About 40 % of the tropical and subtropical land on Earth is covered by forests, of which 42 % are Tropical Dry Forests (TDF) (Murphy and Lugo 1986). Although generally speaking these ecosystems house a lower biological diversity than tropical rain forests (Gentry 1995), in some areas they have equal or even higher plant diversity than some moist forests (Hubell 1979; Gentry 1982; 1988; Janzen 1988). While tropical rain forests have received considerable attention from conservation biologists over the last decades, TDF have only recently become visible on the conservationists’ maps, where their high deforestation rates are a source of concern. In Latin America 12 % of the area originally covered by TDF was lost between 1980 and 2000 (Miles et al. 2006). In Mexico, of the ca. 270,000 km2 of TDF that originally covered its territory, only 27 % remain as intact forest (Trejo and Dirzo 2000). Most of these forests have been transformed to agricultural fields and pastures for cattle ranching. When abandoned, these areas may take decades to recover, or may not recover at all given the profound soil compaction and erosion they have suffered, aggravated by the highly seasonal and torrential rainfall pattern characteristic of these areas (Murphy and Lugo 1986). Therefore, restoration programs seem the appropriate option for these situations.
Restoration ecology is based on the idea that ecological succession may be assisted to reestablish ecosystem functioning (including species composition, biotic interactions, and ecosystem processes) to a point in which it resembles the pre-disturbance conditions, particularly where this is not happening naturally (Luken 1990; Noss 1990; Lamb 1998). A strong emphasis has been placed on the use of native species for these purposes, as they are adapted to the local climatic conditions and may have a positive impact on the ecosystem by attracting local fauna (Montalvo et al. 1997; Lamb 1998), facilitating recruitment of other plant species, and decreasing soil erosion (Lamb 1998; D’Antonio and Meyerson 2002). Also, when chosen native species are useful to local inhabitants, it is more likely that they will participate in tending them, thus aiding in their establishment. Nevertheless, if the land subject to restoration is too degraded, the reestablishment of original vegetation elements becomes highly unlikely, notwithstanding being assisted (Murphy and Lugo 1986). In such situations, ecologists may choose to use stress-tolerant native species. Yet, most stress-tolerant plants are relatively slow growing (Lamb et al. 2005; Taiz and Zeiger 2006), therefore their use in restoration programs is sometimes discouraged.
Considering all of the above, some species in the Agave L. genus may prove useful for the ecological restoration of Mexican TDF. They exhibit a host of biological features that make them potentially successful: they are adapted to withstand relatively stressful environmental conditions (e.g. drought, high temperatures, direct solar radiation, low nutrient availability), they are relatively fast growing compared to other stress tolerators, many propagate vegetatively and some may offer valuable resources to local inhabitants (e.g. fibers, alcoholic beverage production - Gentry 1982; Nobel 1988; Colunga-García Marín et al. 2007). In addition, they perform an important role at the community level as nurse plants and as a food source for pollinators (Gentry 1982; Nobel 1988). As Mexico is the center of origin and diversification of the whole Agavaceae family, there are many species that can be used for restoration purposes (García-Mendoza and Galván 1995; Eguiarte et al. 2000; García-Mendoza 2007). A few species have already been used in soil erosion control programs in severely degraded areas (CONAFOR 2007; Sinisterra et al. 2011).
Agave angustifolia Haw. is commonly found in seasonally dry shrublands, TDFs and Quercus-Pinus forests from the north of Mexico to Central America (Gentry 1982; CONAFOR 2012). This species is locally used for mezcal production (see below). A restoration program carried out in Tembembe, in central Mexico (see Study Site below) is considering the use of A. angustifolia for ecological restoration. However, the performance of its populations at the site must first be examined to evaluate the potential success of such restoration efforts.
Currently, plant population dynamics are generally modeled through population projection matrices. Such matrices contain the probability of individuals in each state category remaining in that category and/or moving or contributing to other categories from one year to the next (Caswell 2001). When categories are expressed in terms of plant size, the resulting matrix is called a Lefkovitch matrix (Lefkovitch 1965). Matrix analysis consists of iterating the matrix (multiplying it by a vector representing population structure and then by the resulting vectors) until it reaches a stable population structure (corresponding to the right eigen-vector of the matrix) and a stable finite population growth rate value (λ, which corresponds to the dominant eigen-value of the matrix). Thus, matrix analysis offers a description of the potential behavior of the studied population if the present demographic conditions were to remain constant over time (i.e., a projection); it provides information on whether the population would potentially grow (λ > 1), or decrease (λ < 1) or remain numerically stable (λ = 1) (Caswell 2001). It also offers valuable theoretical information on how λ would be expected to change if matrix entries were altered (i.e. elasticity analysis; de Kroon et al. 1986), and retrospectively, how λ actually changed given the observed variation in matrix entries (i.e. life table response experiments, LTRE; Tuljapurkar and Caswell 1997; Caswell 2001). Additionally, matrices may be used to carry out ‘theoretical experiments’ (i.e. numerical simulations) in which particular matrix entries are modified in specific ways to simulate different management strategies, and the potential results of such strategies are evaluated by the response of λ to the simulated changes (Contreras and Valverde 2002).
As any other modeling tool, population projection matrices have limitations. The information contained in a matrix reflects the population dynamics (driven by births and deaths only, ignoring migration) in a specific time period, and the projection of this behavior through time assumes that it remains constant, which is clearly an unrealistic assumption. With full awareness of these limitations, projection matrix results may be properly interpreted and used as an aid for the management and conservation of natural populations (Crone et al. 2011).
In this study we address the demographic behavior of two natural populations of Agave angustifolia, one in a well preserved TDF, and another one at the site under restoration: a pasture area that has long been used for cattle ranching and that was originally covered by TDF. We followed the fate of individuals from one year to the next and built two Lefkovitch matrices. These were used to project the numerical behavior of the populations through time and calculate the finite population growth rate to which they would converge. In addition, retrospective and prospective analyses (elasticity matrices and Life-Table Response Experiments - LTRE) as well as numerical simulations were used to evaluate the potential results of different management practices at the restoration site (Illsley et al. 2007). The results of these analyses will throw light into the population processes that are relevant for restoration and that would increase the likelihood of success of future restoration programs based on this species.
Materials and methods
Study species. Agave angustifolia (Agavaceae) produces large rosettes with green-grayish narrow leaves (60-120 cm long), which are stiffly and erect and show moderately spaced lateral spines and a terminal dark spine. Large rosettes frequently produce lateral shoots via rhizomes resulting in vegetative propagation; thus, although individual rosettes are semelparous, genets may be iteroparous. Inflorescences are produced from January to May; they are paniculate, 3-5 m tall, and bear greenish-yellow flowers. Fruits ripen between May and July; they are brownish ovoid capsules, ca. 5 cm long and 3 cm wide. Seeds are black, flat, and triangular in shape, ca. 9-12 mm × 7-8 mm. Each fruit may produce up to ca. 150 seeds.
Agave angustifolia has a relatively widespread geographic distribution, spanning from northern Mexico to southern Costa Rica. It is found in xerophytic shrublands, tropical dry forests and Quercus-Pinus forests (Gentry 1982; CONAFOR 2012). As this species is traditionally used for mezcal production (mezcal is an alcoholic spirit similar to tequila), it is frequently favored by local peasants, and in some areas -e.g. in the Mexican state of Morelos- it is actively cultivated. Cultivation is often carried out by replanting vegetatively produced offshoots. Mezcal production involves severing the sprouting inflorescence and extracting whole plants, from which the leaves are removed in order to process the stem (called ‘piña’) by fermentation and distillation (Gentry 1982; Palma 1999).
Study sites. This study was carried out at two different sites in the Mexican state of Morelos, near Cuernavaca city (ca. 70 km south of Mexico City; Figure 1). These sites differ in various aspects: they have slightly different climate and vegetation types, and they also differ in their land use history and degree of conservation.
Figure 1 Location of the two study sites in the Mexican state of Morelos. The state’s capital city is Cuernavaca, marked with a star.
