Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista latinoamericana de química
versión impresa ISSN 0370-5943
Rev. latinoam. quím vol.41 no.1 Naucalpan de Juárez abr. 2013
Behavioral and biochemical effects of Cannabis Sativa and their modulation by antidepressant drugs
Omar M.E.Abdel-Salam1*, Rehab Fawzy Abdel-Rahman2, Alaa El-Din M. Gaafar3
1 Department of Toxicology and Narcotics, National Research Centre, Cairo.
2 Department of Pharmacology, National Research Centre, Cairo.
3 Department of Photochemistry, National Research Centre, Cairo.
* Correspondence:
Omar M.E. Abdel Salam.
Department of Toxicology and Narcotics,
National Research Centre,
Tahrir St., Dokki, Cairo, Egypt.
E mail: omasalam@hotmail.com. FAX: 202-33370931.
Received September 2012.
Accepted January 2013.
ABSTRACT
We aimed to study the effect of Cannabis sativa on brain oxidative stress and determine whether behavioral responses caused by cannabis could be reversed by standard antidepressant drugs. Cannabis sativa (5, 10 or 15 mg/kg) (expressed as Δ9-tetrahydrocannabinol) was given alone or with fluoxetine, sertraline or imipramine, once daily subcutaneously (s.c.) for 24 days. In the forced-swimming test, the immobility time, was significantly increased in mice treated with cannabis (5-15 mg/kg, s.c.) starting from the 9th day post-injection. Fluoxetine (20 mg/kg, s.c.) coadministered with cannabis (5 mg/kg, s.c.) resulted in significant decrease in the immobility time by the day 21 of the study compared with the cannabis only group. Mice co-administered sertraline or imipramine with cannabis were not statistically different from the vehicle control group as regards their immobility time. Cannabis resulted in a significant decrease in the rearing activity which was ameliorated by either fluoxetine or sertraline. Cannabis sativa increased brain reduced glutathione, but decreased the level of nitric oxide. Fluoxetine, sertraline or imipramine given with cannabis decreased malondialdehyde and increased reduced glutathione. In conclusion: The administration of cannabis decreases brain oxidative stress but exerts depressive-like effect and decreases rearing activity which can be reversed by antidepressant drugs.
Keywords: Cannabis; antidepressants; brain oxidative stress.
RESUMEN
Nos propusimos estudiar el efecto de Cannabis sativa sobre el estrés oxidativo en cerebro y determinar si las respuestas causadas por canabis sobre el comportamiento podrían revertirse por fármacos antidepresivos estándar. Cannabis sativa (5, 10 o 15 mg/kg) (expresada como Δ9-tetrahidrocanabinol) se administró subcutáneamente (s.c.) sola o con fluoxetina, sertralina o imipramina una vez al día por 24 días. En la prueba de nado forzado, el tiempo de in movilidad, se incrementó significativamente en los ratones tratados con canabis (5-15 mg/kg, s.c.) a partir del 9° día post-administración. Fluoxetina (20mg/kg, s.c.), coadministrada con canabis (5 mg/kg, s.c.), dio como resultado una disminución significativa en el tiempo de inmovilidad alrededor del día 21 del estudio comparado con el grupo tratado sólo con canabis. Los ratones co-administrados con sertralina o imipramina y canabis no fueron estadísticamente diferentes del grupo control de vehículo respecto al tiempo de inmovilidad. Canabis provocó una disminución significativa en la actividad de levantamientos que fue aumentada tanto por fluoxetina como por sertralina. Cannabis sativa incrementó la glutationa reducida en cerebro y disminuyó los niveles de óxido nítrico. Flouxetina, sertralina o imipramina administrados con canabis disminuyó la malondialdehido e incrementó la glutationa reducida. En conclusión: La administración de canabis disminuye el estrés oxidativo cerebral pero ejerce un efecto parecido a la depresión y disminuye la actividad de levantamientos que puede revertirse por fármacos antidepresivos.
Palabras clave: Cannabis; antidepresivos; estrés oxidativo en cerebro.
INTRODUCTION
The cannabis preparations marijuana and hashish are the most common illicit drugs worldwide. These are derived from the female plant of Cannabis sativa L (family Cannabinaceae). Marijuana is prepared from the dried flowering tops and leaves; hashish consists of dried cannabis resin and compressed flowers. Marijuana and hashish are usually smoked but may be also eaten or used in a tea form (Ashton, 2001). The administration of cannabis in man produces a spectrum of psychoactive effects including euphoria and relaxation, perceptual alterations, time distortion, and the intensification of ordinary sensory experiences, such as eating, watching films, and listening to music. Short-term memory and attention, motor skills, reaction time, and skilled activities are impaired while a person is intoxicated (Hall et al., 1994). There is also elevated fatigue, drowsiness, dizziness and even severe transient psychotic symptoms with cannabis consumption (Kaufmann et al., 2010). Nevertheless, cannabis is usually self-administered for its mood-altering properties, and has been described as an addictive, dependence-producing drug due to the production of euphoria, the presence of reversible psychological impairment, an abstinence syndrome, and tolerance. A mixture of depressant and stimulant effects is noted at low doses; cannabis acts as a CNS depressant at high doses (Huestis, 2002). These psychomotor effects of cannabis are mainly attributed to the Δ9-tetrahydrocan-nabinol (Δ9-THC), the major psychoactive constituent in Cannabis sativa plant by acting on the cannabinoid (CB1) receptor that is highly expressed in the basal ganglia, the cerebral cortex and the cerebellum (Ameri, 1999; Pertwee, 1997, 2005; Svizenska et al., 2008). Cannabinoid receptors are also activated by endogenous ligands, the en-docannabinoids, a family of endogenous arachidonic acid derivatives, including N-arachidonylethanolamide (anandamide) and the 2-arachidonoylglycerol which is synthesized by the cell membrane (Sugiura et al., 2002).
Cannabis sativa is being prescribed and used in several medical conditions. It is used in the management of chemotherapy-induced nausea and vomiting among cancer patients (Machado Rocha et al., 2008) and for relief of spasticity in multiple sclerosis patients (Sastre-Garriga et al., 2011). Medical cannabis is also used to alleviate chronic pain and arthritis (Swift et al., 2005) and neuropathic pain caused by diabetes (Selvarajah et al., 2010) and to improve the wellbeing in patients with depression (Denson and Earleywine, 2006).
