Introduction
Rather than replacing tissue or organs damaged by disease, trauma, or hereditary reasons, the current clinical approach focuses on organ transplantation which, despite great improvements in their success rate, still poses a rejection risk from the recipients, and the life-long need for immunosuppressants1)(2)(3)(4)(5.
For a long time, the regeneration or manufacture of fully functional, living tissues was conceived as a product of imagination. Nonetheless, regenerative medicine (RM) arose two decades ago as a branch of medicine focused on the treatment of pathologies for which the only treatment available was the use of prosthetics, organ transplantation, or the removal of damaged tissue2)(4)(6.
Over time, this area has divided into two main medical strategies4: cell therapy, which seeks to restore the vital functions of the damaged tissue through drugs applied in vivo7, and tissue engineering, which makes use of scaffolds fabricated via additive manufacturing (AM) to provide cells with support and favorable conditions for proliferation and differentiation3)(4)(8)(9)(10)(11)(12)(13)(14)(15, and the biological functions of the tissue or organ to be replaced. This represents a challenge, as the number of apropos available materials is greatly reduced.
The construction and optimization of scaffolds is not without challenges16. It requires multidisciplinary efforts spanning from materials science to medicine2. One of the most ambitious issues regarding tissue engineering is the construction of highly complex, porous structures that imitate the biological function of the extracellular matrix13)(17)(18)(19. Developments in this area have been driven by the application of AM; particularly, 3D printing8)(20)(21)(22, a materials engineering technique to form intricate structures by depositing fine threads of material, layer by layer11)(21)(22)(23)(24)(25. This has made it possible to manufacture parts with customized morphological characteristics18)(26, intended to fulfill specific biological functions. Other advantages of this approach are scalability, control of physical characteristics, cost-benefit relationship27, and the possibility of manufacturing parts with greater complexity than those that could be achieved with classical manufacturing techniques28.
The first AM systems, developed in the 1980s, were aimed at the production of small prototypes25; only until recently they been extensively applied in the field of tissue engineering, implants for cranial and spinal surgery, and prosthetics, among others8)(11)(21. Despite of it, the extensive application of additive manufacturing for medical purposes is hindered by several issues like the need to fabricate highly porous structures, the development of biocompatible materials suited for AM, the need to improve mechanical strength, and the need to improve printing resolution2)(16)(17.
In conclusion, RM has made great strides in recent years, and the development of AM has played a key role in this progress. However, there is still much work to do to increase the availability of RM therapies based on this manufacturing technology. With continued research and development, it is likely that we will see more and more innovative applications of AM in the medical field.
This search aimed to gather relevant information on the limitations, significance, and applications of additive manufacturing in regenerative medicine.
Materials and methods
The method employed in the development of this review article involved a systematic literature search to identify recent advances in tissue engineering and the utilization of additive manufacturing techniques. The following steps were undertaken:
Literature Search
Comprehensive bibliographic research was conducted to identify relevant articles, research papers, reviews, and conference proceedings related to tissue engineering, additive manufacturing, and regenerative medicine. Multiple academic databases were utilized to ensure a wide coverage of the literature. Keywords and phrases used in the search included "tissue engineering," "regenerative medicine," "additive manufacturing," "3D printing," "bioprinting," "scaffolds," and "recent advances." In the composition of this review article, an exhaustive examination of scholarly literature was undertaken, encompassing a comprehensive array of 104 distinct works. Within this assemblage, a discerning selection process led to the incorporation of 61 articles that not only facilitated the synthesis of pertinent information but also served as foundational references for the present work. The curation of these sources was meticulously governed by predefined inclusion criteria, the succinct explication of which shall be provided.
Inclusion and Exclusion Criteria
Articles were screened based on predetermined inclusion and exclusion criteria. Inclusion criteria included peer-reviewed articles published within the last five years to ensure the inclusion of recent advancements. The articles that focused on the application of additive manufacturing techniques in tissue engineering and regenerative medicine were considered for further analysis. Articles not written in English or lacking relevance to the topic were excluded.
Data Extraction and Analysis
The selected articles were carefully reviewed, and relevant information regarding additive manufacturing techniques, materials, and their applications in regenerative medicine were extracted. The information extracted included details about the types of materials used, fabrication methods, bioprinting techniques, and their impact on cell viability and tissue regeneration. Additionally, information related to challenges and future directions in the integration of additive manufacturing technologies with regenerative medicine was also extracted.
Data Synthesis and Manuscript Organization
The extracted information was synthesized and organized into coherent sections, following the logical flow of the review article. The sections were structured to provide a comprehensive understanding of recent advances in additive manufacturing techniques for regenerative medicine. Special attention was given to the categorization and presentation of the materials used, additive manufacturing techniques employed, and their integration with regenerative medicine.