The first study site is located within the Ecological Restoration Station of the Tembembe River Basin (referred to as Tembembe from now on). This is a transitional zone that spans an altitudinal gradient from 1,700 m, where temperate forests are usually found, to 1,500 m, where TDF are common (Figure 1). The climate is warm and seasonally dry, with a summer rainfall period; mean annual temperature is 21.6 ºC and annual precipitation is ca. 960 mm (according to the Cuentepec Meteorological station, located at an altitude of 1,487 m; Camacho 2004). Its lower part is slightly warmer and dryer that the higher one. The predominant soils are haplic feozems followed by pelic vertisols. The terrain has slopes from 5 to 50 % (Camacho-Rico 2004; Bonfil et al. 2016). The ecotone vegetation includes some 153 plant species (in 98 genera and 42 families). Leguminosae is the most abundant plant family (25.5 % of the species), followed by Asteraceae (15 %), Rubiaceae (5.8 %) and Burseraceae (5.2 %). The most common shrub species are Lantana hispida and Acacia farnesiana. This site is currently a pastureland used for cattle grazing (its main land use at least for the last ca. 200 years, according to the records found by Alavez-Vargas 2010), and is considerably degraded. Only the inaccessible parts of the canyons (on very steep slopes), where the remaining oak and tropical dry forests are distributed, are relatively well preserved (García-Flores 2008). Part of the Tembembe site (97 ha) was protected by a fence in 2008; thus, the fenced area has been free of cattle and the vegetation has been recovering since then. The Agave angustifolia population studied at this site was within this cattle exclusion.
The well preserved site is located in the surroundings of the archeological site of Xochicalco, Morelos, which was fenced when declared a protected area in 1994 (Figure 1 - Diario Oficial de la Federación 1994). It is at an altitude of 1,199 m. The climate is warm and seasonally dry, with a summer rainfall period; mean annual temperature is 22.87 ºC and annual precipitation is 1,055 mm (Camacho-Rico 2004). The soils are rendzinas and the terrain has slopes between 25 and 48 % (CETENAL 1976 a,b). The vegetation at Xochicalco main archaeological site is a secondary TDF in a good conservation status. The best represented plant families are Leguminosae, Burseraceae, Asteraceae, Anacardiaceae, Sapindaceae and Convolvulaceae, and the most important species are Bursera copallifera, B. glabrifolia and Lysiloma divaricatum (Piña 2005).
Field Methods. In this section we describe the different experiments performed to obtain the main demographic data needed to build the population projection matrices for each site, in particular for the individuals in the early life-cycle stages, which are generally scarce in the field. We also wanted to gain a deeper understanding of the factors that affect these processes in natural conditions (i.e. differences between study sites, and between microsites with distinct characteristics), since restoration programs usually work precisely with these life-cycle stages. In addition, we describe how we used our observations of the fate of juveniles and adults to estimate the relevant matrix entries.
a) Early life-cycle stages. At the end of May 2010, just before the onset of the rainy season, we carried out a germination experiment to evaluate the probability of germination under natural conditions. At each site we collected fruits from different agaves that were in the sampled area. In Tembembe we collected 17 fruits from two individuals and in Xochicalco 29 fruits from three individuals. The seeds of all the fruits collected in each area were mixed and groups of 50 seeds were scattered on the bare soil in exposed microsites (five replicates) and protected microsites (five replicates; under the crown of a nurse plant, mainly Acacia spp. or Lysiloma spp. trees). To prevent seeds from being carried away by wind or water flow, each seed group was scattered within a fine metal mesh cylinder (20 cm in diameter) that had been previously attached to the soil (the mesh did not protect seeds from predators). Seed germination was recorded monthly during the rainy season (from June to August).
Also, a seedling establishment experiment was performed. Seeds of Agave angustifolia were collected from the study sites (see above) and sown on a mixed substrate (black soil and agrolite, 3:1) in February and April 2009, and were kept in a greenhouse until July 2009. The resulting seedlings were temporarily transplanted to small pots and then taken to the field. Thus, we had seedlings of two different ages (three and five months old), which were introduced in Xochicalco and Tembembe in the same contrasting microsites considered in the germination experiment: a) exposed microsites, and b) protected microsites (under the crown of Acacia spp. or Lysiloma spp. trees). Five replicates of ten three-month old seedlings, and five replicates of five-month old seedlings were introduced in each microsite and study site. The survival of these seedlings was recorded monthly for a year. Seed germination and seedling survival data were used to estimate some entries of the Lefkovitch matrices, as explained below.
b) Survival, growth and fecundity of juvenile and adult individuals. In both Tembembe and Xochicalco, a population sample of Agave angustifolia individuals were located, tagged and measured to register their growth, survival and reproduction between May 2009 and May 2010. In Xochicalco, individuals of A. angustifolia were founded in specific areas with varying densities; different sized transects (permanent plots) were drawn in each of these areas to sample the population at this site. A total of 204 A. angustifolia individuals were found within a sample area of ca. 5,094 m2; individuals in each transect were tagged and their positions recorded through x,y coordinates in order to relocate them the following year. In Tembembe, the distribution of A. angustifolia individuals was rather spread out, thus a total sample area of ca. 8,276 m2 was covered in which 215 individuals were found and their positions recorded with a GPS. In May 2009, all the sampled individuals at the two study sites were measured (rosette diameter) and their reproductive status was recorded. In addition, we also counted the number of offshoots (daughter rosettes) emerging from each adult rosette.
In May 2010, all sampled plants were relocated and their diameter was measured again and new individuals from sexual (seedlings) and vegetative (offshoots) origin were searched for in the study plots. These data were used to construct two Lefkovitch matrices (see below).
In addition to survival and growth data, we also recorded reproduction. In May 2009, our original population sample (204 and 215 individuals in Xochicalco and Tembembe, respectively) had only a few reproductive individuals. Several agaves show mast seeding years in which many individuals reproduce, followed by years in which only a few individuals reproduce (Arizaga and Ezcurra 1995) and 2009 was not a mast seeding year for Agave angustifolia. Thus, for this section of the study we needed to expand our sampled area to include a higher number of reproductive plants and estimate reproductive probabilities per category. Therefore, the fecundity values were estimated from a sample of 235 and 240 individuals (in Xochicalco and Tembembe, respectively). All individuals - from all size categories - found in the expanded area were quantified. We also recorded the number of fruits produced per inflorescence (in a sample of seven plants) and the number of seeds per fruit (in a sample of 44 fruits) to estimate the fecundity value (as described below).
Numerical Methods. In this section we describe statistical analyses performed on the data obtained from the seed germination and seedling establishment experiments. We also describe the procedures followed to build and analyze the population projection matrices, from which the λ values were obtained to evaluate the performance of the two populations studied. Additionally, other analyses (elasticity, LTRE, and numerical simulations) were carried out to identify the most critical demographic processes and life-cycle stages in the population, and to theoretically test the potential results of different management strategies, all aimed to inform future restoration programs.
a) Early life-cycle stages. A two-way ANOVA was used to test the effect of site (Xochicalco and Tembembe) and microsite (exposed and protected) on seed germination percentage at the end of the rainy season (arcsine transformed for normality). A three-way factorial ANOVA was used to evaluate the effect of site (Xochicalco and Tembembe), microsite (exposed or protected) and age (three and five months old) on seedling survival percentage (arcsine transformed for normality). Both seed germination and seedling survival probabilities from these experiments were incorporated in the demographic analyses as components of particular matrix entries, as explained below.
b) Projection matrix analysis. To construct the Lefkovitch matrices, we subdivided the population in six size categories, according to rosette diameter (Table 1). We followed the fate of individuals in each category and estimated the probability of remaining in the same category, moving to a different one (by growing to a larger size category, or regressing to a smaller size category due to a reduction in plant diameter after the loss of leaves, for instance), or dying, from the proportion of individuals following each fate from May 2009 to May 2010. We also recorded offshoot production (emergence of daughter rosettes), as well as reproduction, and integrated them in the matrices.
Table 1 Size categories defined to evaluate the population structure and construct Lefkovitch matrices for the Agave angustifolia populations studied.