Several studies have suggested a potential antidepressant effect for cannabis, its constituents (El-Alfy et al.,, 2010; Zanelati et al., 2010) or CB1 receptor agonists (Bambico et al., 2007). Basal serum concentrations of the endocannabinoid ligands N-arachidonylethanolamide and 2-arachidonoylglycerol were significantly reduced in women with major depression relative to matched controls, indicating a deficit in peripheral endocannabinoid activity (Hill et al., 2009). Meanwhile, the intake of cannabis has itself been associated with increased prevalence of depressive disorders (Bovasso, 2001). Cannabis-dependent subjects consumed greater amounts of cannabis, alcohol, and a variety of other drugs. They also had lower levels of motivation, happiness, and satisfaction with life, with higher levels of depression (Looby and Earleywine, 2007).
In view of these reported differences in effects of Cannabis sativa as regards depression, the aim of the study was to: (1) examine the effect of cannabis in the forced-swim test (a test for depressive-like behavior) (Porsolt et al., 1978) in normal mice and after treatment with the selective serotonin reuptake inhibitors (SSRIs) fluoxetine and sertraline as well as the tri-cyclic drug imipramine with view to a possible modulatory effect of antidepressant drugs; (2) observe the effect of cannabis on exploratory behavior (rearing); (3) evaluate the effect of cannabis administration on antioxidant reserve and oxidative stress markers reduced glutathione (GSH), ma-londialdehyde (MDA) and nitric oxide in brain under these experimental paradigms since people who suffer from depression have abnormal levels of oxidative stress (Yager et al., 2010; Sarandol et al., 2007). A total extract from Cannabis sativa was used based on the fact that the effect of the whole plant which is abused by humans differs from that of THC in view of its content of other cannabinoids, terpenoids and flavonoids (Russo and McPartland, 2003).
MATERIALS AND METHODS
Animals
Swiss male albino mice 25-30 g of body weight were used. Standard laboratory food and water were provided ad libitum. Mice were obtained from animal house colony of the National Research Centre, Cairo. All animals were acclimatized to the laboratory conditions for 7 days before the beginning of the experiments. Animal procedures were performed in accordance with the Ethics Committee of the National Research Centre and are in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 8523, revised 1985).
Drugs and chemicals
Cannabis sativa L. plant was supplied by the Ministry of Justice- Egypt. Fluoxetine hydrochloride (Amoun Pharmaceutical Co., Cairo, A.R.E.), sertraline hydrochloride (Pfizer Egypt, Cairo, A.R.E.) and imipramine hydrochloride (Novartis Pharma, Cairo, A.R.E.) were used and dissolved in isotonic (0.9% NaCl) saline solution immediately before use.
Preparation of Cannabis sativa extract
Cannabis sativa extract was prepared from the dried flowering tops and leaves of the plant. The method of extraction followed that described by Turner and Mahlberg (1984) with modification. In brief, 10 g of dried cannabis was ground with a mortar and pestle. Decarboxylation of the plant material was achieved by placing the sample in a glass test tube (30 mL) and covering it with aluminum foil. The test tubes were placed in boiling water bath (100 °C) for 2 h. Ten milliliters of analytical grade chloroform was added and allowed to react for 1 h. The dried cannabis was extracted three times and fractions were combined, filtered over filter paper and collected in a 100 mL volumetric flask. The filtrate was evaporated under a gentle stream of nitrogen (on ice and protected from light and stored at 4 °C) and protected from light in an aluminum-covered container, which provided the dry extract as residue.
The residue (dry extract) is suspended in 2 mL of 96% ethanol and the total volume in the volumetric flask increased to 100 mL by adding distilled water. The extract was injected s.c. at doses of 5, 10 and 15 mg/kg (expressed as Δ9-THC). The injection volume was 0.3 ml/mouse. Tetrahydrocannabinol (THC) content was quantified using "GC mass spec". The dry extract contained 10% of the Δ9-tetrahydrocannabinol (Δ9-THC).
Cannabinoids are enzymatically biosynthesised in the plant as their corresponding carboxylic acid forms (Taura et al., 2007). Neutral cannabinoids are formed via decarboxylation (loss of CO2) of the acidic cannabinoids during exposure to light, heat (e.g. smoking), or as a result of prolonged storage (Thakur et al., 2005) (Fig. 1).
The decarboxylation is carried out by heat in water bath at 100 oC for time. The 'H-NMR proves that there is no signal corresponding to the carboxylic acid (COOH) around 12-13 ppm (Fig. 2).
STUDY DESIGN
Experiment 1: Effect of cannabis alone
Twenty eight mice were randomly divided into four groups; each of 7 animals. Mice of the 1st (normal control) group were injected s.c. with an ethanol/distilled water (5 ml/ kg). Animals of the 2nd, 3rd, 4th groups were injected s.c. with cannabis extract (5, 10, 15 mg/kg, respectively) daily for 24 days.
Experiment 2: Effect of antidepressant drugs
Mice were treated with ethanol/distilled water (1st; normal control), fluoxetine (20 mg/kg) (2nd group), sertraline (20 mg/kg) (3rd group) or imipramine (20 mg/kg) (4th group) s.c. daily for 24 days (n = 7/group).
Experiment 3: Effect of cannabis in combination with antidepressant drugs
Mice received ethanol/distilled water (1st; normal control), fluoxetine (20 mg/kg) + cannabis (5 mg/kg) (2nd group), sertraline (20 mg/kg) + cannabis (5 mg/kg) (3rd group) or imipramine (20 mg/kg) + cannabis (5 mg/kg) (4th group) s.c. daily for 24 days (n = 7/group).
In the three previously mentioned experiments, behavioral tests (rearing activity and forced swimming test) were done before the start of treatment (baseline), and then were examined twice weekly for 24 days. At the end of experimental period, mice were euthanized by decapitation under ether anesthesia. The brain of each mouse was excised and kept at -80 °C for estimation of reduced glutathione (GSH), lipid peroxidation (MDA) and nitric oxide determination in brain homogenate.
Behavioral tests
All behavioral tests were conducted in quiet rooms 1h after drug injection.
Forced-swimming test
This test was conducted according to the method of Porsolt et al. (1978). In brief, mice were placed individually in a glass cylinder (diameter 12 cm, height 24 cm) filled with water at a height of 12 cm, without the possibility of escaping. Water temperature was maintained at 25 ± 2°C. The animal was forced to swim for 6 min and the duration of immobility was measured. Mice were judged immobile when floating motionless or making only those movements necessary to keep its head above water. The duration of immobility was measured by an observer blind to the treatment conditions (Porsolt et al., 1978) after treatment with vehicle, only cannabis (5, 10 or 15 mg/kg, s.c.), fluoxetine, sertraline, imipramine or after cannabis (5 mg/kg) co-administered with the antidepressant drugs. Water was changed after every swimming test to eliminate urine, excrement, and fur. After the swimming session, the mice were removed from the cylinder, dried with towels, and placed gently under a heating lamp for 15-30 min.
