Introduction
México has a great geological, topographic and climatic diversity, which generates a wide distribution of bryophytes in numerous micro-environments (Delgadillo and Cárdenas 1990). According to Goffinet and Shaw (2009), bryophytes are the second most abundant group of terrestrial plants after angiosperms, calculating an approximate diversity of 25 000 described species (Quan et al. 2021). Although their biomass is higher in mesotrophic environments, bryophytes are found in all habitats that vascular plants can grow (Nadhifah et al. 2018), with the exception of marine waters. Generally, bryophytes are ignored in floristic inventories, as well as at any geographic scale (Cornwell et al. 2019).
The diversity of bryophytes varies with the environments, since there are gradients such as vegetation type, topography, humidity, and soil type, which influences the distribution of bryophytes (Delgadillo-Moya et al. 2022). In general, bryophytes have
adapted a strategy of tolerance to desiccation, photosynthesis and growth during wet periods and reduction or suspension of the metabolism during times of drought (Proctor 2000). In addition, bryophytes play a fundamental role in the ecosystem, as they are able to colonize a large amount of substrate and give way to early soil development (Gerson 1982, Gradstein et al. 2001, Nadhifah et al. 2018). In Mexico, the arid zones of the country comprise more than 50% of the territory and are characterized by low precipitation and extreme climate; however, these zones have a great variation in their life forms and plant communities (González 2012). The high elevations have not presented variations in the minimum temperature (CNA 2017). The area has presented warm periods mainly in the 1990s, so that the low elevations experienced higher temperatures than in the 1970s, while the average elevations of the 2010s registered cold temperatures. Low elevations recorded the wettest periods in the 1980s and 2010s, while the 1970s was the driest period. Similarly, the mid and high elevations of the 1960s and 1990s presented dry conditions (CNA 2017). In arid and semi-arid areas, bryophytes help in edaphic formation and stabilization, erosion prevention, surface soil isolation, enhancement of the chemical weathering process, microhabitat for invertebrates as well as facilitators for the colonization process of other vascular plants (Downing and Selkirk 1993, Eldridge 1993, Eldridge and Tozer 1997, Belnap and Lange 2001).
For Mexico, there is significant bryofloristic information in three main research axes: floristics, taxonomy, and phytogeography (Delgadillo and Equihua 1990, Delgadillo 2003). Besides, bryofloristic explorations have been carried out mainly in communities of the Transversal Volcanic Axis (Delgadillo 1987). However, despite their evolutionary and ecological importance such as water balance and nutrient cycling, the study of these plants in floristic terms in Mexico is still limited. In the present study, bryofloristic diversity and habitat preference were determined in an altitudinal gradient of the tourist area of the semi-arid region called Puente de Ojuela in the municipality of Mapimí, Durango.
Materials and methods
Study area
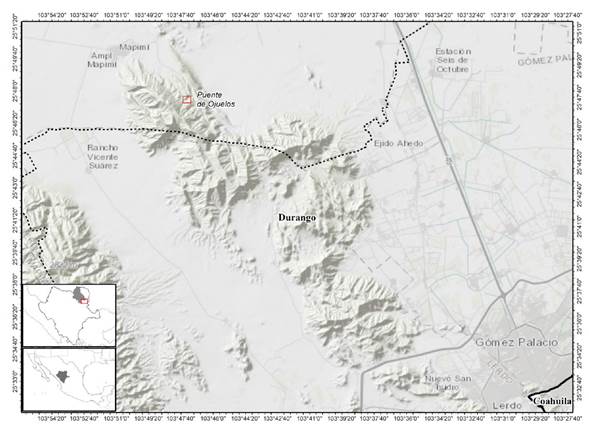
The tourist area called Puente de Ojuela is located in the sierra of Mapimí mountain range south of the municipality of Mapimí (Figure 1) at the coordinates 25° 48’ 3” north latitude, 103° 48’ 29" west longitude. It has an average elevation of 1 876 m (INEGI 2013). In general, high elevations (1 700-1 900 m a.s.l.) tend to be cold with temperatures of -5°C and more humid than the mid-altitudes (1 300-1 700 m a.s.l.) and low-altitudes (<1300 m a.s.l.) that are located in the study area (González-Elizondo et al. 2006). Cold periods have been recorded for the region during the 1980s at low and middle elevations, while the following decade occurred for high elevations.
