Introduction
Habanero pepper (Capsicum chinense Jacq.) is considered one of the most pungent peppers, reaching values between 100,000 and 600,000 Scoville Heat Units (SHU), equivalent to 7-40 mg g-1 DW capsaicinoids (CAPs), the active compounds of peppers’ typical hot flavor [1]. CAPs are solely synthetized and accumulated in the epidermal cells of the placenta, the tissue holding the seeds inside the fruit [2]. The pathway leading to CAPs synthesis involves shortening of the C3 chain of phenylalanine, through the phenylpropanoid route, to produce an aromatic amine (vanillylamine), which would be condensed with a 10-C acyl unit, derived from a branched amino acid (either leucine or valine; Fig. 1). The last reaction in CAP synthesis involves the formation of an amide bond between the amine group of vanillylamine and the acyl chain, catalyzed by capsaicinoid synthase [1]. Most cDNA’s of genes coding the corresponding enzymes involved in CAPs biosynthesis have been isolated [3] and transcriptional profiles during fruit development revealed that most of them are simultaneously accumulated previous to maximal CAPs accumulation [4]. Moreover, regulatory enzymes involved in the synthesis of the required amino acids are coordinately activated with those of CAP synthesis during fruit development [5,6].
Besides CAPs, the phenylpropanoid pathway produces a number of different phenolic compounds. Each intermediary between Phe and vanillylamine (see Fig. 1) might be used as the initial compound of branches leading to different products [7]. In this way, the formation of this amine represents a committed step towards CAPs synthesis and could function as a regulatory step [1]. In here, we compared the efficiency of Habanero pepper’s placentas to transform externally supplied ferulic acid (FA) and vanillin (V) into CAPs to that of a mild C. annuum cultivar. These two compounds were selected since both are involved in critical steps of the biosynthetic pathway. The propanoic lateral chain of FA must be cleaved to produce V (3-methoxybenzaldehyde), which is the direct vanillylamine precursor (Fig. 1). Our results showed that, even though externally supplied V was equally transformed in both pepper species, C. chinense placentas were more efficient in transforming FA into CAPs than those of C. annuum, suggesting that the channeling of these late intermediaries may play a role in defining pepper pungency.