Rearing activity
The open field was an acrylic round device (30 cm height) with a central circle (10 cm diameter). Mice were individually placed in the centre of the device. During the next 5 min the number of rears (R) (number of times seen standing on hind legs or on the wall) were recorded (Carbajal et al., 2009).
BIOCHEMICAL ANALYSIS
Lipid peroxidation
The lipid peroxides content in brain homogenate was determined by monitoring the thiobarbituric acid reactive substance formation as described by Ruiz-Larea et al. (1994). In brief, 0.5 ml of the supernatant of brain homogenate was added to exactly 4.5 ml of working reagent (1 volume from 0.8% thiobarbituric acid + 3 volume from 20% trichloroacetic acid). The mixture was incubated for 20 minutes in boiling water bath then left to cool and centrifuge at 4000 rpm for 5 minutes. The pink color (malondialdehyde) was measured at 535 nm against blank (distilled water instead of sample). Lipid peroxidation was expressed in terms of MDA equivalents using an extinction coefficient of 1.56><105 M-1 cm-1 and the results were expressed as nmol MDA/g tissue.
Reduced Glutathione
Determination of reduced glutathione content (GSH) in brain homogenate was determined according to the colorimetric method of Ellman (1959) modified by Bulaj et al. (1998). In brief, the brain was homogenized in phosphate buffered saline (pH 8.0) using a homogenizer. The homogenate was then centrifuged at 4000 rpm/5 minutes at 4 °C in a cooling centrifuge then 0.5 ml of the supernatant was added to 0.5 ml of trichloro-acetic acid 10%. The mixture was vortex-mixed and centrifuged at 4000 rpm/5 min at 4 °C. In a clean test tube; 1.8 ml of phosphate buffer pH 8.0 was added to 0.1 ml of the supernatant and 0.1 ml of Ellman reagent. The absorbance was read at wave length 412 nm after exactly 5 minutes against blank (distilled water instead of sample). Reduced glutathione levels were calculated using the extinction coefficient of 1.36x104 M-1 cm-1. The results were expressed in jimol GSH/g tissue.
Nitric Oxide
Nitric oxide was determined in rat brain homogenate using a colorimetric method based on the Griess reaction according to the method of Miranda et al. (2001) with minor modification. Briefly, in Eppendorf tubes; zinc sulphate 30% was used for deproteinization of the samples (0.5 ml of supernatant of brain homogenate + 0.5 ml of ZnSO4); the mixture was centrifuged at 4000 rpm/15 minutes. In 96-well plate; 100 |il of the obtained supernatants were placed in each well, followed by rapid addition of 50 µl sulfanilamide (2% (w/v) in 5% (v/v) HCl) and 50 µl: N-(1-naphthyl) ethylenediamine dihydrochloride (0.1% (w/v) in distilled water). The plate was then incubated at 37 °C for 45 minutes in a shaker water bath then cooled and the absorbance of the pink colored chromophore was measured at 540 nm using ELISA reader, against a blank treated in a similar manner to the test but using 100µl distilled water instead of the sample. A standard curve was constructed using serial dilutions ranging from 1.56100 µM of sodium nitrite (NaNO2) and was assayed using the previous method of nitrite determination. From the standard curve; an equation for calculation of nitrite concentration (µM) was determined. The level of total nitrite/nitrate in the brain homogenate was expressed as |imol/g tissue.
Statistical Analysis
Data are expressed as mean ± SEM. The data were analyzed by one way ANOVA and by repeated measures ANOVA, followed by Dunnett's test for multiple group comparisons, using SPSS software (SAS Institute Inc., Cary, NC). A probability value of less than 0.05 was considered statistically significant.
RESULTS
Behavioral tests
Forced-swimming test
Effect of cannabis alone
The immobility time in the Porsolt's forced-swimming test after the repeated administration of cannabis at doses of 5, 10 or 15 mg/kg is shown in figure 3A. The floating time was significantly increased in mice treated with cannabis (5-15 mg/kg, s.c.) starting from the 9th day post-injection and afterwards. The immobility time increased 79.6, 87.7 and 89.3% at the end of the study by cannabis given at 5, 10 or 15 mg/kg, respectively. There was a significant drug effect (F3,24 = 42.76; P = 0.001), time effect (F8,192 = 24.81; P= 0.001) or treatment x time interaction (F24,192 = 5.75; P = 0.001).
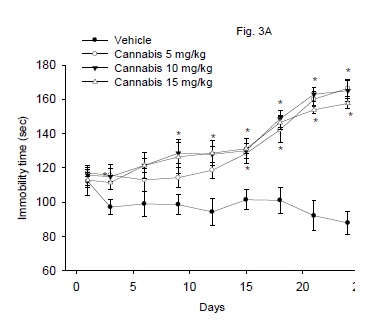


Effect of antidepressant drugs
Treatment of mice with fluoxetine, sertraline or imipramine resulted in significant decrease in the immobility time compared to vehicle control group (Fig. 3B). There was a significant drug effect (F3,24 = 38.42; P < 0.001), but no significant time effect (F8,192 = 0.95; P= 0.47) or treatment x time interaction (F24 192 = 0.63; P = 0.91).
Effect of cannabis in combination with antidepressant drugs
The immobility time in mice co-administered with fluoxetine (20 mg/kg, s.c.) and cannabis (5 mg/kg, s.c.) was significantly increased compared with the vehicle control group as well as with the cannabis only-treated group. By the day 21 of the study, however, the immobility time of the fluoxetine-cannabis group was significantly decreased compared with the cannabis only-treated group. Mice co-administered sertraline or imipramine with cannabis (20 mg/kg, s.c.) were not statistically different from the vehicle control group as regards their immobility time. Sertraline or imipramine significantly decreased the immobility time in cannabis-treated mice by the day 16 and 18 of the study, respectively. There was a significant drug effect (F4, 30 = 22.02; P < 0.001), time effect (F8,240 = 7.58; P= 0.001) or treatment x time interaction (F32,240 = 4.59; P = 0.001) (Fig. 3C).