Bryophyte sampling
The delimited area contemplated the tourist corridor that goes from the 1 200 until little more than 1 900 m a.s.l. Suitable places were sought where bryophytes could develop according to Delgadillo and Cárdenas (1990). For sampling, the site where the bryoflora was present was considered. The sample size was obtained through the collection criteria for moss according to Franco-López et al. (1996) as well as the size of the mat given that some samples had dimensions less than five cm2. The collected specimens were photographed and assigned a unique numerical record to form part of the photo library. Ecological data of date, cover (cm2), association with other plants, altitude, exposure, azimuth, abundance defined di visu criteria (very scarce, scarce, abundant and very abundant) and type of substrate where it develops were taken from each sample. The preparation of permanent flakes of each specimen was carried out for its subsequent characterization. For identification at the generic and/or species level, taxonomic keys from Crum and Anderson (1981), Allen and Crum (1994), Sharp et al. (1994) and Gradstein et al. (2001) were consulted. The nomenclature was following Delgadillo 2003. Finally, the samples were deposited in the JAAA Herbarium of the Faculty of Biological Sciences of the Juárez University of the State of Durango.
Estimation of bryofloristic diversity
Bryofloristic diversity is directly related to environmental conditions such as precipitation, temperature and topographic variables, so its distribution is aggregated, because the environment is heterogeneous and the limited availability of water resources forces taxa to concentrate in specific sites (Sun et al. 2013, González et al. 2017). Considering the aforementioned, we carried out a frequency distribution analysis to establish the altitudinal ranges. The number of altitudinal intervals or ranges, as well as the length of each interval were obtained according to the frequency distribution for a continuous variable described by Franco (2007).
Furthermore, specific diversity is an emergent property of biological communities that are related to the variety within them. This attribute is known as species richness, which is the number of species present in the community (Hurlbert 1971). The ideal way to measure specific richness is to have a complete inventory that allows us to know the total number of species (S) obtained by a community census (Moreno 2001). Under this criterion, diversity was analyzed by evaluating different indices according to the best fit the study.
Sampling effort
An analysis of the sampling effort was carried out in order to assess the real effort on the registration of the species. Species accumulation curves are a useful tool for estimating species richness (Chao 1984). The methods for estimating the diversity of a population are of two types: parametric and non-parametric. The latter’s use presence-absence data or abundance data focusing on infrequent or rare species (Colwell and Coddington 1994, Moreno 2001). The curves were obtained in Excel from presence-absence data evaluating the non-parametric estimators of Chao 1, Chao 2, Jackknife 1, Jackknife 2, ICE and Bootstrap with the purpose of observing differences of the real effort on the registry of the species.
Cluster analysis
The habitat preference of the species was determined from the ecological attributes of height, slope and azimuth degrees using the following sample statistics: mean, variance and standard error relating it to the t-Student test at a significance level of P < 0.05 (Brower and Zar 1998, Muro-Pérez et al. 2009) and “t” student’s factor. With the presence and absence data along the altitudinal gradient, a data matrix was elaborated assigning the value one for presence and 0 for absence (Gauch 1982, Manly 1986, Estrada-Castillón et al. 2011). The agglomerative polythetic hierarchical method was applied using the Sorensen similarity index (Gauch 1982, Estrada-Castillón et al. 2011) through the WPGMA clustering method with the minimum variance technique (Ward 1963) expressed as 2C/A+B, where C: number of common species in the sites under comparison, A: total number of species on site A, y B: total number of species on site B.
Results and discussion
Bryofloristic diversity through the altitudinal gradient
13 species belonging to three families, 14 genera and four varieties were recorded (Table 1), of which 15 belong to the Bryophyta division and two to the Marchantiophyta division. The remaining four species were identified to genus (17 total species), two of which belong to Anoectangium sp., and Weisiopsis sp. (mosses). The other two species belong to the hepatic group, Asterella sp. and Reboulia sp. The moss species identified belong to the Bryaceae and Pottiaceae families, while two species of liverworts are from the Aytoniaceae family.