Fecundity was estimated as the mean number of seedlings that would potentially emerge in May 2010 resulting from the reproduction of individuals and the germination of seeds in 2009. Its calculation comprised four variables: the probability of reproduction of individuals in category x (termed R x ) obtained as the proportion of reproductive individuals in the category in May 2009; the average number of seeds that would be produced by a reproductive individual in category x (termed S x ), estimated by multiplying the mean number of fruits per inflorescence times the mean number of seeds per fruit (in Xochicalco, reproductive plants produced an average of 335 fruits per plant, and 151 seeds per fruit; while in Tembembe reproductive plants produced an average 293 fruits per plant, and 112 seeds per fruit). Finally, we obtained the probability of seed germination (termed G) and seedling survival in the field (termed P) from the summer of 2009 up to May 2010 from the field experiments described above, for each site. For the estimation of G and P, we averaged the results of the protected and exposed microsites, assuming that seeds may fall evenly in either type of microsite. Thus, the fecundity values (F x ) incorporated in the Lefkovitch matrices were given as:
As no seedlings were observed in the field, the survival and transition probabilities for the seedling category were also obtained from the results of the field experiment. And finally, the production of new rosettes was also incorporated in the matrices as the average contribution of adult individuals to the second size category.
Matrices were analyzed to obtain their dominant eigen-value (finite population growth rate, λ) and their right and left eigen-vectors (stable population size structure and size specific reproductive values, respectively). The λ values of the two matrices were compared given their confidence intervals. The latter were obtained through the bootstrap resample technique, in which 2000 new matrices and λ values were calculated to estimate the actual distribution for λ and its 95 % confidence intervals (as described in Caswell 2001). To evaluate the differences between the stable and the observed population structures, and between the observed population structure in 2009 and 2010, paired chi-square tests were run.
c) Prospective analyses: elasticity. The elasticity measures the proportional sensitivity of λ to proportional changes in matrix entries. As the sum of all the elements of the elasticity matrix equals unity, each entry of the elasticity matrix may be interpreted as the proportional contribution of its related a i,j to λ. The entries of the elasticity matrices were obtained as described in de Kroon et al. (1986) and Caswell (2001).
d) Retrospective analysis: life-table response experiments (LTRE). This analysis assesses the contribution of each matrix entry to the actual variation in λ. It involves building a contribution matrix in which each entry represents the contribution of its respective a i,j to an increase or a decrease (i.e. positive or negative contributions, respectively) in the λ value, compared to a reference average matrix. The contribution matrices for each site were calculated as follows (Pulido et al. 2007):
where α i,j (m) is the value of the contribution of each a i,j of the matrix of the site m; a ij (m ·) is the a i,j of a matrix obtained by averaging the transition matrix of the site m with the average matrix obtained from both sites (A (..) ); a i,j (··) is the a i,j of the average matrix; and s i,j (m·) is the sensitivity of a i,j (m ·).
e) Numerical Simulations. Agave angustifolia is commonly used for mezcal production. Therefore, in some natural populations, the flowering stalks are severed during their early developmental stages, and entire plants are later extracted, which results in a decrease in fecundity. To explore the effect of this extractive management in the Tembembe population (which is where extraction takes place), numerical simulations were performed on the population projection matrix for this site. This simulation consisted in decreasing fecundity entries by 20, 50, 80 and 100 % compared to their original value, and recalculating λ for each new matrix.
Additionally, numerical simulations were carried out to evaluate the potential effect of juvenile introduction (plants in the second category) as part of an ecological restoration project in Tembembe. The matrix for this site was modified to simulate the introduction of 10, 50, 100, 500 and 1,000 juveniles in the sample area. After each matrix modification, λ was recalculated.
Results
Early life-cycle stages. Final seed germination percentage was higher in Tembembe than in Xochicalco (F = 5.301, d.f. = 1, 16, p = 0.035) and was favored by the presence of nurse plants (F = 30.076, d.f. = 1, 16, p < 0.00001). The interaction between site and treatment was also significant (F = 18.674, d.f. = 1, 16, p < 0.0001): in Xochicalco the contrast between seed germination in the protected and the exposed sites was very dramatic (52.40 and 7.20 %, respectively), while in Tembembe the effect of microsite was considerably attenuated (39.20 % in protected and 32.0 % in exposed sites, Figure 2).
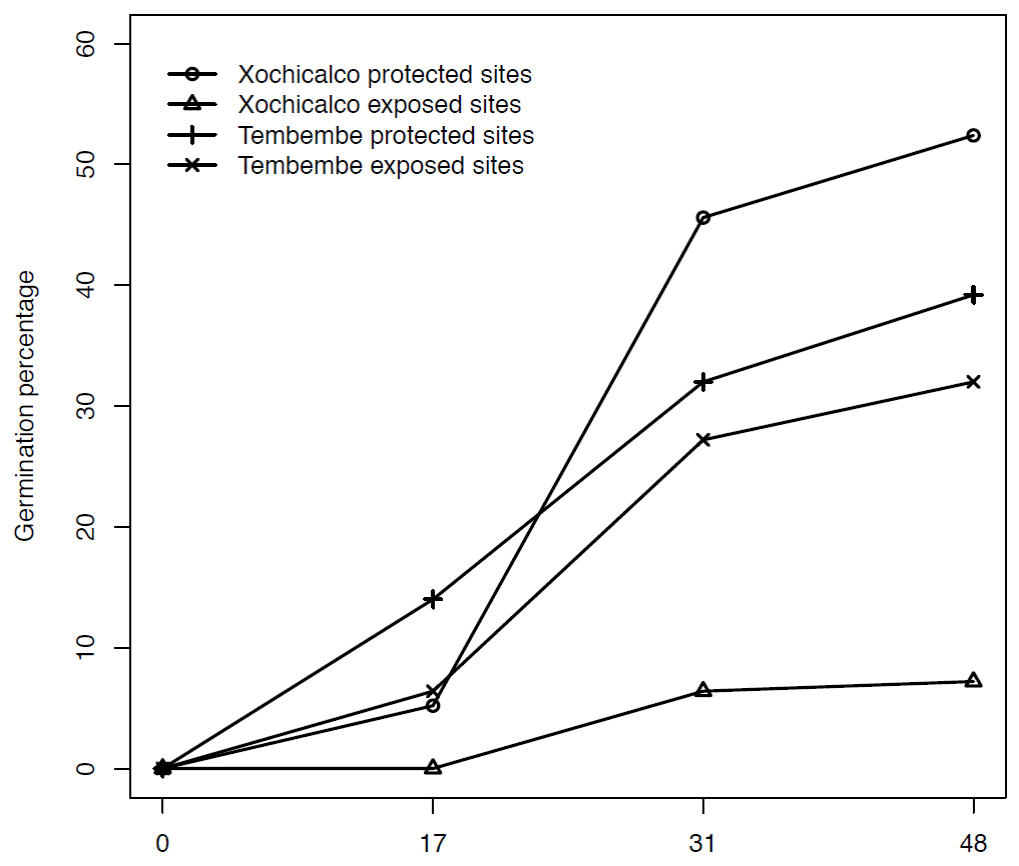
Figure 2 Cumulative germination percentage of Agave angustifolia seeds in two treatments (protected and exposed sites) in Xochicalco and Tembembe. The horizontal axis shows the number of days after the start of the experiment: 0 (May 21), 17 (July 7), 31 (July 21) and 48 (August 8, 2009) days.
Regarding seedling survival, the seedlings at Tembembe had a higher survival rate than those planted in Xochicalco (F = 19.185, d.f. = 1, 32, p < 0.001). The interaction between site and treatment was also significant (F = 4.617, d.f. = 1, 32, p = 0.039): the survivorship was highest in the protected sites at Tembembe. Finally, the effect of microsite and seedling age were marginally significant: there was a tendency for seedlings to show a higher survival rate in protected than in exposed microsites (F = 4.008, d.f. = 1, 32, p = 0.053), and five month old seedlings had a slightly higher survival rate than three-month old ones (F = 3.067, d.f. = 1, 32, p = 0.089).
In Xochicalco, three-month old seedlings had a very high mortality rate during the first month in exposed microsites, and then they all died the following month (Figure 3a). On the other hand, in these microsites five-month old seedlings had a high mortality rate during the first month (80 %), but most of the remaining seedlings survived until the following April (Figure 3a). In the protected microsites at Xochicalco seedlings died more gradually; however, at first three-month old seedlings had a slightly higher mortality rate compared to five-month old seedlings (Figure 3a). Yet, by May all five-month old seedlings had died in the protected microsites at this location, while the three-month old ones at these microsites showed an 8 % survival at the end of the experiment (Figure 3a).
