the study. A time-by-treatment interaction was observed for the serum Ca level (P < 0.05). The serum Ca concentration increased by day 7 postpartum in the cows that were treated with the synthetic PGF2α analog compared with the controls (P < 0.05). The percentage of cows with subclinical hypocalcemia is shown in Table 2. There were no cases of parturient paresis.

Figure 1 Serum Ca, P and Mg concentrations in dairy cows treated with 500 µg of sodium cloprostenol at 1 and 48 hours postpartum (n = 10) and in control cows (n = 10) (mean values ± SD). There was a time-by-treatment interaction for the Ca values (P < 0.05). Different letters indicate a significant difference from the control group at the same time of sampling, determined using the Bonferroni adjustment (P < 0.05).
Table 2 Proportion (percentage) of cases of subclinical hypocalcemia (serum Ca < 2.0 mmol/L) observed in control Holstein dairy cows vs those treated with 500 µg of sodium cloprostenol at different times postcalving.

The concentrations of serum P and Mg were not different in the two groups of cows and were unchanged throughout the sampling period (P > 0.05).
There were no differences between the treated and control cows in serum calcidiol concentration (Fig. 2). For serum calcitriol, there was a time-by-treatment interaction effect (P < 0.05, Fig. 2). The calcitriol concentration was increased at 48 h and 7 d postpartum with respect to value at the first hour postpartum in the PGF2α-treated group (P < 0.05). In contrast, in the control group, the concentration of serum calcitriol was increased at 24 and 48 hours after calving compared with the value at the first sampling; however, at 7 days postpartum, the level had returned to the original value (P < 0.05) (Fig. 2).
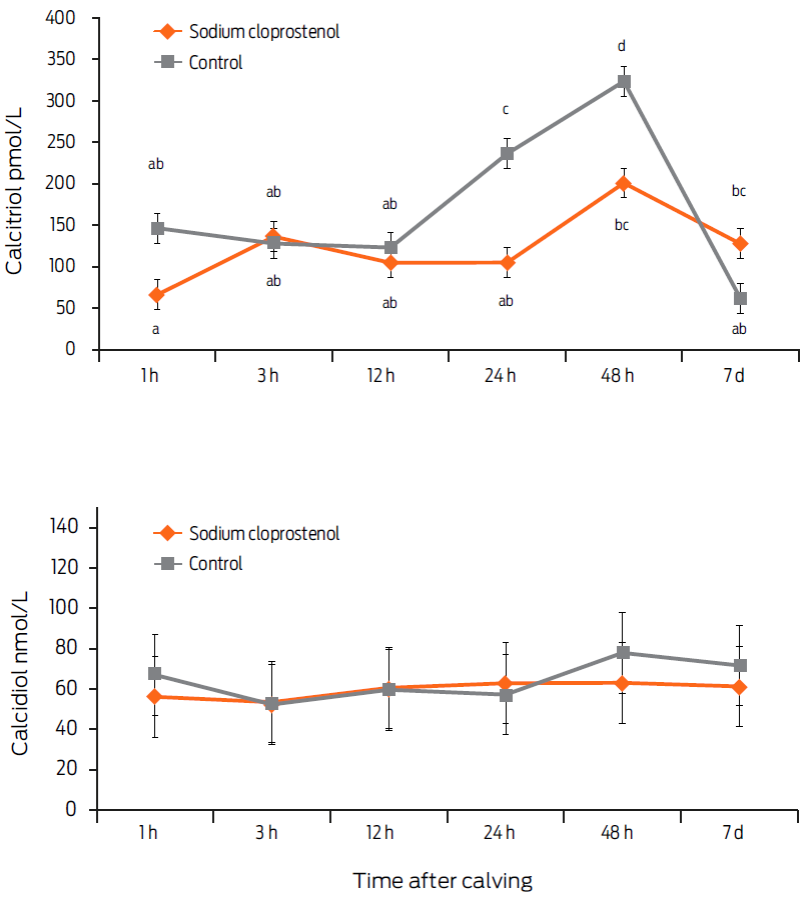
Figure 2 Serum calcitriol and calcidiol concentrations in dairy cows treated with 500 µg of sodium cloprostenol at 1 and 48 hours postpartum (n = 10) and in controls (n = 10) (mean values ± SD). For calcitriol, there was a treatment-by-time interaction (P < 0.05). Different letters indicate a significant difference between the mean values, as determined using the Bonferroni adjustment (P < 0.05).