Table 1 List of species found along an altitudinal gradient (1353-1850 m a.s.l.) in the tourist area Puente de Ojuela, Mapimí, Durango.
| Family | Genus | Specie | Altitude range |
|---|---|---|---|
| Bryaceae | Bryum | argenteum Hedw. | 5, 7 |
| Pottiaceae | Anoectangium sp. Schwaegr | 1, 2, 5, 6 | |
| Barbula | orizabensis C. Müller.* | 6, 7 | |
| Crossidium | crassinervium (De Not.) Jur. | 6 | |
| Didymodon | rigidulus var. gracilis (Schleich. ex Hook. & Grev.) Zand. | 5, 6 | |
| Didymodon | rigidulus var. rigidulus Hedw. | 5 | |
| Didymodon | vinealis var. brachyphyllus (Sull. in Whipple & Ives) Zand. | 1, 2 | |
| Husnotiella | revoluta Card. | 4, 5 | |
| Hymenostylium | recurvirostrum (Hedw.) Zand* | 5 | |
| Neohyophila | sprengelii. var. stomatodonta (Card.) Zand.* | 2 | |
| Pseudocrossidium | replicatum (Tayl.) Zand. | 1,5, 6 | |
| Syntrichia | bravipes (Lesq.) Broth* | 5, 6 | |
| Syntrichia | princeps (De Not.) Mitt.* | 4, 5, 6, 7 | |
| Tortella | humilis (Hedw.) Jenn.* | 5, 6 | |
| Weisiopsis sp. Broth. | 1,2, 3, 4, 5, 6, 7 | ||
| Aytoniaceae | Asterella sp. Beauv. | 2, 4, 6 | |
| Reboulia sp Raddi.* | 5, 6, 7 |
* New record for the arid and semi-arid zone of Durango. 1 = 1353-1423; 2 = 1424-1494; 3 = 1495-1565; 4 = 1566-1636; 5 = 1637-1707; 6 = 1708-1778; 7 = 1779-1850 m.a.s.l.
A histogram was prepared, which grouped the data into seven classes considered as altitudinal ranges of 70 meters each (Table 2). The greatest diversity of bryophytes was found between 1 637-1 707 m a.s.l. with 12 species, followed by the altitudinal range 1 708-1 778 with 11 species, followed by the interval 1779-1850 with seven species. The diversity indices of Margalef, Menhinick, Shannon and Simpson were evaluated to know the altitudinal range where the greatest bryophyta richness occurs (Table 3). Rank six is the one with the highest richness and rank three the one with the lowest species richness. Authorssuch asAh-Peng et al. (2012), Da Costa et al. (2015), Smith (2013) and Sun et al. (2013) mention that the number of species increases as the altitude increases. Lloret (1986) determined three tiers of bryofloristics diversity distributed in 102 species (78 mosses and 24 liverworts) and distributed along an altitudinal gradient in the Santa Fe of Montseny Valley in Barcelona, Spain. The first level from 100-450 m was defined by species with a wide range of distribution, both in altitude and humidity. The second level of 450-1000 m is formed by species with a preference for a certain altitudinal range (high or low) and finally, the third level of 1000-1600 m is formed by species linked to a certain level of humidity, where species that prefer dry or humid environments were found. In the present study, despite the fact that the bryofloristic diversity is totally different; the registered species presented a distribution preference in both humid and dry areas as reported Lloret (1986). Something similar was reported by Song et al. (2015), who established that the diversity calculated in 140 mosses and 86 liverworts in the mountains of Yunnan in China, is correlated with the availability of water and the altitude increase divided into three areas; sub-mountain (800-1 400 m a.s.l.), mountain (2 000-2 600 m a.s.l.) and sub-alpine (3 200-3 800 m a.s.l.). However, from 2 700 meters above sea level, the number of species began to decrease as in the present study, which, from the altitudinal range of 1 637-1 707, the richness decreased, coinciding with that reported by Morales (2009), Ramírez (2013) and Song et al. (2015). In this sense, Stevens (1992) mentions that in general the availability of water increases according to the altitude, so in the present study the diversity of bryophytes from 1707 m a.s.l., began to decrease because to the fact that at higher altitude the availability of water is reduced reflecting in a decrease in diversity. In the case of the semi-arid area of Durango, at altitudes of 1 700-1 900 meters above sea level, climatic conditions tend to present lower temperatures and higher humidity than in categories of 1 300-1 700 and 1 100-1 300 meters above sea level (CNA 2017). The geographical area where the Puente de Ojuela is located corresponds to a semi-desert (Sánchez et al. 2014), which presents historical natural variable conditions of temperature and precipitation on a scale of ten-year periods. This extreme variability is related to the torrential rainy season in summer that cause atmospheric and oceanic circulation patterns called Pacific decadal oscillation (PDO), which generate prolonged drought conditions, especially at elevations of 1 300-1 500 meters above sea level, as happened in the 60s and 90s or generate periods of extraordinary rainfall as in the 80s and 2010s (Stahle et al. 2016) that were reflected throughout the semi-arid region of Durango. The bryophytes in this area are perfectly adapted, possibly to the fact that stable thermal conditions are present at elevations above 1300 meters above sea level since the second half of the 20th century (CNA 2017), the main reason why in this region the taxa is particularly sensitive to changes in humidity that occur throughout the day and the rainy seasons. On the other hand, Bell (1982) concerns the presence of laminated papillae, rolled margins, thick shoreline and the presence of hyaline hairs at the ends of the lamina; this as adaptations that favor the establishment of bryophytes in arid and semi-arid environments as in the case of Crossidium crassinervium, Pseudocrossidium replicatum, Syntr ichia bravipes and S. princeps described in the present study.