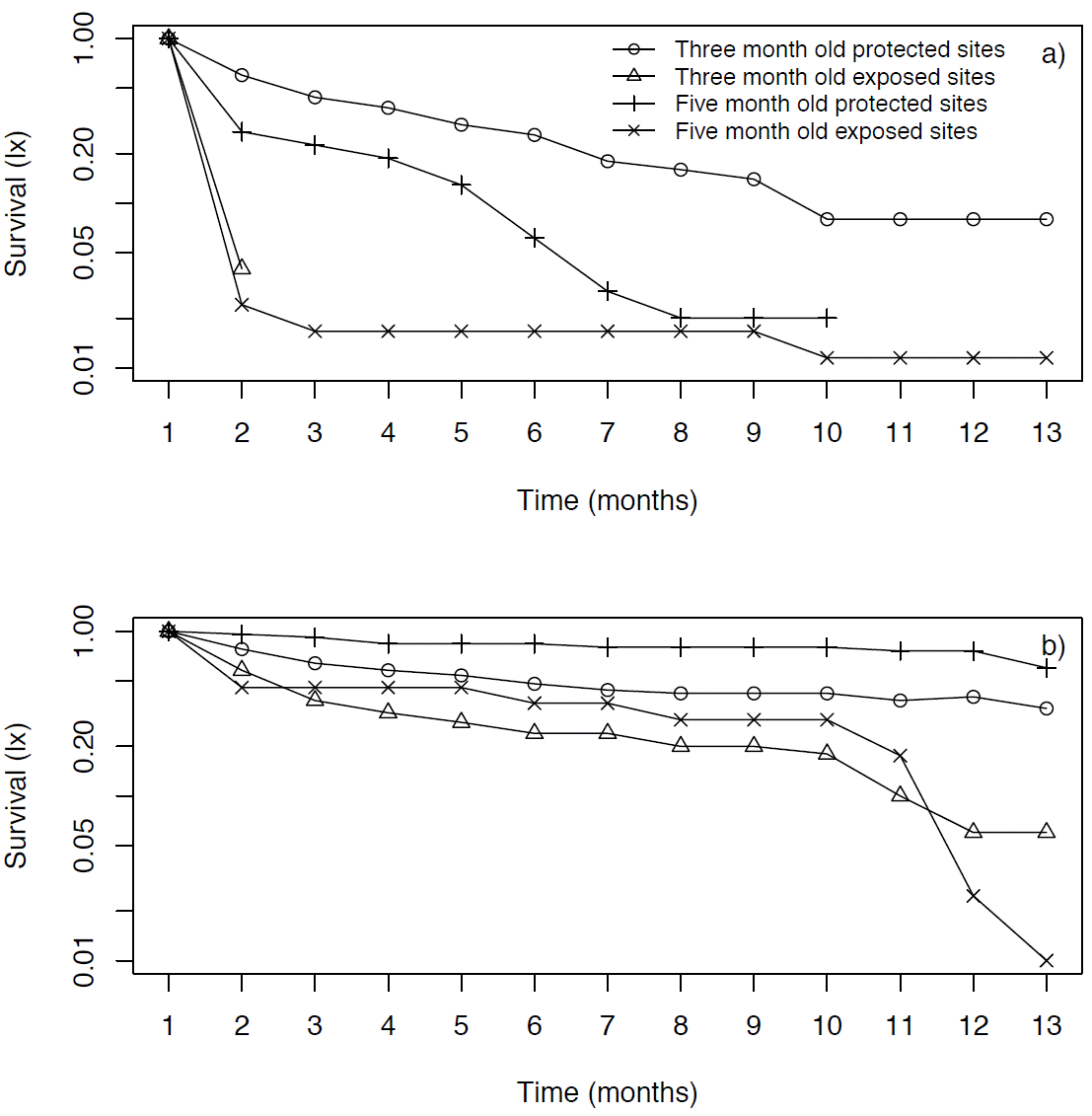
Figure 3 Survivorship curves (lx, on a log scale) of Agave angustifolia seedlings of different ages introduced to the field in July 2009, in two types of microsites (exposed and protected), at Xochicalco (a) and Tembembe (b).
In Tembembe, three month old seedlings in both protected and exposed sites died gradually, but in exposed sites they died more rapidly (Figure 3b). The five month old seedlings in exposed sites had a high mortality rate during the first two months and then died gradually, having a final survivorship of 36 % (Figure 3d), while seedlings in protected sites had the highest final survivorship (60 %; Figure 3d).
Population structure and density. Population density was significantly higher at Xochicalco (5.90 plants/100m2) than in Tembembe (2.40 plants/100m2; t = 4.53, d.f. = 9, p < 0.001). In both populations the smallest size category was the least frequent, while plants in the intermediate size categories dominated numerically with a relative decrease of individuals in the largest size category (Figure 4). The chi-square test showed that there were no significant differences in the population size structure observed in 2009 and in 2010, neither in Xochicalco (χ2 = 9.60, d.f. = 5, p = 0.08), nor in Tembembe (χ2 = 2.90, d.f. = 5, p = 0.71).
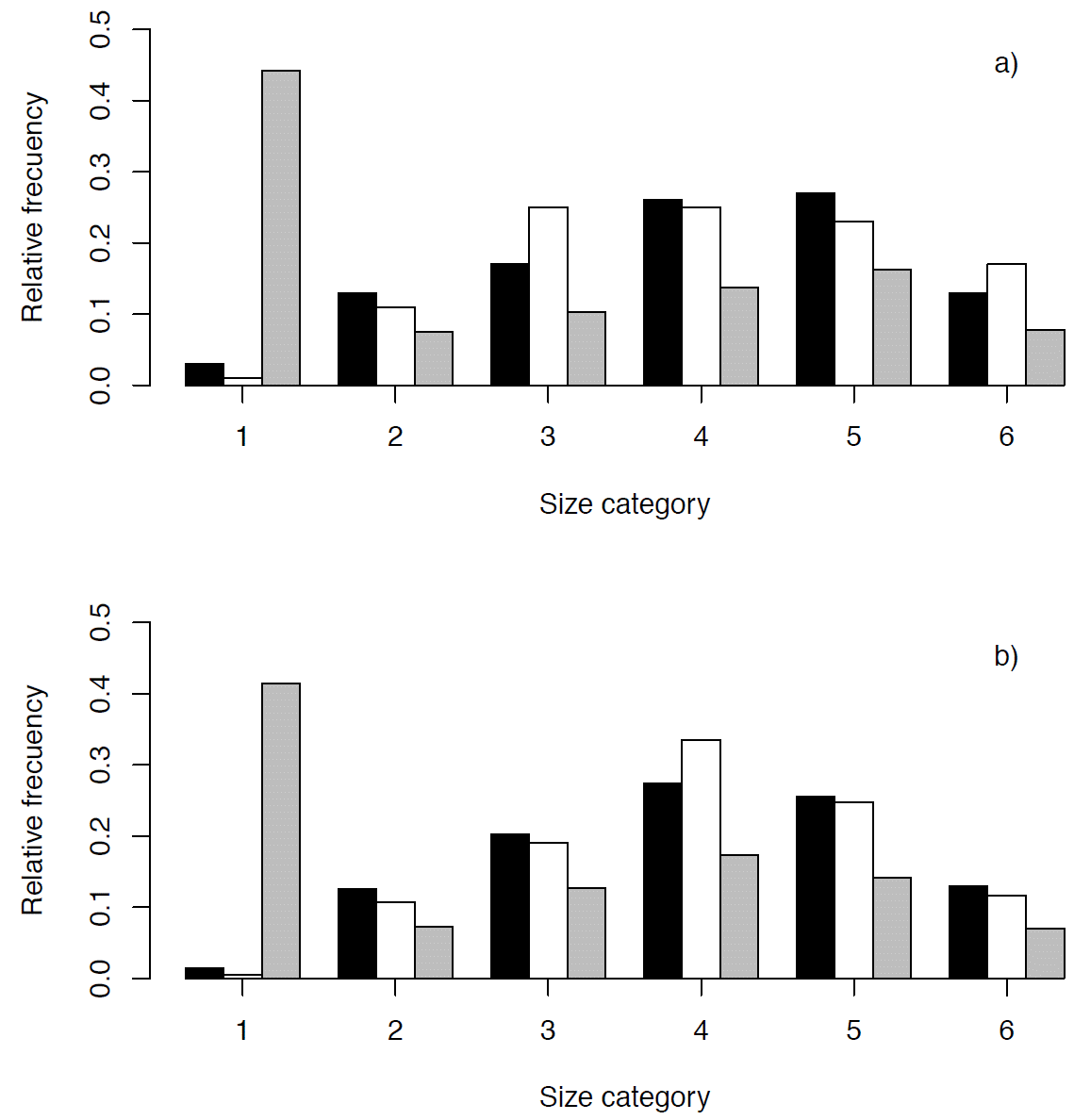
Figure 4 Size structure of Agave angustifolia populations at Xochicalco (a) and Tembembe (b), in 2009 (observed, black bars), 2010 (observed, white bars), and stable (i.e., projected, gray bars).