Result and discussion
Biomaterials
Biomaterials are characterized by their capability to be used within or in conjunction with biological organisms. It is necessary that these materials have characteristics such as biocompatibility, resistance to corrosion, apropos mechanical properties, and absence of carcinogenic factors15)(29)(30. Current literature clearly distinguishes between natural and synthetic materials9)(10)(23. These, in turn, can be classified as polymers, ceramics, glasses, and metals31, which we proceed to discuss in the following sections.
Metals
Some metals and alloys are biocompatible and have been widely used in the medical field as support structures due to their high mechanical strength, good conformity, and resistance to corrosion. However, some of their main disadvantages are low biocompatibility and high corrosion in biological environments, shortening the useful lifetime of these materials.
Applications where metals are mostly used include, fracture repair screws, and replacement prosthetics for limbs and joints.
Stainless Steel
Stainless steel represents a set of alloys with a high chromium content and different concentrations of nickel. Chromium is an element with a high affinity for oxygen, which is why it forms a rich oxygen layer on the surface of the piece, preventing it from advancing to the internal areas. The first compound used as an implant was stainless steel 316. Later, this alloy was substituted by a low-carbon version known as the 316L. However, this alloy also has a high nickel content, which can cause allergic reactions. Nitrogen has also been found to be a stabilizer of the austenitic phase of iron, so it could serve as a replacement for this element in the 316L SS, forming the ASTM 1686 variant, even though it does not have sufficient resistance to corrosion; thus, it is only used as a temporary implant. For this reason, new alloys are currently being sought that meet the demands of clinical medicine.
Cobalt-based alloys
These alloys are specially used in applications where resistance to wear is required. This alloy was first used in the aerospace sector, but due to its higher resistance to corrosion, compared with stainless steel, and excel-lent mechanical properties, it began to be used to manufacture medical implants.
Titanium-based alloys
Titanium is a low-density material that hardens considerably when alloyed, or by mechanical treatment. It is widely used in the manufacture of prosthetics and implants due to its high resistance to corrosion. Titanium alloys are part of the category of bioinert materials, which means they do not interact with sur-rounding tissue.
Polymers
Polymers are long chains of smaller carbon molecules, called monomers. These exist both naturally and synthetically25)(32)(33. Biopolymers are a special class of naturally derived polymers.
Natural Polymers
The main advantage of these materials is that cells can easily adhere and proliferate, and that they also have excellent biocompatibility6. One of the disadvantages, compared to synthetic polymers, is their reduced mechanical properties13)(30, difficulty in processing, and reduced availability34. Still, it is possible to use both types of polymers by combining the mechanical stability of synthetic polymers and the biocompatibility of natural ones3.
Alginate
It is a polysaccharide derived mainly from marine algae. It has been widely used in bioprinting due to its low cost, good biocompatibility, and rapid gelation35. Alginate undergoes a sol-gel reaction in the presence of Ca2+ ions, which are found in compounds such as CaCl2 and CaSO430, making it suitable for certain additive manufacturing techniques such as drop printing, which will be addressed later.
Synthetic Polymers
These materials usually allow for more efficient control of degradation rate and mechanical properties9)(13. For this reason, they have been developed as rapidly as biomaterials in recent years36. Of the synthetic polymers that have been used, the following stand out:
Polycaprolactone (PCL)
Polycaprolactone is a biodegradable polymer37 with a low melting point17. It is one of the most used polymers due to its physical and chemical properties, as well as its pliability38 most importantly: PCL has been approved by the Food and Drug Administration (FDA) for use in humans 39. The use of this material in combination with other elements such as hydrogel has been studied to form scaffolds with physical and chemical characteristics that are more suitable for certain applications, such is the case of Liang Dong, et al. who created one of these hybrid scaffolds for use in bone tissue 40.
Polylactic acid (PLA)
PLA is a biodegradable aliphatic polyester. It is a hydro-phobic material with a relatively long degradation period41. Due to its mechanical properties, various applications for this material have been explored, ranging from tendon regeneration41)(42 to the design of temporary implants that release drugs at a given place, while degrading43.
Pluronic F-127
Pluronic F-127 is a synthetic copolymer based on three blocks: polyethylene glycol, polypropylene glycol, and polyethylene glycol (PEO-PPO-PEO)35)(44. This substance has the characteristic of forming micelle structures that solidify at a certain temperature, called the micelle temperature. At lower temperatures, this sub-stance is in a liquid state35.
Bioinks
These materials consist of admixtures of hydrogels and cells22. Hydrogels are polymeric substances capable of absorbing large amounts of water26)(30)(45 and play an important role in tissue engineering providing mechanical support for the cells. Although they have been tried extensively, they still lack certain mechanical properties such as rigidity and high Young's modulus, necessary for applications requiring support for high mechanical loads46. However, their physical and chemical characteristics are quite malleable to fit a wide range of tissue engineering needs47; additionally, it has been possible to create hybrid bioinks that promote proliferation and differentiation48. Conductive hydrogels are being developed to be used as biosensors49.