Table 2 Distribution of bryophyte species along the altitudinal gradient.
| Altitude range (m a.s.l.) | 1353-1423 | 1424-1494 | 1495-1565 | 1566-1636 | 1637-1707 | 1708-1778 | 1779-1850 |
|---|---|---|---|---|---|---|---|
| Range | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Bryophyte species | 4 | 5 | 1 | 4 | 12 | 11 | 6 |
Habitat preference of bryoflora along the altitudinal gradient
The species accumulation curves showed that the non-parametric estimators behaved differently (Figure 2). These did not present a defined asymptote, however, the estimators that showed a high initial growth of the curve were ICE, followed by Chao 1, Chao 2 and Jackknife 2 (Figure 2). The estimators that most closely approximated the asymptote of species richness were Bootstrap and Jackknife 1, these in turn, showed less bias because both the estimator and the species richness behave in a similar way.
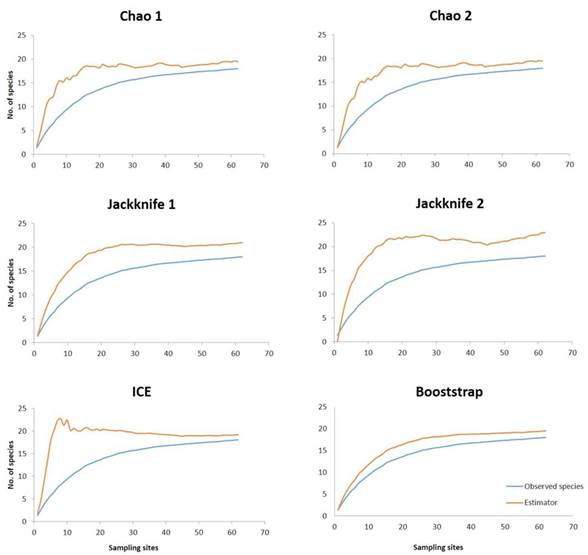
Figure 2 Species accumulation curves of observed richness and richness estimated by six non-parametric estimators for bryophytes according to increasing sampling efforts. The orange line of the graph indicates the estimator evaluated and the blue line indicates the species observed.
A correlation of r = 0.95 was obtained and two main groups with four subgroups were defined. Group I present a similarity of 25% (15 species) with respect to group II with 18% similarity (7 species), however, these groups share genera such as Anoectangium, Didymodon and Weisiopsis. Group I. It is formed by four altitudinal ranges where five species are shared, originating two subgroups (1a and 1b) and contains most of the registered species. Subgroup 1a. It shares a 52% similarity (15 species) distributed between the altitudinal ranges of 1 637-1 707 and 1 708-1 778. Subgroup 1b. It shares a 35% similarity since only five species are distributed between the altitudinal ranges 1 566-1 636 and 1 779-1 850. The genera and species that are shared in the group are Asterella sp., Bryum argenteum, Syntrichia princeps and Weisiopsis sp. Group I was formed from the dissimilarity of 10 species (Table 4). Group II. It is made up of three altitudinal ranges: 1 353-1 423, 1 424-1 494 and 1 495-1 565. This was formed by the absence of the taxa Didymodon vinealis var. brachyphyllus and Neohyophyla sprengelii var. stomatodonta in the group I (Table 4). This group is made up of two subgroups. Subgroup 2a. It is made up of a single species, constituting the group with the greatest dissimilarity. Subgroup 2b. It shares 46% similarity since 33% of the species are distributed between the altitudinal ranges 1 353-1 423 and 1 424-1 494 (Figure 3). Delgadillo et al. (2022) mention that bryophytes inhabit diverse substrates such as rocks, soil, tree trunks or branches, preferring humid and protected places. In this regard, Delgadillo and Cárdenas (1990) consider that bryophytes can be found in numerous micro environments and highly variable conditions, as in the case of bryophytes in the Puente de Ojuela. According to García and Mercado 2017, climatic variations are considered filters to determine the assembly mechanisms of the diversity of taxa in a plant community. In this sense, Pinzón and Linares (2006) determined that bryophytes, mainly from families Pottiaceae y Bryaceae, they are found from environments exposed to the sun to certain less exposed microclimates, which generates compact mats of variable size where scrub areas and glens present a wide coverage. In the current