Reproduction. The proportion of reproductive individuals was quite low in both populations. In Tembembe only four individuals (out of 240) were observed reproducing in early 2010, one in size category 5 and three in category 6. In Xochicalco only three individuals (out of 235) were observed reproducing, all in size category 6. The mean number of fruits per reproductive plant was 336.7 ± 5.0 s.d. in Xochicalco and 292.2 ± 349.2 s.d. in Tembembe, with no significant differences between them (t = -0.24, d.f. = 5, p < 0.82). The number of seeds per capsule was significantly higher in Xochicalco than in Tembembe (Xochicalco: 151 seeds/fruit ± 43 s.d., n = 28 fruits; Tembembe: 112 seeds/fruit ± 51 s.d., n = 17 fruits; t = 2.59, d.f. = 42, p < 0.01), which suggests that pollination efficiency is lower in the latter.
Population projection matrices. Population dynamics differed in several aspects between sites. The highest mortality was observed in the first size category in both sites, but it was substantially higher in Xochicalco than in Tembembe (Table 2). In contrast, the rest of the size categories had a much lower mortality rate, which tended to be higher in Tembembe than in Xochicalco. Also, important differences between sites were observed with regard to reproduction. The relatively higher seed germination rates and seedling survival probabilities in Tembembe resulted in much higher fecundity values at this site compared to Xochicalco.
Table 2 Population projection matrices for Agave angustifolia at Xochicalco (a) and Tembembe (b). The projected λ values are given above each matrix (along with their 95 % confidence intervals). The stable size structure (vector w) and the size-specific reproductive values (vector v) are reported. The two bottom lines refer to the size-specific mortality rate (qx) and sample size per category (N). Matrix entries marked with * correspond to vegetative propagation, while those marked with + are compound (i.e. vegetative propagation and retrogression).
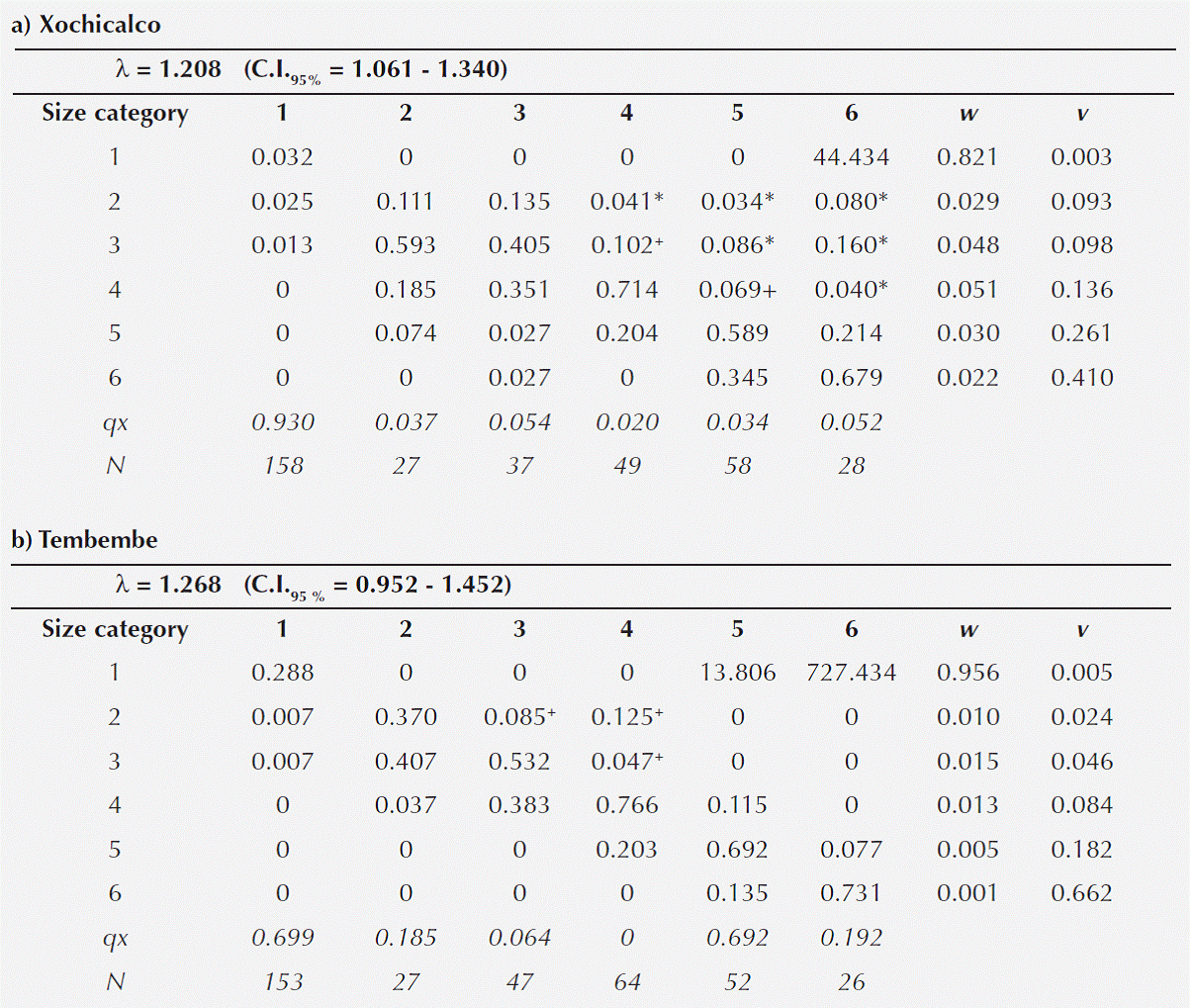
Vegetative spread through the production of daughter rosettes was observed in both populations. This process is reflected in the contribution of individuals in categories 4, 5 and 6 in 2009, to categories 2, 3 and 4 in 2010 (i.e. the matrix entries above the main diagonal). In Xochicalco individuals in these three size categories were observed producing daughter rosettes of different sizes (i.e. in categories 2, 3 and 4), while in Tembembe produced daughter rosettes, which tended to be smaller (i.e. in categories 2 and 3).
In Tembembe the λ value was slightly higher (λT = 1.268) than in Xochicalco (λX = 1.208), though their confidence intervals overlapped. Even though the Tembembe population had a slightly higher λ value, it did not differ significantly from unity given its wide confidence interval (Table 2), suggesting that this population is close to numerical stability. However, the relatively lower λ value of the Xochicalco population was indeed significantly higher than unity, suggesting that the population is growing.
The projected population size structure showed that the two populations would be dominated by individuals in the first size category if the present demographic conditions were to persist over time, and that relative abundances would decrease towards larger categories (Table 3, Figure 4). The observed and the stable size structure differed significantly in both sites (Xochicalco: χ2 = 322.84, d.f. = 5, p < 0.05; Tembembe: χ2 = 389.961, d.f. = 5, p < 0.05). The size-specific reproductive values showed a similar behavior in both populations, with low values in the first category and increasing towards larger categories (Table 2a and b). The reproductive value of category 6 represented almost half the total reproductive value, which was expected given the semelparity of individual rosettes, and was higher in Tembembe than in Xochicalco.