Rearing activity
Effect of cannabis alone
Figure 4A illustrates the effect of cannabis extract on rearing activity. Repeated measures ANOVA indicated a significant treatment effect: F3,24 = 64.23; P < 0.001, significant time effect, F8,192 = 63.99; P = 0.001 and a significant treatment x time interaction, F24,192 = 2.97; P = 0.001. The rearing activity was significantly decreased by cannabis compared to vehicle control group. This decrease in the rearing behavior was dose and time-dependant and started 3 days after treatment with cannabis extract and continued throughout the study. The rearing activity decreased at the end of the study by 33.3, 51.1 and 58.9% after cannabis given at 5, 10 or 15 mg/kg, respectively.


Effect of antidepressant drugs
The results are shown in Fig. 4B. Repeated measures ANOVA indicated a significant treatment effect, F3,24 =62.58; P < 0.001, significant time effect, F8,192 = 55.03; P = 0.001 and significant treatment x time interaction, F24,192 = 3.02; P = 0.001. The rearing activity in mice treated cannabis was markedly and significantly increased by sertraline or fluoxetine compared with the cannabis-only treatment group. The number of rears in mice given cannabis + fluoxetine showed no significant difference from the vehicle control group. Sertraline resulted in significant increase in number of rears in mice after 12 days of treatment till the 24th day (28.6% increase vs. vehicle control group, p<0.05). Meanwhile, the administration of imipramine to mice resulted in significant decrease in the rearing behavior after 6 days of treatment till the end of the study, as compared to the vehicle control group (34.4% decrease vs. vehicle control group, p<0.05).
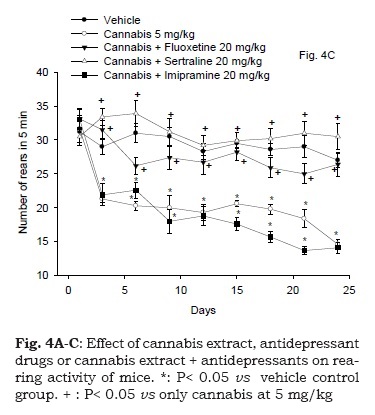
Effect of cannabis in combination with antidepressant drugs
Figure 4C demonstrates the rearing activity in mice following cannabis treatment in combination with antidepressant drugs. Repeated measures ANOVA indicated a significant treatment effect, F4,30 = 82.72; P < 0.001, significant time effect, F8,240 = 21.6; P = 0.001 and significant treatment x time interaction, F32,240= 4.18; P = 0.001. The administration of fluoxetine or sertraline at the dose of 20 mg/kg in combination with cannabis extract (5 mg/kg) resulted in no significant difference in the rearing activity of animals compared to vehicle control group. Mice treated with cannabis extract (5 mg/kg) and imipramine (20 mg/ kg) showed a decrease in rearing activity compared with the vehicle treated group. The decrease in the rearing activity of mice that received imipramine and cannabis extract started after 6 days of administration till the end of the study (47.8% decrease vs. vehicle control group, p<0.05).
Biochemical studies
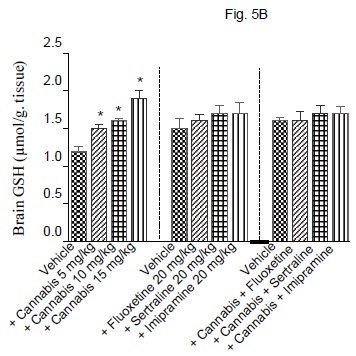
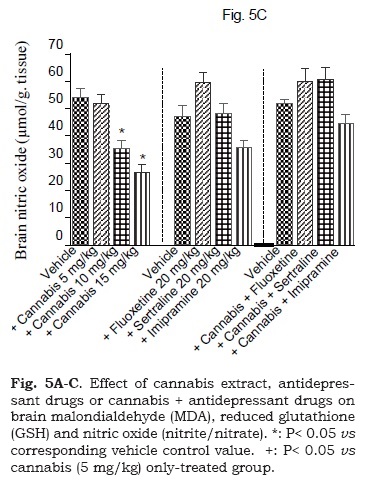
The administration of cannabis altered the redox status in brain with the effect being significant with the higher doses of the extract. Brain MDA was significantly decreased by the higher dose (15 mg/kg) of the extract (Fig. 5A). Brain GSH showed significant increase by 25, 3.3 and 58.3% after cannabis at 5, 10 or 15 mg/kg, respectively (Fig. 5B). Meanwhile, the administration of cannabis at 10 or 15 mg/ kg resulted in a significant decrease in brain nitrite by 34.5 and 50.5% (Fig. 5C). Brain MDA or GSH were not significantly altered by fluoxetine, sertraline or imipramine alone (Figs. 5A and 5B). The level of nitric oxide, however, increased by 26.8% after fluoxetine treatment, but decreased by 23.8% following impiramine (Fig. 5C). There was a significant decrease in lipid peroxidation by 18.1, 28.3 and 26.6% after co-administration of cannabis with either fluoxetine, sertraline or imipramine, respectively when compared with the vehicle control group (Fig. 5A).

DISCUSSION
This study investigated the effect of cannabis extract with known Δ9-THC content on the rearing activity and immobility in the forced swimming test in mice. The present findings indicate that the single and repeated daily administration of the extract at doses corresponding to 5, 10 and 15 mg Δ9-THC/kg resulted in a significant increase in immobility time. The administration of cannabis extract thus resulted in depressive-like effects. The increased immobility time was reversed by sertraline or imipramine, thereby, suggesting alteration of serotonergic and/or noradrenergic neurotransmission by cannabis. These results also indicate the ability of sertraline or imipramine to alleviate chronic stress due to repeated forced swimming. The SSRIs share the common property of inhibiting the reuptake of serotonin at synaptic terminals (Fuller, 1994). There is also an evidence suggesting inhibition of noradrenaline and dopamine reuptake by fluoxetine (Pozzi et al., 1999; Bymaster et al., 2002) and of dopamine reuptake by sertraline (Kitaichi et al., 2010). The tricyclic drug imipramine, on the other hand, is a dual inhibitor of the reuptake of both noradrenaline and serotonin (Felton et al., 2003).