The number of cases of retained placenta (P = 0.15), metritis (P= 0.15) and endometritis (P = 0.8) in the two groups were not different (Fig. 3).

Figure 3 Percentage of cases of placenta retention, metritis and endometritis in dairy cows treated with 500 µg of cloprostenol sodium at 1 and 48 h postpartum (n = 38) and in controls (n = 42).
The mean and median numbers of open days were 94.66 ± 4.4 and 98, respectively in the sodium cloprostenol-treated group and 107.9 ± 4.9 and 100.5 in the control group. Analysis of the survival curves showed a significant effect of the treatment (P = 0.02; Fig. 4).
Discussion
The increase in serum Ca concentration in the cows that were treated with PGF2α is the expected result, based on studies in rats, rabbits and chickens, as well as in children with the syndrome of prostaglandin E over-production (Welch, 1997). To the authors' knowledge, there is no previous study in cows on the effect of PGF2α on the serum Ca level with which to compare these results. In some studies conducted with rats treated with PGE2 (Yamada et al., 1983) or rabbits treated with PGE1 (Velásquez-Forero et al., 2006) and in children with the PGE1 over-production syndrome, the serum calcitriol level and the urinary excretion of Ca increased, whereas the serum Ca concentration was stable. However, the serum Ca concentration increased in vivo in a study of rabbits treated with PGE1 (Veláquez-Forero et al., 2006); the discrepancy between these results may lie in the different concentrations of the prostaglandin compounds used, the specific needs of each species, the dose and the frequency of application of the prostaglandin. In this study of cows with subclinical hypocalcemia, an increase in the serum calcium concentrations at 7 days postpartum was observed in the cows that were treated with PGF2α. As shown in Table 2, the number of cases of subclinical hypocalcemia was lower (P < 0.03) at 7 days postpartum in the cows treated with PGF2α than in untreated cows, and at 3 and 48 hours postpartum there was a tendency (P = 0.08) for lower number of cases of subclinical hypocalcemia in the PGF2α-treated cows than in untreated cows. These results support the positive effect of PGF2α on serum Ca, although more cows and higher doses are needed for better evaluation. The increase in serum Ca levels in the PGF2α-treated cows was observed at day 7 postpartum. In order to achieve this effect in a more timely manner, PGF2α could be administered several days prepartum, but its negative effect on placental retention makes it necessary to start treatment only after calving.
The serum concentrations of phosphorus (P) and magnesium (Mg) were unaffected by the treatment. This result is very interesting because, physiologically, serum P concentrations decrease when Ca concentrations are low (Kojouri, 2003), whereas in this study, the concentration of P did not decrease even though hypocalcemia developed in cows. This phenomenon occurs when hypocalcemia stimulates parathyroid hormone (PTH) production and this hormone stimulates an exchange mechanism in the kidney by which it retains Ca and secretes P, causing a decrease in the P concentration in the blood, as well as secretion of both minerals into the colostrum (Kojouri, 2003). Recently, Fibroblast Growth Factor-23 (FGF-23) has been described as an important regulator of P metabolism, so that when the P level increases, the secretion of FGF-23 also increases. FGF-23 inhibits PTH synthesis and activates 1α-hydroxylase enzyme (1α-OHase), which is necessary to convert calcidiol into calcitriol (Shimada et al., 2004); at the level of the parathyroid gland, elevation in the concentration of calcitriol also inhibits PTH synthesis, as demonstrated by a study of cultured bovine parathyroid cells (Krajisnik et al., 2007). In contrast, in the kidney, FGF-23 inhibits 1α-OHase, and as a result, calcitriol production decreases (Fukumoto and Yamashita, 2002). In this study, there was no decrease in the serum P level; in contrast, it was observed that the P concentration increased slightly at 12 and 24 h postpartum in the group treated with PGF2α. The mechanism through which this occurred is difficult to explain because the calcitriol concentration was higher in the control group at 12 and 24 hours postpartum.