Table 3 Elasticity matrices for the Agave angustifolia populations at Xochicalco (a) and Tembembe (b). The three highest elasticity values in each matrix are highlighted in bold.
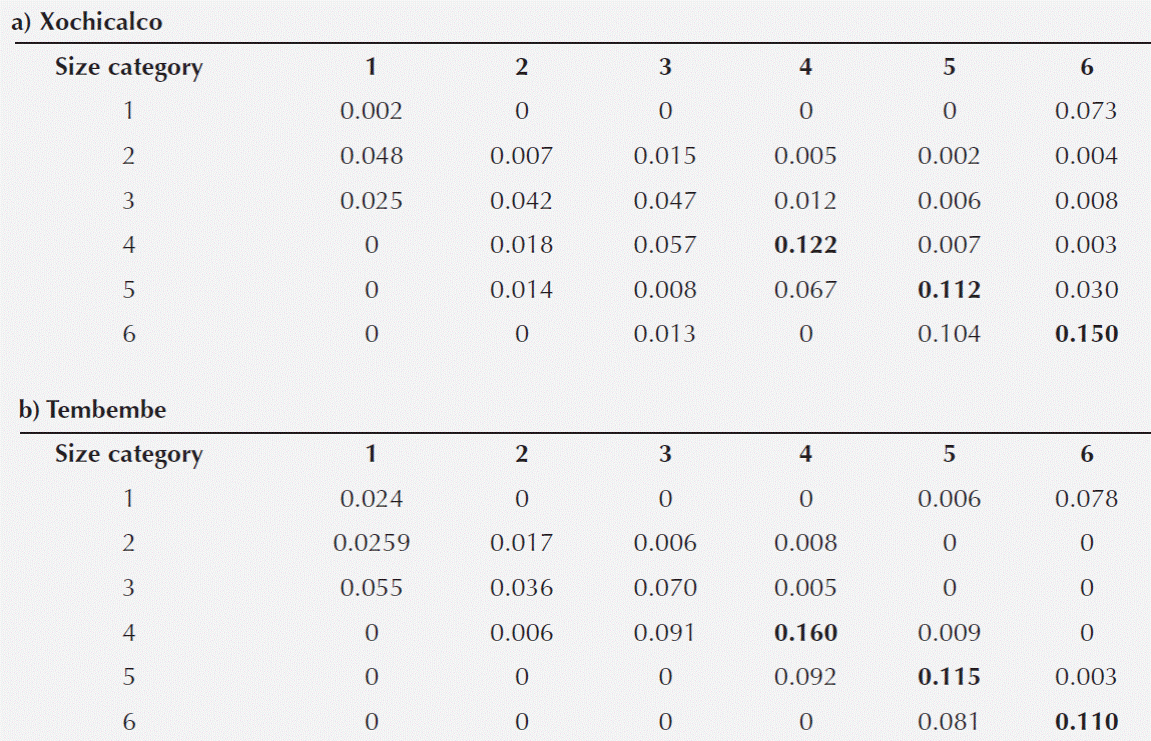
Prospective analyses: elasticity. In both sites, the highest elasticity values corresponded to the persistence (stasis) of individuals in categories 4, 5 and 6. These entries of the elasticity matrix were slightly higher for the Xochicalco than for the Tembembe population (Table 3). On the other hand, the elasticity values for fecundity were quite low (less than 8 % of total elasticity), and those corresponding to vegetative spread were even lower (Table 3).
When adding up the elasticity values corresponding to the same demographic processes, we observed a similar pattern in both sites: stasis and growth were the most important demographic processes, while retrogression, fecundity, and vegetative spread had low values. It is important to note, however, that fecundity had higher elasticity in Tembembe (the site with a relatively higher λ value) than in Xochicalco, while the opposite was true for retrogression and vegetative spread (Figure 5).
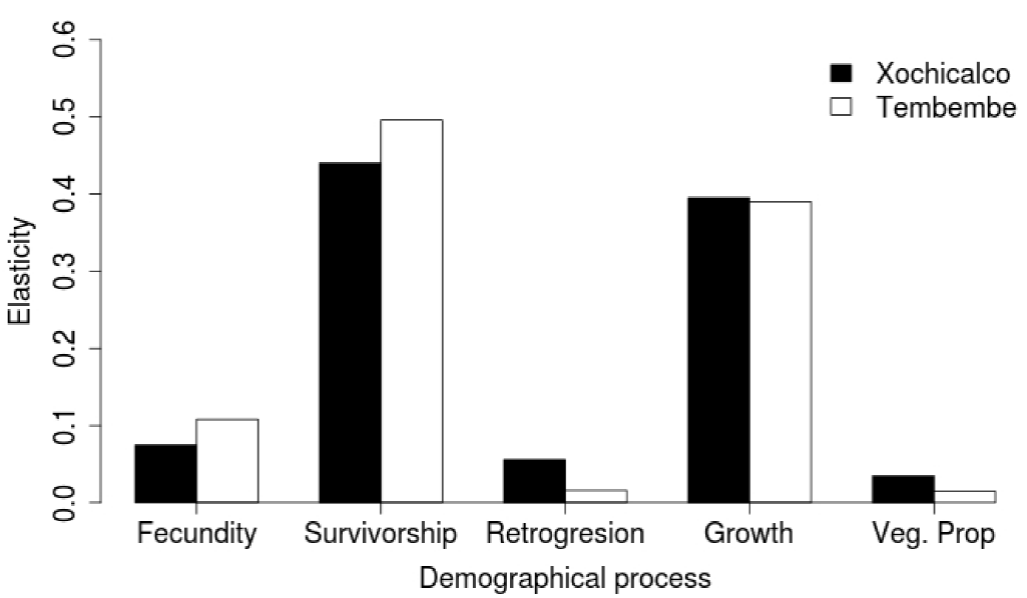
Figure 5 Sum of the elasticity values corresponding to different demographic processes for the Agave angustifolia populations studied in Xochicalco (black bars) and Tembembe (white bars).
Retrospective analysis: life-table response experiments (LTRE). This analysis is based on a comparison between the matrices obtained for each site with an average matrix, which oddly had a higher λ value than the other two (λAverage = 1.363; while λX = 1.208; λT = 1.268; see discussion). According to the LTRE, the λ value of the Xochicalco matrix was lower than the average matrix mainly due to its low fecundity (Figure 6). The stasis elements of the Xochicalco matrix also contributed to a decrease in λ, while the same demographic process contributed to an increase in λ in Tembembe (Figure 6). Most of the growth elements made a positive contribution to λ in Xochicalco, while the contrary occurred in Tembembe. The most important growth contributions were those of the a2,1 and a6,5 transitions, which were positive in Xochicalco and negative in Tembembe (Figure 6). Similarly, most of the contributions referring to vegetative spread were positive in Xochicalco, and negative in Tembembe, except for a4,5 (Figure 6).