Other researchers have shown that treatment with an endocannabinoid uptake inhibitor or CB1 receptor agonist induced comparable decreases in immobility in the forced swim test in rats (Hill and Gorzalka, 2005). In addition, the principal psychoactive component of marijuana Δ9-THC (2 and 6 mg/kg, i.p.) significantly prolonged the immobility time forced swim test; the effect being mediated by CB1 receptor- and 5-HT1A receptors (Egashira et al., 2008). Studies also suggested that females may be more sensitive to the effects of THC than males. Adolescent female rats treated with (THC) for 11 days and left until adulthood presented significant "behavioral despair' (forced swim test) paralleled by anhedonia (sucrose preference). In contrast, male rats showed no behavioral despair but did present anhedonia (Rubino et al. 2008). Daily injections of THC (2 mg/kg ) caused locomotor depression in both male and female rats dosed during early adolescence but only in female animals dosed during late adolescence (Harte et al., 2010). Chronic (20 days) adolescent but not adult daily injection of CB (1) receptor agonist led to anxiety and depression-like behavior in the forced swim and sucrose preference test in rats. Serotonergic hypoactivity and noradrenergic hyperactivity were observed (Bambico et al., 2010). It has been suggested that significant alterations in serotonergic systems may be rather related to acute activation of the endogenous cannabinoid system or to cannabis dependence accompanied by manifest depressive symptoms (Rose et al., 2009). Studies in CB1-knockout on the other hand showed the opposite results. Thus, reduced exploration of the open arms of the plus-maze apparatus by CB1-knockout compared with wild-type animals was reported (Haller et al., 2002). CB1 knockout mice also showed a higher sensitivity to exhibit depressive-like responses in the chronic unpredictable mild stress procedure suggesting an increased susceptibility to develop an anhedonic state (Martin et al., 2002). In humans, inconsistent data exists as regards whether cannabis causes depression or not. In one survey, daily or once weekly adult users of cannabis reported less depressed mood and more positive affect than non-users (Densona and Earleywine, 2005). Other studies suggested that cannabis abuse was a risk factor for the development of depressive symptoms (Gregory and Bovasso, 2001) and that cannabis dependence was highly associated with independent depression (Dakwar et al., 2011). Cannabis-dependent subjects had lower levels of motivation, happiness, and satisfaction with life, with higher levels of depression (Looby and Earleywine, 2007). Depressive disorders were Behavioral and biochemical effects of Cannabis Sativa and their modulation by antidepressant drugs Rev. Latinoamer. Quím. 41/1(2013) 31 frequent in adolescents with substance (alcohol and cannabis) use disorder (Findling et al., 2009) and the intake of cannabis has been associated with increased prevalence of depressive disorders (Bovasso, 2001), anxiety and mood disorders (Cheung et al., 2010). Moreover, among patients with bipolar disorder, cannabis users exhibited less compliance and higher level of overall illness severity compared with non-users (van Rossum et al., 2009).
The present findings also indicated that cannabis administration was associated with decreased rearing activity of mice. The latter is a frequently used measure of anxiety-like behavior (Henderson et al., 2004); those with less exploratory activity (i.e. rearing) were considered more anxious (Bouwknecht et al., 2007; Bogdanov et al., 2012). It has been suggested that rearing is a useful marker of environmental novelty, that the hippocampal formation is a crucial component of the system controlling rearing in novel environments (Lever et al., 2006). In other studies, decreased rearing has been taken as an indication of reduced anxiety (González-Trujano et al., 2006). The results of the present study are in accordance with other studies showing decreased rearing activity after chronic THC treatment in rats (Miczek, 1976, 1979; Miczek and Dixit, 1980). The rearing activity in rats was also decreased by the endogenous endocannabinoid anandamide (Fride and Mechoulam, 1993). Studies also showed that THC strongly affects rearing activity more strongly than locomotion with tolerance being evident to the latter, but with no recovery of the reduced rearing activity when exposure to THC was continued for weeks (Miczek and Dixit, 1980; Miller and Drew, 1974). In the present study, the decrease in rearing activity was ameliorated by the SSRIs fluoxetine or sertraline, suggesting amelioration of the effect of cannabis on exploratory behavior by the antidepressant drugs.
Oxidative stress has been implicated in pathogenesis of depression and several other brain disorders such as Parkinson's disease, Alzheimer's disease, bipolar disorder, major depression and schizophrenia (Sian et al., 1994; Schulz et al., 2000; Yao et al., 2006; Lavoie et al., 2011; Gawryluk et al., 2011). In addition, there is evidence to support a benefit from increasing brain glutathione levels (e.g., via the glutathione precursor N-acetylcysteine) in conditions such as schizophrenia (Ng et al., 2008; Duarte et al., 2011). In the present study, the repeated administration of cannabis for 24 days was associated with significant and dose-dependent increase in brain GSH. The latter is a major antioxidant and plays an important role in the maintenance of the redox status of the cell and in protecting against oxidative damage by reactive oxygen species (Wang and Ballatori, 1998). Malondialdehyde, a marker of lipid peroxidation (Gutteridge, 1995) showed a significant decrease in brain by the highest dose of cannabis, suggesting an antioxidant effect for the extract. Moreover, the co-administration of fluoxetine, sertraline or imipramine with cannabis was associated with a significant decrease of brain MDA. Indeed, a neuroprotective effect of cannabis has been suggested. In rat cortical neuronal cultures, Δ9-THC and cannabidiol, a nonpsychoactive component of Cannabis sativa, exhibited antioxidant activity and reduced glutamate toxicity by cannabinoid receptor-independent mechanism (Hamp-son et al., 1998).
In the present study, nitric oxide (the concentrations of nitrite/nitrate) is also decreased in the brain following cannabis injection. Lifetime major depressive disorder patients had lower total plasma nitrite/ nitrate concentrations compared to healthy controls (Wagner et al., 2011; García et al., 2011). Other studies, however, reported elevated plasma nitrate concentrations in depression (Suzuki et al., 2001). In the present study; nitric oxide is increased in the brain by fluoxetine. In contrast, nitric oxide is decreased by imipramine. The coadministration of fluoxetine or sertraline with cannabis was associated with near normal values of nitric oxide. In several studies antidepressant drugs have been shown to modulate nitric oxide release within the brain (Ha et al., 2006; Liu et al., 2011; Krass et al., 2011). Studies also suggested the involvement of nitric oxide in depression and in the mood elevating action of antidepressant drugs. Thus pre-treatment with L-arginine counteracted the antidepressant-like effect of imipramine, venlafaxine and bupropion (Krass et al., 2011), while inhibition of nitric oxide levels within the hippocampus can induce antidepressant like effects (Joca and Guimaracs, 2006). In those with lifetime major depressive disorder antidepressant medications were associated with higher NO in plasma (Wagner et al., 2011). Meanwhile, the antidepressant action of imipramine and venlafaxine involves suppression of nitric oxide synthesis (Krass et al., 2011). Rats exposed to chronic forced swimming test showed depressive-like behavior with alteration in platelet morphology, activity and platelet nitric oxide synthesis, and/or in 5-HT concentrations; these changes were prevented by fluoxetine (González-Trujano et al., 2012).