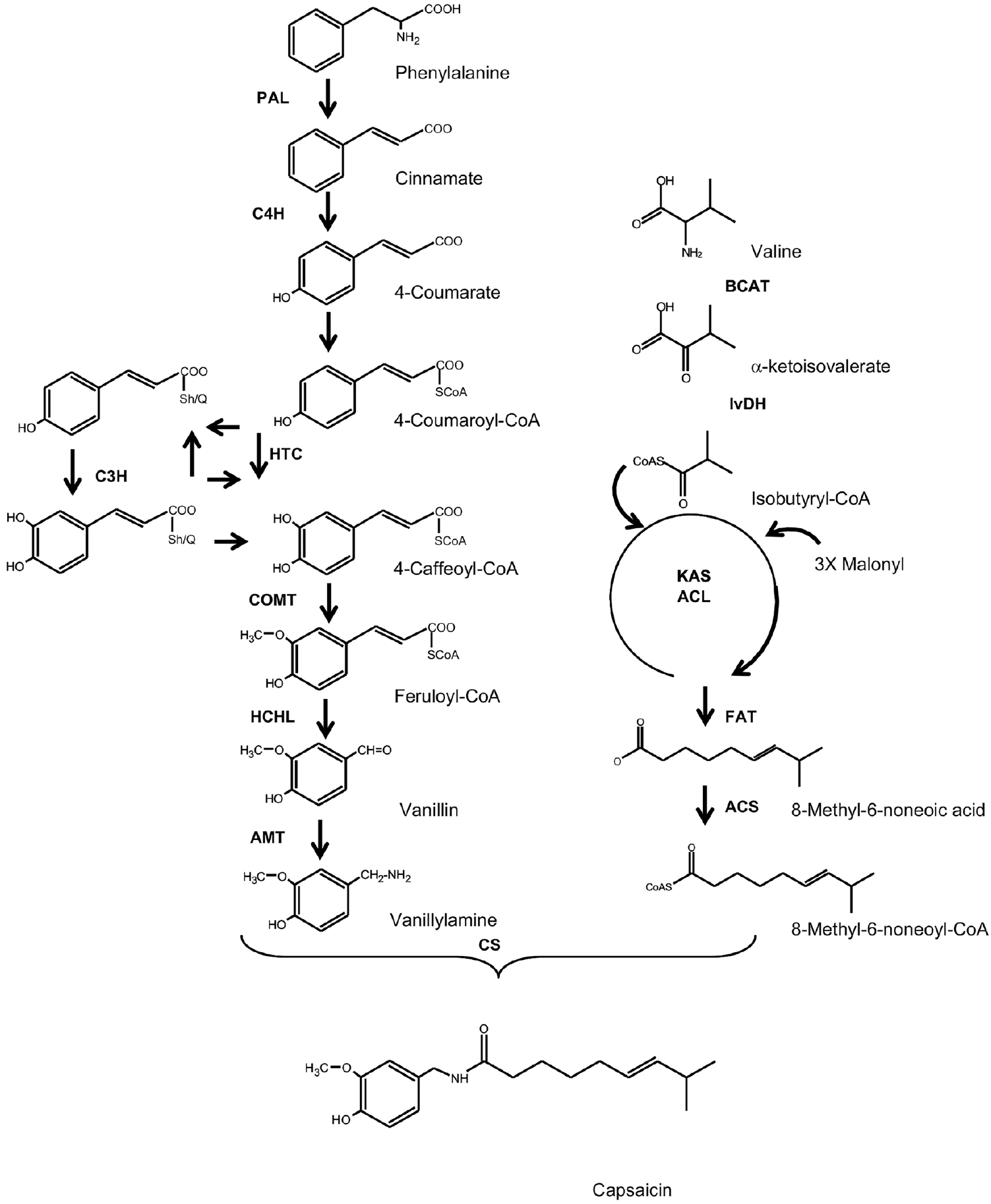
Figure 1 CAP biosynthetic pathway. PAL phenylalanine ammonia lyase; C4H cinnamate 4-hydroxylase; 4CL 4-coumaryl-CoA ligase; HCT hydroxycinnamoyl shikimate/quinate 3-hydroxylase; COMT, caffeic acid methyltransferase; HCHL hydroxycinnamoyl-CoA hydratase/lyase; AMT, vanillin aminotransferase; BCAT branched-chain amino acid transferase; KAS ketoacyl-ACP synthase; ACL acyl carrier protein; FAT acyl-ACP thioesterease; ACS acyl-CoA synthase; CS capsaicinoid synthase. Modified from [1].
Results and Discussion
Total CAPs (capsaicin plus dihydrocapsaicin) content in intact placentas from C. chinense and C. annuum cultivars used in our experiments were 21.4 ± 1.63 and 8.6 ± 1.31 µmoles g-1 DW, respectively. These values decreased markedly, up to 2.3 and 0.7 µmoles g-1 DW respectively, upon introduction to in vitro culture due to damage caused to the external surface of the placentas, where CAPS are stored (see Fig 2). However, placentas recovered after their introduction to in vitro culture, remaining metabolically active as it has been shown previously [8]. Two different concentrations of the intermediaries were assayed (50 and100 µM) for up to 48 h. Mock water-fed placentas were used as controls. Similar effects were recorded for both intermediaries’ concentrations, 50 and 100 µM, although in a less marked fashion at the lower concentration. Therefore, the results for 100 µM are shown only. Moreover, in water-exposed isolated placentas (controls), CAPs levels were maintained with little variation between 2.0 and 4.0 and between 0.6 and 0.8 µmoles g-1 DW for C. chinense and C. annuum, respectively (data not shown). In general, low levels of the analyzed intermediaries (FA and V) were detected in intact placentas from both species (at least two orders of magnitude lower than CAPs; Fig. 2) and this trend was maintained after introducing them into culture. The external supply of 100 µM FA to isolated placentas had different responses, depending on the Capsicum species employed.