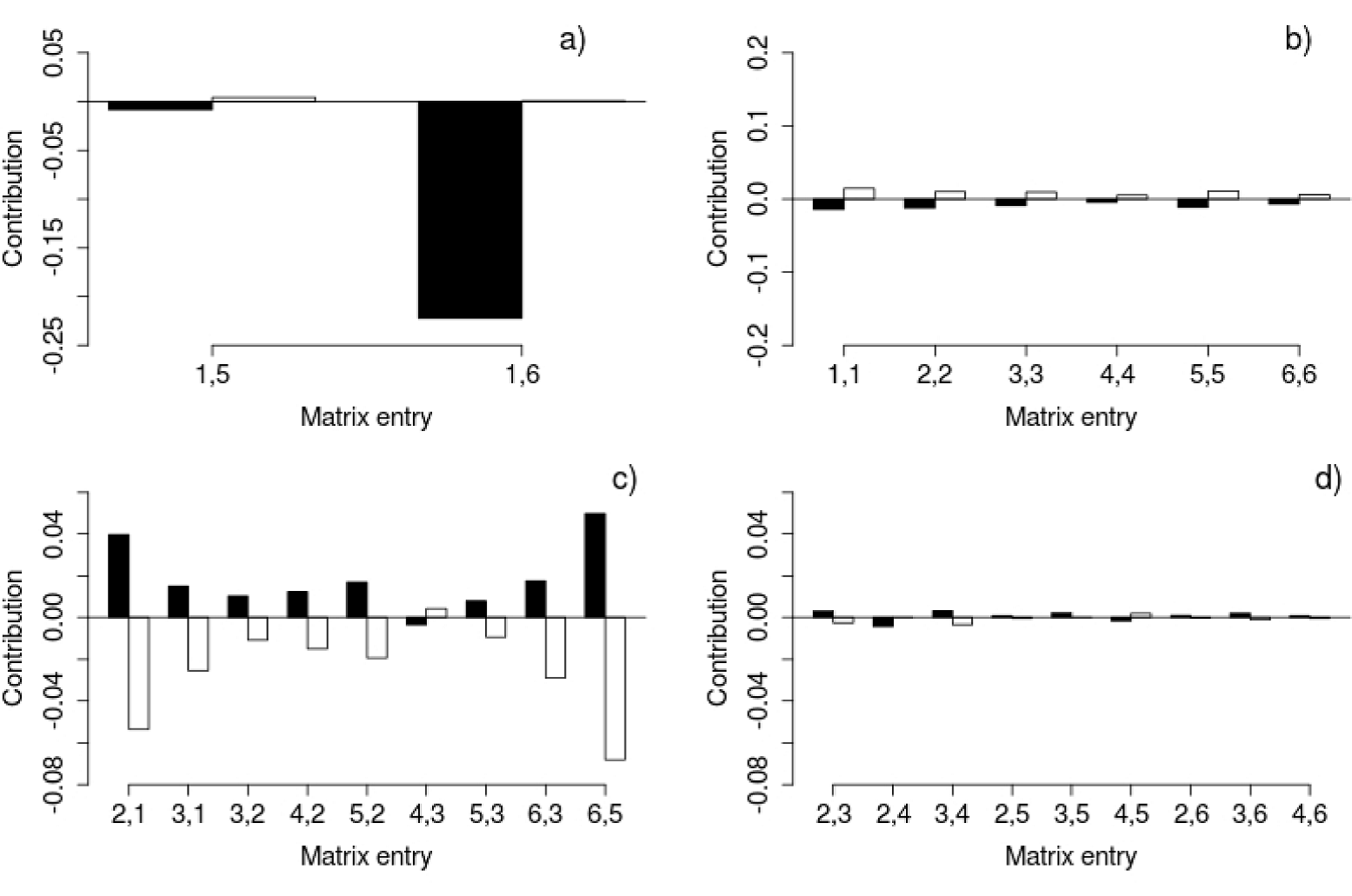
Figure 6 Results of the LTRE. Bars represent the contribution of each matrix entry to the increase (positive contributions) or decrease (negative contributions) of the λ value of the Xochicalco (black bars) or Tembembe (white bars) matrices compared to the λ value of the average matrix. Different demographic processes are depicted in the different graphs: a) Fecundity, b) Stasis, c) Growth, and d) Vegetative spread. Matrix entries are represented by their sub-indices, where i,j are the transitions or contributions from category j to category i from time t to t+1. Note the differences in the scale of the y axis between plots.
Numerical Simulations. The purpose of the numerical simulations was to theoretically evaluate the effect of different management scenarios on the Tembembe population. This is a highly degraded site given its long history of cattle ranching and is currently being subjected to ecological restoration. Additionally, Agave angustifolia plants are sometimes harvested for mezcal production in the area. The results of the numerical exercises simulating inflorescence removal and plant harvesting suggest that only a very dramatic reduction in fecundity would decrease λ to values below unity (Figure 7). On the other hand, yearly introduction of juvenile plants (in size category 2) would produce a susbstantial increase in λ (Figure 7).
Discussion
Population growth rate did not vary significantly between the two sites, suggesting that the difference in disturbance level between sites does not have an important effect on the population dynamics of Agave angustifolia. Yet, the λ at the well preserved site (Xochicalco) was significantly above unity, while the λ of Tembembe did not differ from unity. Other Agave populations that have been studied demographically have yielded λ values close to unity, as was the case for Agave cupreata and A. marmorata (Illsley et al. 2007; Jiménez-Valdés et al. 2010). The same has been observed in other succulent plants from arid and semi-arid environments, such as the cacti Echinocactus platyacanthus, Neobuxbaumia macrocephala, N. tetetzo and N. mezcalaensis (Esparza-Olguín et al. 2002; Jiménez-Sierra et al. 2007). Some of these studies have addressed the effect of disturbance on population dynamics, and have noted that they tend to be either negative or nil. For instance, A. marmorata in the Tehuacán desert (Mexico) showed a decrease in λ in a disturbed compared to a well preserved site (λ = 0.780-0.814 and 0.900-1.156, respectively; Jiménez-Valdés et al. 2010). Also in the cacti Coryphantha werdermannii and Mammillaria magnimamma populations were negatively affected by disturbance (Valverde et al. 2004; Portilla 2007). On the other hand, in A. cupreata the λ value did not show a noticeable variation between sites, irrespective of their conservation status (Illsley et al. 2007).
In our study, the slight differences in the demography of the two populations cannot be accounted for only as a result of disturbance level, as the two sites also vary in relation to climate and soil. The LTRE allowed us to identify which demographic processes varied between sites that caused the slight variation in λ. These were the variations in the fecundity entries (as discussed below), which were in turn determined by a higher seed germination and seedling establishment probability in Tembembe compared to Xochicalco, probably due to the higher altitude and thus lower temperatures that prevail at Tembembe.
In addition to variation between populations, it is important to study demographic variation in time. Our results are based on a single year of study, so the projected λ values reflect the demographic potential of these populations under the particular conditions of the study period. Although it is generally thought that slow growing plants from arid and semi-arid environments show little plasticity in growth and reproductive patterns in response to environmental variation, it has been observed that other succulents do vary in their demographic behavior through time depending on the weather conditions of each year. For instance, in the cacti Mammillaria magnimamma very contrasting λ values were obtained in years with different rainfall patterns (Valverde et al. 2004). In our study sites, there is marked inter-annual variation in precipitation and temperature, which surely affects the demographical behavior of Agave angustifolia between years. In addition, demographic variation through time in Agave populations may also reflect variation in inflorescence removal for mezcal production. In Agave marmorata, for instance, stalk removal had an important impact on several demographic processes (fecundity, growth, and seedling establishment) resulting in a lower λ value compared to a population where no stalks were severed (Jiménez-Valdés et al. 2010). In addition, many Agave populations present mast seeding years (Arizaga and Ezcurra 1995), which would be a further source of demographic variation through time.
Another important result of matrix analysis is the stable size distribution to which the population would converge if the current demographic behavior remained constant over time (i.e. the right eigen-vector of the matrix). In our analyses, both stable size distributions were characterized by a very large proportion of size 1 individuals (i.e. seedlings); yet the observed population structures differed significantly from the projected ones precisely due to the small number of size 1 individuals found in the field. It is difficult to conclude regarding the actual form of the population structure, as seedlings are very difficult to observe in the field; thus, their small numbers in our sample do not necessarily imply that they are that scarce. In fact, without talking into account the seedling stage, the observed and the projected population structures show a reasonable resemblance. This is important, as a higher resemblance between the observed and the stable population structures implies that the projected λ value may adequately represent the actual behavior of the population.
According to our elasticity analysis, stasis and growth were the two demographic processes with a higher impact on λ in both study sites. Thus, the studied populations are located in the central right-hand area of the demographic triangle (sensu Silvertown et al. 1993), near the region where iteroparous herbs and forest shrubs are generally found (Figure 8). This elasticity pattern contrasts with the results of most long-lived plants of arid and semi-arid environments (i.e. cacti and other agaves), for which stasis is by far the single most important demographic process (Contreras and Valverde 2002; Esparza-Olguín et al. 2002; Godínez-Álvarez et al. 2003; Méndez et al. 2004; Valverde et al. 2004; Valverde and Zavala-Hurtado 2006; Illsley et al. 2007; Jiménez-Sierra et al. 2007; Jiménez-Valdés et al. 2010). Yet, Tembembe and Xochicalco are not really semi-arid environments: the annual precipitation at Xochicalco and Tembembe (ca. 1,000 mm) is twice that of a typical semi-arid area at these latitudes. Therefore, it is not surprising that the relatively milder conditions allow for growth to become an important process for population dynamics.