The psychoactive effects of cannabis preparations are largely mediated by Δ9-THC (Gaoni and Mechoulam, 1964). The latter is the major cannabinoid found in marijuana and hashish, and constituted 10% of the extract used in the present work, making it the most likely candidate responsible for the effects of cannabis observed in the present work. Cannabis sativa, however, contains more than 600 different chemical compounds including over 70 different cannabinoids. In addition to Δ9-THC, other important cannabinoids include cannabidiol (CBD), cannabinol (CBN) and tetrahydrocannabivarin (THCV). These can result in different effects from those of, Δ9-THC alone since Δ9-THC, is a CB1 and CB2 receptor partial agonist while Δ9-THCV, behaves either as a CB1 antagonist or, at higher doses, as a CB1 receptor agonist (Pertwee, 2008). Whilst Δ9-THC prolonged the immobility time forced swim test (Egashira et al., 2008), other cannabinoids e.g., cannabidiol exhibited antidepressant-like effects (Zanelati et al., 2010). The involvement of 5-HT1A receptors has been suggested. The chemistry of cannabis is thus a complex one and it is likely that the final effect of the extract will depend on the relative abundance of different cannabinoids, their interaction as well as interaction with other non-cannabinoid constituents.
CONCLUSIONS
The administration of cannabis for 24 days in mice resulted in decreased brain oxidative stress but induced a significant increase in immobility in the forced-swimming test and a decrease in the rearing activity, suggesting a depressant- and anxiety-like effect. These latter effects were improved by antidepressant drugs.
CONFLICTS OF INTEREST
There are no conflicts of interest.
ACKNOWLEDGMENTS
For the supply of Cannabis sativa plant the authors are indebted to the Ministry of Justice of the Arab Republic of Egypt.
REFERENCES
Ameri A (1999) The effects of cannabinoids on the brain. Progress in Neurobiology 58:315-348. [ Links ]
Ashton CH (2001) Pharmacology and effects of cannabis: A brief review. British Journal of Psychiatry 178:101-106. [ Links ]
Bambico FR, Nguyen NlT, Katz N, Gobbi G (2010) Chronic exposure to cannabinoids during adolescence but not during adulthood impairs emotional behavior and monoaminergic neurotransmission. Neurobiology of Disease 37:641-55. [ Links ]
Bogdanov VB, Bogdanova OV, Koulchitsky SV, Chauvel V, Multon S, Makarchuk MY, Brennan KC, Renshaw PF, Schoenen J (2013) Behavior in the open field predicts the number of KClinduced cortical spreading depressions in rats. Behavioural Brain Research 236:90-93. [ Links ]
Bouwknecht JA, Spiga F, Staub DR, Hale MW, Shekhar A, Lowry CA (2007) Differential effects of exposure to low-light or high-light open-field on anxiety-related behaviors: relationship to c-Fos expression in serotonergic and non-serotonergic neurons in the dorsal raphe nucleus. Brain Research Bulletin 72:32-43. [ Links ]
Bovasso GB (2001) Cannabis abuse as a risk factor for depressive symptoms. American Journal of Psychiatry 158:2033-2037. [ Links ]
Bulaj G, Kortemme T, Goldenberg DP (1998) Ionization-reactivity relationships for cysteine thiols in polypeptides. Biochemistry 37:8965-72. [ Links ]
Bymaster FP, Zhang W, Carter PA, Shaw J, Chernet E, Phebus L, Wong DT, Perry KW (2002) Fluoxetine, but not other selective serotonin uptake inhibitors, increases norepinephrine and dopamine extracellular levels in prefrontal cortex. Psychopharmacology (Berl) 160: 353-361. [ Links ]
Carbajal D, Ravelo Y, Molina V, Mas R, Arruzazabala ML (2009) D-004, a lipid extract from royal palm fruit, exhibits antidepressant effects in the forced swim test and the tail suspension test in mice. Pharmacology Biochemistry and Behavior 92: 465-468. [ Links ]
Cheung JT, Mann RE, Ialomiteanu A, Stoduto G, Chan V, Ala-Leppilampi K, Rehm J (2010) Anxiety and mood disorders and cannabis use. American Journal of Drug & Alcohol Abuse 36:118-22. [ Links ]
Dakwar E, Nunes EV, Bisaga A, Carpenter KC, Mariani JP, Sullivan MA, Raby WN, Levin FR (2011) A Comparison of Independent Depression and Substance-Induced Depression in Cannabis-, Cocaine-, and Opioid-Dependent Treatment Seekers. American Journal on Addictions 20: 441-446. [ Links ]
Denson TF, Earleywine M (2006) Decreased depression in marijuana users. Addictive Behaviors 31:738-742. [ Links ]
Duarte JM, Kulak A, Gholam-Razaee MM, Cuenod M, Gruetter R, Do KQ (2011). N- N-Ace-tylcysteine normalizes neurochemical changes in the glutathione-deficient schizophrenia mouse model during development. Biological Psychiatry 2011 [Epub ahead of print] [ Links ]
El-Alfy AT, Ivey K, Robinson K, Ahmed S, Radwan M, Slade D, Khan I, ElSohly M, Rossb S (2010). Antidepressant-like effect of Δ9-tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa L. Pharmacology Biochemistry and Behavior 95: 434-442. [ Links ]
Egashira N, Matsuda T, Koushi E, Higashihara F, Mishima K, Chidori S, Hasebe N, Iwasaki K, Nishimura R, Oishi R, Fujiwara M (2008) Δ9-tetrahydrocannabinol prolongs the immobility time in the mouse forced swim test: Involvement of cannabinoid CB1 receptor and serotonergic system. European Journal of Pharmacology 589:117-121. [ Links ]
Ellman GL (1959) Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics 82: 70-77. [ Links ]
Felton TM, Kang TB, Hjorth S, Auerbach SB (2003) Effects of selective serotonin and serotonin/noradrenaline reuptake inhibitors on extracellular serotonin in rat diencephalon and frontal cortex. Naunyn Schmiedebergs Arch Pharmacol 367: 297-305. [ Links ]
Fride E, Mechoulam R (1993) Pharmacological activity of the cannabinoid receptor agonist, anandamide, a brain constituent. European Journal of Pharmacology ; 231 :313-314. [ Links ]