Figure 2 Accumulation of capsaicinoids and biosynthetic intermediaries in C. chinense (A-B) and C. annuum (C-D) isolated placentas exposed to 100 µM ferulic acid. Contents of ferulic acid and vanillin in C. chinense (A) and C. annuum (C) placentas. Contents of total capsaicinoids in C. chinense (B) and C. annuum (D) placentas. Average of triplicates with standard deviation.
In C. chinense placentas, the addition of FA increased the initial value of V (Fig. 2A), its direct biosynthetic product, as well as that of FA (Fig. 1). This was more evident after 12 h, but always remaining in the nanomol range (Fig. 2A). This data suggest that FA was incorporated and transformed into V under the experimental conditions used. CAPs content showed maximal accumulation (12.3 µmoles g-1 DW) 24 h after exposure (Fig. 2B). Although those values represented over a five-fold increase of the initial in vitro values (0 h in Fig. 1B), they only accounted for nearly 60 % of those found in intact tissues (21.4 ± 1.63 µmoles g-1 DW). In isolated placentas of C. annuum, the amounts of FA and V were comparable (Fig. 2C) to those in C. chinense. Interestingly CAPs levels slightly increased in response to FA exposure (Fig. 2D) and maximal accumulation in C. annuum (1.4 µmoles g-1 DW) occurred 48 h after exposure. This represented a two-fold increase over the initial in vitro values, which in turn represented a 16% recovery of the contents of the intact tissues (8.6 ± 1.31 µmoles g-1 DW). In this way, placentas from both C. chinense and C. annuum increased FA and V levels, in response to the addition of FA, suggesting its incorporation to tissues and further utilization. In contrast, those from C. annuum showed a limited transformation rate to CAPs (Fig. 2D).
The external supply of V, which is a closer intermediary to CAP formation than FA (Fig. 1), increased V, but not FA contents in C. chinense placentas (Fig. 3A). CAPs levels also increased in the same extent as those observed in placentas exposed to FA, following a similar trend (Fig. 3B). C. annuum placentas also increased V contents when exposed to this compound (Fig. 3C). Furthermore, a noticeable increase in the accumulation of CAPs occurred after 12 h of exposure, reaching values comparable to those of intact placental tissues (ca. 5 µmoles g-1 DW; Fig. 3D). All together, these data suggest that Habanero pepper (C. chinense) higher pungency, compared to mild C. annuum cultivars, might be related to a higher efficiency in transforming FA into subsequent intermediaries.

Figure 3 Accumulation of capsaicinoids and biosynthetic intermedi aries in C. chinense (A-B) and C. annuum (C-D) isolated placentas exposed to 100 µM vanillin. Contents of ferulic acid and vanillin in C. chinense (A) and C. annuum (C) placentas. Contents of total capsaici noids in C. chinense (B) and C. annuum (D) placentas. Average of triplicates with standard deviation.
Undifferentiated cell cultures of both C. chinense and C. annuum presented a low capacity for CAPs accumulation [9]. In an attempt to promote CAPs formation, cell suspensions were treated in the same way as isolated placentas (Fig. 4 and 5). As expected, the two species showed very low CAPs levels. Interestingly, C. chinense cell cultures accumulated a fair amount of V under normal conditions (around 100 nmoles g-1 DW; Fig. 5A), contrasting to those of C. annuum (under 20 nmoles g-1 DW; Fig. 5B). Under the assayed conditions, no increases in CAPs accumulation were recorded in response to the external supply of either FA (Fig. 4A and B) or V (Fig. 5A and B). Nevertheless, FA as well as V contents, increased in the corresponding treatments (Fig. 4 and 5), suggesting that, to a certain extent, both intermediaries were taken up by the exposed cell cultures. Interestingly, a slight increase in V levels could be observed in the FA-treated C. chinense cells, but not in those from C. annuum, indicating that some of the externally supplied FA could have been converted into V, without inducing CAPs accumulation (Fig. 4 and 5).