The level of calcitriol increased at 24 and 48 hours after calving in the control group, which is contrary to the expected results. In chicken kidney cell cultures, PGE1 and PGF2α have been shown to increase the levels of calcitriol (Treschel et al., 1980). In rabbits, both in vitro and in vivo, daily administration of PGE1 for 20 days increased the rate of conversion of calcidiol to calcitriol (Velásquez-Forero et al., 2006). The results of this experiment do not support the hypothesis that PGF2α increased the level of serum calcitriol in dairy cows, at least at the dose and frequency used. Further research using higher doses and greater frequency of PGF2α administration is needed. The calcidiol concentrations were similar in the two groups at all of the sampling times, which indicated that the treatment did not affect the metabolism of this precursor of calcitriol. It is not possible to compare this result to others because, to the author's knowledge, there are no reports in the literature mentioning any effect of PGF2α or PGE1 on calcidiol synthesis in cattle or in other species. Recently, the normal serum calcidiol concentrations in humans have been discussed, although this value is not well defined. The serum calcidiol concentration of the dairy cows in this study was greater than the normal concentrations reported for humans. Although there are no studies on the normal values for dairy cows, the high incidence and exposure to solar radiation in the region where this study was conducted suggests that the animals do not have a deficiency of calcidiol (Salgado et al., 2014a). Furthermore, the dose of PGF2α used has been commonly used to treat uterine diseases and to induce luteolysis. However, Treschel et al. (1980) observed a dose-dependent response in cultures of chicken tubular kidney cells. It is necessary to determine whether higher doses or a greater frequency of application could trigger a response to calcitriol in cows.
Another possible mechanism for why the increased serum Ca concentration did not decrease the serum P concentration during the first postpartum hours in the cows that were treated with PGF2α, despite their having a lower serum calcitriol concentrations, could be the presence of an elevated number of vitamin D receptors (VDRs), which would increase the efficiency of the effect of calcitriol on the intestinal absorption of Ca and P. Humans with idiopathic hypercalciuria were shown to have normal calcitriol concentrations; however, they had increased intestinal uptake of Ca and the hypercalciuria was found to be due to the increased expression of VDRs (Favus et al., 2004). The primary factors that stimulate the expression of these receptors are calcium and calcitriol (Carrillo-Lopez et al., 2009), so that a slight increase in the concentrations of calcitriol and Ca after the application of PGF2α could result in the greater efficiency on the actions of calcitriol. Studies designed to examine whether these changes occur in dairy cows are needed to facilitate understanding of the mechanism of action of PGF2α and demonstrate with greater certainty the usefulness of PGF2α treatment to prevent hypocalcemia.
The number of cases of placental retention, metritis and endometritis of the two groups were not different, although a numerically better performance was observed in the cows in the treated group. This result could be due to the number of animals that were used for this study because Ortega et al. (2012) performed a study using a larger number of animals (n = 180) and found significant differences between the animals that were treated or not treated, with numerical results similar to those of this study. The use of PGF2α in the early hours postpartum is controversial; its mechanism of action has been thought to be causing the lysis of the corpus luteum, which causes a decrease in the progesterone levels (Lander Chacin et al. 1990). However, in the early days postpartum, the cows have no corpus luteum upon which PGF2α could act. In a study of intact and ovariectomized sheep treated with exogenous progesterone, it was observed that uterine susceptibility to experimental challenge with Arcanobacterium pyogenes and E. coli was lower in ewes treated with PGF2α regardless of the absence or presence of progesterone; so it is believed that PGF2α has direct immune-modulatory effects (Lewis et al., 2006). The increase in serum Ca concentrations caused by the activity of PGF2α, in addition to the direct action of PGF2α, could partially explain the positive effects of the immune system and the prevention of metritis and placental retention observed in some studies.
The administration of sodium cloprostenol increased the cumulative curve of pregnant cows in this experiment. Ortega et al. (2012) showed that the application of sodium cloprostenol at 12 and 48 hours postpartum decreased the incidence of placental retention, shortened the interval from parturition to first estrus and increased the pregnancy rate as evaluated at 90 days postpartum; these findings are similar to our results. The mechanism by which PGF2α had positive effects on reproduction in this study is difficult to explain. A positive effect of PGF2α in the treatment of metritis was observed by Melendez et al. (2004). Most likely, the effect of PGF2α on the immune system improved uterine health and fertility (Lewis et al., 2006).
Conclusions
In this study, the administration of PGF2α at the first hour and at 48 hours after calving increased the serum Ca level and decreased the number of cases of subclinical hypocalcemia at day 7 postpartum. It also reduced the number of open days in dairy cows. Therefore, this treatment could be used to prevent reproductive problems in dairy cows.











 texto en
texto en 