Fuller RW (1994) Uptake inhibitors increase extracellular serotonin concentration measured by brain microdialysis. Life Sciences ence 55:163-167. [ Links ]
Gaoni Y, Mechoulam R (1964) Isolation, structure and partial synthesis of an active constituent of hashish. Journal of the American Chemical Society 86: 1646-1647. [ Links ]
García RG, Zarruk JG, Barrera C, Pinzón A, Trillos E, Arenas WD, Luengas C, Tomaz C, López-Jaramillo P (2011) Plasma nitrate levels and flow-mediated vasodilation in untreated major depression. Psychosomatic Medicine 73:344-349. [ Links ]
Gawryluk JW, Wang JF, Andreazza AC, Shao L, Yatham LN, Young LT (2011) Prefrontal cortex glutathione S-transferase levels in patients with bipolar disorder, major depression and schizophrenia. International Journal of Neuropsychopharmacology 14:1069-1074. [ Links ]
González-Trujano ME, Alvarado-Vásquez N, Mendoza-Sotelo J, López G, Estrada-Camarena E, Martínez-Mota L, Moreno J (2012). Alterations on the morphology, nitric oxide synthesis and activity of platelets reproduced in rats as possible biomarkers for depression are reversed by fluoxetine. Pharmacology Biochemistry and Behavior 102:349-56. [ Links ]
González-Trujano ME, Martínez AL, Reyes-Ramírez A, Reyes-Trejo B, Navarrete A (2006) Palmitone isolated from Annona diversifolia induces an anxiolytic-like effect in mice. Planta Medica 72:703-707. [ Links ]
Gutteridge JMC (1995) Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clinical Chemistry 41: 1819-1828. [ Links ]
Ha E, Jung KH, Choe BK, Bae JH, Shin DH, Yim SV, Baik HH (2006) Fluoxetine increases the nitric oxide production via nuclear factor kappa B-mediated pathway in BV2 murine microglial cells. Neuroscience Letters 397: 185-189. [ Links ]
Hall W, Solowij N, Lemon J (1994) The health and psychological consequences of cannabis use. National Drug Strategy Monograph Series no 25, Canberra: Australian Government Publishing Service. [ Links ]
Haller J, Bakos N, Szirmay M, Ledent C, Freund TF (2002) The effects of genetic and pharmacological blockade of CB1 cannabinoid receptor on anxiety. European Journal of Neuroscience 16:1395-1398. [ Links ]
Hampson AJ, Grimaldi M, Axelrod J, Wink D (1998) Cannabidiol and (-)D9-tetrahydrocannabinol are neuroprotective antioxidants. Proceedings of the National Academy of Sciences USA 95: 8268-8273. [ Links ]
Harte LC, Dow-Edwards D (2010) Sexually dimorphic alterations in locomotion and reversal learning after adolescent tetrahydrocannabinol exposure in the rat. Neurotoxicology and Teratology 32:515-524. [ Links ]
Henderson ND,Turri MG, DeFries JC, Flint J (2004) QTL analysis of multiple behavioral measures of anxiety in mice. Behavior Genetics 34:267-293. [ Links ]
Hill MN, Gorzalka BB (2005) Pharmacological enhancement of cannabinoid CB1 receptor activity elicits an antidepressant-like response in the rat forced swim test. European Neuropsychopharmacology 15: 593-599. [ Links ]
Hill MN, Miller GE, Carrier EJ, Gorzalka BB, Hillard CJ (2009) Circulating endocannabinoids and N-acylethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology 34:1257-1262. [ Links ]
Huestis MA (2002) Cannabis (Marijuana)-Effects on human behavior and performance. Forensic Science Review 14:15. [ Links ]
Joca SR, Guimarães FS (2006) Inhibition of neuronal nitric oxide synthase in the rat hippocampus induces antidepressant-like effects. Psychopharmacology (Berl) 185: 298-305. [ Links ]
Kaufmann RM, Kraft B, Frey R, Winkler D, Weiszenbichler S, Backer C, Kasper S, Kress HG (2010) Acute psychotropic effects of oral cannabis extract with a defined content of Delta9-tetrahydrocannabinol (THC) in healthy volunteers. Pharmacopsychiatry 43:24-32. [ Links ]
Kitaichi Y, Inoue T, Nakagawa S, Boku S, Kakuta A, Izumi T, Koyama T (2010) Sertraline increases extracellular levels not only of serotonin, but also of dopamine in the nucleus accumbens and striatum of rats. European Journal of Pharmacology 647:90-96. [ Links ]
Krass M, Wegener G, Vasar E, Volke V (2011) The antidepressant action of imipramine and venlafaxine involves suppression of nitric oxide synthesis. Behavioural Brain Research 218: 57-63. [ Links ]
Lavoie S, Allaman I, Petit JM, Do KQ, Magistretti PJ (2011) Altered glycogen metabolism in cultured astrocytes from mice with chronic glutathione deficit; relevance for neuroenergetics in schizophrenia. PLoS One 6:e22875. [ Links ]
Lever C, Burton S, O'Keefe J (2006) Rearing on hind legs, environmental novelty, and the hippocampal formation. Review of Neuroscience 2006; 17:111-133. [ Links ]
Liu D, Wang Z, Liu S, Wang F, Zhao S, Hao A (2011) Anti-inflammatory effects of fluoxetine in lipopolysaccharide (LPS)-stimulated microglial cells. Neuropharmacology 61: 592-599. [ Links ]
Liu WM, Fowler DW, Dalgleish AG (2010) Cannabis-derived substances in cancer therapyan emerging anti-inflammatory role for the cannabinoids. Current Clinical Pharmacology 5:281-287. [ Links ]
Looby A, Earleywine M (2007) Negative consequences associated with dependence in daily cannabis users. Substance Abuse Treatment, Prevention, and Policy 2:3. [ Links ]
Machado Rocha FC, Stéfano SC, De Cássia Haiek R, Rosa Oliveira LM, Da Silveira D (2008) Therapeutic use of Cannabis sativa on chemotherapy-induced nausea and vomiting among cancer patients: systematic review and meta-analysis. European Journal of Cancer Care (Engl) 17:431-443. [ Links ]
Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O (2002) Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology 159:379-387. [ Links ]
Miczek KA (1976) Mouse-killing and motor activity" effects of chronic Δ9..tetrahydrocannabinol and pilocarpine. Psychopharmacology (Berl) 47: 59-64. [ Links ]
Miczek KA (1979) Chronic Δ9-tetrahydrocannabinol in rats: effect on social Interactions, mouse killing, motor activity, consummatory behavior, and body temperature. Psychopharmacology (Berl) 60: 137-146. [ Links ]