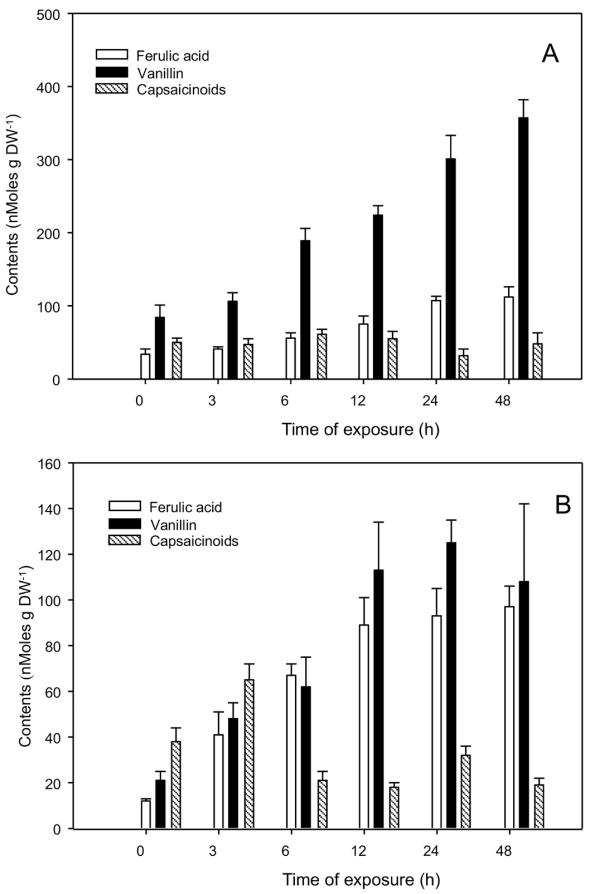
Figure 4 Accumulation of capsaicinoids and biosynthetic intermedi aries in C. chinense (A-B) and C. annuum (C-D) cell suspension cul tures exposed to 100 µM ferulic acid. Contents of ferulic acid and vanillin in C. chinense (A) and C. annuum (C) cultures. Contents of total capsaicinoids in C. chinense (B) and C. annuum (D) cultures. Average of triplicates with standard deviation.

Figure 5 Accumulation of capsaicinoids and biosynthetic intermedi aries in C. chinense (A-B) and C. annuum (C-D) cell suspension cul tures exposed to 100 µM vanillin. Contents of ferulic acid and vanillin in C. chinense (A) and C. annuum (C) cultures. Contents of total cap saicinoids in C. chinense (B) and C. annuum (D) cultures. Average of triplicates with standard deviation.
The capacity to synthetize and accumulate CAPs is exclusively located in epidermal cells of the placental tissue of hot, pungent genotypes of the Capsicum genus [10]. This trait behaves as a single, dominant and epistatic character, controlled by the presence of at least of one functional allele at the Pun1 loci [11]. No clear correlation between CAP accumulation and the genetic condition at Pun1, either homozygous or heterozygous, has been observed [2]. Moreover, most QTL’s associated to CAP accumulation can account only to up to a 20% of the maximal variations [2 and wide differences in CAP amounts have been detected in highly isogenic C. annuum varieties cultured under the same conditions [1]. Therefore, as a quantitative trait, pungency is controlled in a complex way, probably involving a multilevel mechanism. In here, we have shown that the availability of late intermediaries, such as FA and V, may affect the final outcome regarding CAPs accumulation. Cinnamic acid, the first product of the phenylpropanoid pathway (Fig. 1), is used in the synthesis of lignin and number of other metabolites. In fact, when cell cultures of C. annuum var. Tampiqueño were presented with an external supply of Phe, cinnamic, coumaric and caffeic acids, CAPs accumulation did not increase, whereas the addition of FA, V and vanillylamine, the late biosynthetic intermediaries, had an important effect [12]. It should be pointed out that C. annuum cell suspensions are able to incorporate externally supplied FA to CAPs [12, 13], even though the actual intermediary for CAP synthesis is feruloyl-CoA ester [2. C. frutescens immobilized placentas were also able to incorporate FA into CAPs [14].