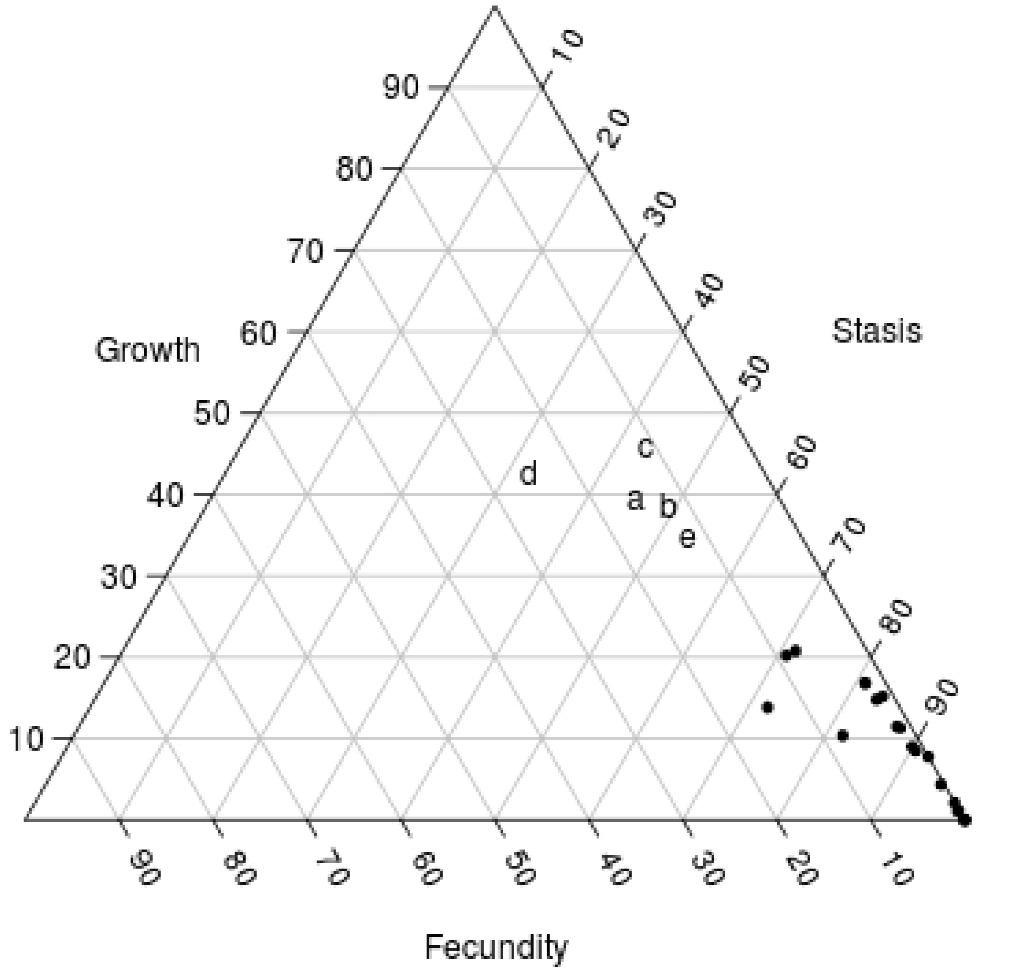
Figure 8 Position of the studied populations in the demographic triangle (a: Xochicalco; b: Tembembe), in which the three main demographic process are represented. Letters c, d and e represent populations in preserved sites of Agave marmorata for the year 2002-2003, M. magnimamma for the year 1997-1998 and Pterocereus gaumeri for the year 1998-1999, respectively. The other dots are different agave and cacti populations (two populations of Agave marmorata; two populations of Mammillaria crucigera; M. magnimamma; M. pectinifera; Neobuxbaumia macrocephala; N. mezcalensis, and N. tetetzo (Contreras and Valverde 2002; Esparza-Olguín et al. 2002; Méndez et al. 2004; Valverde et al. 2004; Valverde and Zavala-Hurtado 2006; Jiménez-Valdés et al. 2010).
In addition, the LTRE showed that, in Xochicalco, growth always contributed to an increase in λ, and stasis contributed to a decrease, while the opposite was true in Tembembe. Also, these analyses showed that the low fecundity of the largest size category contributed importantly to a decrease in λ in Xochicalco. Despite fecundity elasticities being generally low, variation in fecundity usually accounts for actual variation in λ in many populations. Such behavior has also been observed in Agave marmorata, and in the cacti Neobuxbaumia macrocephala, N. tetetzo, N. mezcalaensis and Coryphantha werdermannii (Esparza-Olguín et al. 2002; Portilla 2007; Jiménez-Valdés et al. 2010). In this study, two of the components of the fecundity values were seed germination and seedling establishment probabilities, which were obtained from field experiments. Both germination and seedling establishment were lower in Xochicalco (29.8 and 3.5 %, respectively) than in Tembembe (35.6 and 29.3 %). As a result, overall fecundity was much lower in Xochicalco. The other important component of fecundity was seed production, which was quite low during 2009-2010, given that it was not a mast-seeding year. Thus, occasional massive reproductive events may have important consequences for long term population dynamics, as has been demonstrated for other Agavaceae, such as Furcraea parmentieri (Hernández-Pedrero 2009).
The numerical simulations to evaluate the potential effect of cutting reproductive stalks in Tembembe showed that λ decreased below unity only when all the inflorescences were removed. The results of Jiménez-Valdés et al. (2010) with Agave marmorata point in the same direction. This suggest that these Agave populations are quite resilient to high exploitation levels, as individuals may produce offshoots and replace themselves even in the absence of seed production. This may not be the case for other Agave populations, though. For instance, it is believed that A. potatorum populations may indeed be decreasing due to the extraction of individuals for the production of alcoholic beverages in Tehuacán, Mexico (Delgado-Lemus 2008).
Implications for ecological restoration. The results of our study suggest that Agave angustifolia could successfully be used for the ecological restoration of disturbed fields in the Tembembe area. We base this suggestion on the high seed germination percentage, seedling survival probability and population growth rate obtained in the disturbed site, which imply that the population is barely affected by the high disturbance level which characterizes the area. Even if these results correspond to a single yearly period, it was a year in which the number of reproductive individuals was particularly low. In mast-seeding years the population growth rate should be considerably higher.
It is important to consider that part of the Agave angustifolia population sampled at Tembembe was located inside a cattle exclusion plot that was set at the site one year before the start of the study. This allowed the partial recovery of the plant cover (particularly of grasses and forbs) and may have been an important factor determining the high seed germination and seedling establishment probabilities at this site. In the non-protected areas we have observed grazed A. angustifolia individuals. Thus, any restoration program would have to consider setting some kind of protection from cattle grazing at the area before launching actual restoration activities.
Our numerical simulations suggest that the introduction of Agave angustifolia plants would be a successful strategy to boost population growth rate at Tembembe. This is also supported by the results of the seedling survival experiments, which showed that five months old seedlings had a high survival probability in both exposed and shaded microsites. However, three-month old seedlings did not have such high survival, so plant size (or level of development) would be an important factor to take into account for planning restoration strategies.
Native agave plants, and even cacti, have been used for ecological restoration programs in other parts of Mexico (Sinisterra et al. 2011). Their use with this aim has been considered an economic an ecological viable option, as it is presumed that they may effectively reduce soil erosion. A wider use of Agave species would increase the diversity of plant species used for restoration of Mexican TDF, which is generally very low (Bonfil and Trejo 2010). An additional advantage is that these plants are valuable resources for local inhabitants, given their use for alcoholic beverage production; so their introduction as part of restoration programs would presumably be locally accepted and supported. Of course, their exploitation would have to be strictly regulated to make sure that extracted plants are replaced with new individuals, and that the genetic variation of populations is maintained, as in other areas the introduction of vegetative propagules from limited genetic sources has caused the loss of genetic variation (Eguiarte et al. 1999).











 nueva página del texto (beta)
nueva página del texto (beta)