Miczek KA, Dixit BN (1980) Behavioral and biochemical Effects of chronic Δ9-tetrahydrocannabinol in rats. Psychopharmacology (Berl) 67: 195-202. [ Links ]
Miller LL, Drew WG (1974) Cannabis: Review of behavioral effects in animals. Psychological Bulletin 81: 401-417. [ Links ]
Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for detection of nitrate and nitrite. Nitric Oxide 5: 62-71. [ Links ]
Ng F, Berk M, Dean O, Bush AI (1995) Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. International Journal of Neuropsychopharmacology 11: 851-876. [ Links ]
Pertwee RG, Ross RA (2002) Cannabinoid receptors and their ligands. Prostaglandins, Leuko-trienes and Essential Fatty Acids 66:101-121. [ Links ]
Pertwee RG (2005) Pharmacological actions of cannabinoids. Handbook of Experimental Pharmacology 168:1-51. [ Links ]
Pertwee RG (2008) The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta 9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. British Journal of Pharmacology 153:199-215. [ Links ]
Porsolt RD, Anton G, Deniel M, Jalfre M (1978) Behavioral despair in rats: a new animal model sensitive to antidepressant treatments. European Journal of Pharmacology 47:379-391. [ Links ]
Pozzi L, Invernizzi R, Garavaglia C, Samanin R (1999) Fluoxetine increases extracellular dopamine in the prefrontal cortex by a mechanism not dependent on serotonin: a comparison with citalopram. Journal of Neurochemistry 73: 1051-1057. [ Links ]
Roser P, Gallinat J, Weinberg G, Juckel G, Gorynia I, Stadelmann AM (2009) Psychomotor performance in relation to acute oral administration of Delta9-tetrahydrocannabinol and standardized cannabis extract in healthy human subjects. European Archives of Psychiatry and Clinical Neuroscience 259:284-292. [ Links ]
Rubino T, Vigano' D, Realini N, Guidali C, Braida D, Capurro V, Castiglioni C, Cherubino F, Romualdi P, Candeletti S, Sala M, Parolaro D (2008) Chronic Δ9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology 33: 2760-2771. [ Links ]
Ruiz-Larrea MB, Leal AM, Liza M, Lacort M, de Groot H (1994) Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. Steroids 59: 383-388. [ Links ]
Russo EB, McPartland JM (2003) Cannabis is more than simply D9-tetrahydrocannabinol. Psychopharmacology 165:431-432. [ Links ]
Sarandol A, Sarandol E, Eker SS, Erdinc S, Vatansever E, Kirli S (2007) Major depressive disorder is accompanied with oxidative stress: short-term antidepressant treatment does not alter oxidative-antioxidative systems. Hum Psychopharmacology 22: 67-73. [ Links ]
Sastre-Garriga J, Vila C, Clissold S, Montalban X (2011). THC and CBD oromucosal spray (Sa-tivex®) in the management of spasticity associated with multiple sclerosis. Expert Review of Neurotherapeutics11:627-637. [ Links ]
Schulz JB, Lindenau J, Seyfried J, Dichgans J (2000) Glutathione, oxidative stress and neurodegeneration. European Journal of Biochemistry 267: 4904-4911. [ Links ]
Selvarajah D, Gandhi R, Emery CJ, Tesfaye S (2010) Randomized placebo-controlled double-blind clinical trial of cannabis-based medicinal product (Sativex) in painful diabetic neuropathy. Diabetes Care 33: 128-130. [ Links ]
Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, Jenner P, Marsden CD (1994) Alterations in glutathione levels in Parkinson's disease and other neurodegenerative disorders affecting basal ganglia. Annals of Neurology 36: 348-355. [ Links ]
Sugiura T, Kobayashi Y, Oka S, et al (2002) Prostaglandins, Leukotrienes and Essential Fatty Acids 66: 173-192. [ Links ]
Suzuki E, Yagi G, Nakaki T, Kanba S, Asai M (2001) Elevated plasma nitrate levels in depressive states. Journal of Affective Disorders 63:221-224. [ Links ]
Svízenská I, Dubovy P, Sulcová A (2008) Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures - A short review. Pharmacology Biochemistry and Behavior 90:501-511. [ Links ]
Swift W, Gates P, Dillon P (2005) Survey of Australians using cannabis for medical purposes. Harm Reduction Journal 2:18. [ Links ]
Taura F, Sirikantaramas S, Shoyama Y, Shoyama Y, Morimoto S (2007) Phytocannabinoids in Cannabis sativa: recent studies on biosynthetic enzymes. Chemistry & Biodiversity 4: 1649-1663. [ Links ]
Thakur GA, Duclos RI, Makriyannis A (2005) Natural cannabinoids: templates for drug discovery. Life Sciences 78: 454-466. [ Links ]
Turner JC, Mahlberg PG (1984) Separation of Acid and neutral cannabinoids in Cannabis sativa L. using HPLC. In: Agurell S, Dewey WL, Willete RE (Eds.), Chemical Pharmacol Ther Agents. Academic Press, USA, pp79-88. [ Links ]
van Rossum I, Boomsma M, Tenback D, Reed C, van Os J (2009) EMBLEM Advisory Board. Does cannabis use affect treatment outcome in bipolar disorder? A longitudinal analysis. Journal of Nervous and Mental Disease 197:35-40. [ Links ]
Wagner JA, Tennen H, Finan PH, White WB, Burg MM, Ghuman N (2011) Lifetime History of Depression, Type 2 Diabetes, and Endothelial Reactivity to Acute Stress in Postmenopausal Women. Int J Behav Med [Epub ahead of print] [ Links ].
Wang W, Ballatori N (1999) Endogenous glutathione conjugates: occurrence and biological functions. Pharmacological Reviews 50:335-356. [ Links ]
Yager S, Forlenza MJ, MillerYanik GE, Erel MO, Kati M (2010) Depression and oxidative damage to lipids. Psychoneuroendocrinology 35: 1356-1362. [ Links ]
Yao JK, Leonard S, Reddy R (2006) Altered glutathione redox state in schizophrenia. Disease Markers 22:83-93. [ Links ]
Zanelati TV, Biojone C, Moreira FA, Guimarães FS, Joca SRL (2010) Antidepressant-like effects of cannabidiol in mice: possible involvement of 5-HT1A receptors. British Journal of Pharmacology 159: 122-128. [ Links ]





![Synthesis and anthelmintic evaluation of [2,5']-bis-heterocycles as bengazole analogs](/img/es/next.gif)