In our experiments, Habanero pepper (C. chinense) placentas incorporated FA into CAPs, and no differences were found when V was used (Fig. 2 and 3). Conversely, C. annuum placentas were more efficient using V over FA (Fig. 2 and 3). These data suggest that C. chinense placentas readily synthesized feruloyl-CoA ester (C6-C3) from the exogenously supplied acid, which was quickly transformed into the benzoic derivative (C6-C1), channeling the intermediaries to CAPs and some other phenolic products [1]. This higher ability of C. chinense to use FA, in comparison to the mild C. annuum variety, marks a clear difference, and may explain, at least partially, the higher capacity of the Habanero pepper to produce CAPs. The efficient formation of benzoates in Habanero pepper may reduce the possible diversions of the C6-C3 units to other phenylpropanoid compounds, increasing their channeling towards CAPs formation.
It is interesting to notice that cell cultures, which evidently lack tissue organization, only accumulated very low amounts of CAPs (Fig. 4 and 5). Hence, the role of epidermal cell swelling to form blisters, where these compounds are accumulated, has been clearly established [10]. Such blisters remain intact when placental tissues are cultured in vitro, although some damage could be observed, thus explaining the decrease in CAPs levels after manipulation required for initiating the cultures [8].
Experimental
Biological material and treatments. Placentas were collected from immature pods of C. chinense (local landrace Naranja) and C. annuum (local landrace ‘Katic’). C. chinense corresponds to a highly pungent genotype, whereas the Katic variety of C. annuum is a mild type of pepper (see Results for a comparison). Peppers were collected once they have attained their final dimensions and taken to the laboratory where they were washed in soapy water, rinsed with tap water and disinfested by subsequent 5-min incubations in 70% ethanol and 3 mg L-1 sodium hypochlorite (50% dilution of commercial bleach). After rinsing the pods in sterile distilled water, the entire placentas were exscinded using scalpel and tweezers and rinsed in sterile water. Square sections of ca. 3 mm per side were pre-incubated for 1 h in half strength Murashige and Skoog (MS) medium. After rinsing the tissues with a 10% dilution of the culture medium, they were kept in 125 mL Erlenmeyer flasks, containing 25 mL half strength MS, supplemented with 20 g L-1 sucrose [8]. Placentas were cultivated for 12 h before supplying CAP intermediaries. Ferulic acid (FA) or vanillin (V) (Sigma-Aldrich, St Louis MO) were diluted in water and added to reach final doses of 0 (control, water), 50 and 100 µM. Tissues were incubated for periods shown in the figures, rinsed with sterile distilled water, frozen in liquid nitrogen and kept at -80 °C until analysis. Each treatment was applied in triplicate.
Cell suspension cultures from hypocotyls of C. chinense and C. annuum (local landraces Naranja and ‘Katic’, respectively) were maintained by biweekly subcultures in MS medium as described before [9]. Ten-day cultures were exposed to similar treatments as isolated placentas and kept under constant agitation. Cultures were collected by filtration under vacuum and frozen until analysis.
Analytical procedures. After harvest from the culture flasks, tissues were freeze-dried and one gram was extracted with 12 mL acetronitrile overnight with gentle shaking (50 rpm) at 60°C. Extracts were decanted and centrifuged to eliminate tissue debris, dried under reduced pressure, and the residue was then dissolved in 0.5 mL methanol. Prior to injection (total volume: 20 µL), extracts were centrifuged and filtered through a nylon membrane (pore size: 0.45 mm). Capsaicin, dihydrocapsaicin and intermediaries were quantified by DAD-HPLC [15], using an Agilent Technologies liquid chromatographer (Santa Clara CA), Model 1200 with a diode array UV-visible detector coupled to an Agilent LC Chem Station. A Zorbax C18 ODS column (4.6 x 250 mm, 5 mm particle size) from Agilent, was used as stationary phase. Solvents used for separation were as follows: solvent A, 10% methanol in water; solvent B, 100 % methanol. The separation was carried out isocratically with 70% B plus 30% A, at a flow rate of 1.0 mL min-1 for 10 min. The eluant was monitored at 280 nmµ and capsaicin (Rt 4.25 min) was completely separated under these conditions from dihydrocapsaicin (Rt 5.75 min), ferulic acid (Rt 1.61 min), vanillin (Rt 1.87 min) and vanillylamine (Rt 2.14 min).











 nueva página del texto (beta)
nueva página del texto (beta)