study, it was observed that bryophytes have a certain preference for less exposed environments, with preferential development on rocks and fissures of these. On the other hand, at higher elevation, where there is high radiation, temperature fluctuations and edaphic variations; the development of arboreal plants is limited, however, in the case of the bryoflora it seems that these conditions favor it especially in places with open spaces (rocks and fissures or crevices) where competition with other plants is practically non-existent (Delgadillo and Cárdenas 1990). In the present study it was observed that most of the bryophyte mats were found mainly on rocks and fissures where vascular plants cannot develop. This condition allows them to be pioneers or colonizers of disturbed sites that give way to the establishment of other vascular plants (Delgadillo and Cárdenas 1990). In this regard, moss mats were found growing in disturbed sites where mining extractions were carried out, even becoming the most representative sites of mosses. According to Rzedowski (1968), arid climate regions with topography characteristics, geological substrate and soil exert a greater influence on the distribution of the vegetation that the rainfall itself: for what could be the reason the mosses of the Puente de Ojuela have a narrow preference for shady sites compared to sites with the presence of water.
Table 4 List of species not shared between the main groups.
| Cluster group | Genus or Species |
|---|---|
| I | Brium argenteum Hedw. |
| Barbula orizabensis C. Muller. | |
| Crossidium crassinervium (De Not.) Jur. | |
| Husnotiella revoluta Card. | |
| Didymodon rigidulus var. gracilis (Schleich. Ex Hook. & Grev.) Zand. | |
| D. rigidulus var. rigidulus Hedw. | |
| Reboulia sp. Raddi. | |
| Syntrichia bravipes (Lesq.) Broth. | |
| S. princeps (De Not.) Mitt. | |
| Tortella humilis (Hedw.) Jenn. | |
| II | Didymodon vinealis var. brachyphyllus (Sull. In Whipple & Ives) Zand. |
| Neohyophyla sprengelli var. stomatodonta (Card.) Zand. |
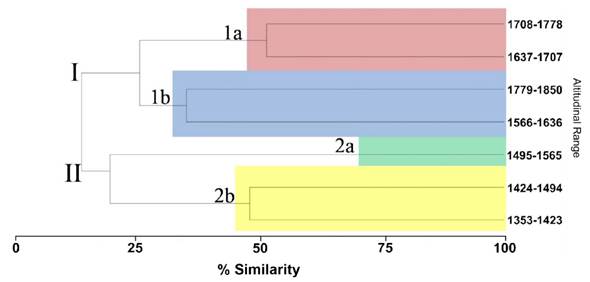
Figure 3 Dendrogram that shows the grouping of the altitudinal ranges in relation to data on the presence and absence of the bryoflora along the altitudinal gradient in the tourist area of the Puente de Ojuela.
The bryofloristic diversity of the Puente de Ojuela tourist corridor increases according to the altitude increase, rather than with the presence of sites with water. That is why the habitat preference of bryophytes in semi-arid environments is not restricted solely to the availability of water since factors such as exposure, slope and altitude are key to their diversity. The taxa in this region showed distribution preferences in an area with extreme climatic conditions, where the solar incidence is indirect and turning them into species of the sciophilic type. Bryoflora is considered a bioindicator of ecosystem health. For this reason, the present diversity could be indicating that, despite being a tourist area with high recurrence, it remains conserved in a certain way. It is necessary to carry out studies on bryophytes to determine the ecosystem role under which they are distributed in the semi-arid zone either as bio-indicators or bio-accumulators. The present study can be considered a pioneer in the knowledge of the bryoflora of semi-arid environments for Durango and a fundamental basis for new lines of research in this taxon.











 nueva página del texto (beta)
nueva página del texto (beta)