Ceramics
Ceramics are high-hardness materials2, suitable for applications requiring high resistance to corrosion and low friction; their main disadvantage is that they are brittle and do not support high mechanical loads.
Hydroxyapatite
Among the ceramics most used in tissue engineering is hydroxyapatite; this material is naturally found in bones and tooth enamel.
Glasses
These are materials composed primarily of silicon, tempered with sodium, calcium, and phosphorus oxides50. The advantage of bio-glasses has been determined for bone regeneration, due to their capability to maintain osteoblasts and bond with both soft and dense tissue31. However, they may have low degradation rates.
Composite Materials
Sometimes a material is not suitable on its own to support high mechanical loads; this is the idea behind composite materials, where the goal is to give the scaffold greater mechanical strength and retain characteristics of interest such as its propensity for cell fixation.
Scaffolds can also be provided with growth factors that interact with cells to attract, differentiate, or guide them in the desired direction34.
Bioprinting
Manufacturing techniques have been designed with the hope of creating increasingly complex morphologies using biomaterials to create different prosthetics and implants impossible to achieve through subtractive manufacturing techniques - as they are known nowadays.
The integration of AM as part of the RM arsenal of solutions has led to the conception of a new medical subarea known as tissue engineering, based on the use of scaffolds, cells and growth factors2)(13)(15)(22)(51. The first three-dimensional printing techniques were not conceived as a tool for regenerative medicine, so materials were used that had a high melting point or cross-linking agents that caused damage to cells52, for this reason new techniques and materials have been developed to forward the field of tissue engineering.
Each of these materials processing forms has its advantages and disadvantages26. The most used techniques are based on extrusion, laser and drop11)(33)(53)(54.
Drop Printing
It was the first bioimpression technique used27 although there are variations in this technique, they all have a common ink reservoir. The ink is conducted to a small orifice or nozzle, where, due to the liquid’s surface tension, it cannot come out.
To force the ink out through the nozzle, pressure is applied by means of thermal, acoustic, or piezoelectric methods11)(30)(52. In the first method, a thermal actuator is used to heat the solution. This increase in temperature causes the material to expand, which generates the necessary pressure to expel the drop11. It has been found that the increase in temperature can cause cell damage, reducing cellular viability54.
In the second case, a piezoelectric actuator drives the drop out, through the nozzle. Although this method has the advantage of uniformity in drop size, it is possible to cause lysis to cells if used at high frequency11.
Finally, there is the acoustic actuator, it is designed to converge sound waves at the tip of the nozzle, forcing the drop down.
The disadvantage in this system is that the material must be in liquid form to form the drops. However, this problem can be solved by using a prepolymer. Which, as its name implies, would be a more fluid molecular antecedent of the intended polymer.
Once the prepolymer is expelled, cross-linking between molecules is facilitated to induce material gelation. With this reaction, the newly gelled polymer molecules will remain fixed at the desired location. It is important to note that the deposited layer must be fully gelled before printing a new layer on top of it. Therefore, the gelation time must be shorter or like the drop deposition time55.
Different configurations have been explored to facilitate cross-linking. These range from printing the prepolymer directly onto the cross-linking agent, to spraying the prepolymer with the cross-linking agent53.
This technique has the advantage of high resolution, high speed, and low equipment cost. These advantages have allowed commercial inkjet printers to be modified to work with bio-inks, as is the case of Arai et al. (2011)56, who modified a commercial printer to work with a prepolymer (sodium alginate), which was deposited in two-dimensional patterns, dictated by a bitmap image. In the end, the ability of the equipment to create complex structures in three dimensions was demonstrated, using simple cross-sections.
The viscosity of the bioink is one of the important parameters during the printing of alginate with the technique described in this section. However, this has been easily modified by adding a small percentage of the cross-linking agent30)(57.
Finally, one of the main problems that has been tried to solve is that this technique is based on the use of hydrogels, which lack the mechanical properties necessary to be used in in vivo applications. Therefore, to increase the effectiveness of this technique, new materials must be developed, which must have mechanical properties like the tissue they intend to replace11.
Extrusion
Printers that use extrusion create pressure on the material by means of pneumatic valves or mechanical pressure30)(32)(57)(35. They have the disadvantage of low resolution compared to other techniques35. However, due to the simplicity of operation and low fabrication cost, these have become widely disseminated.
Fused deposition modeling
This is the most widely spread and popular technique. It is based on the deposition of melted thermoplastic material through a small opening or nozzle8)(23. The material deposition follows a calculated trajectory using CAD software18)(52. Material rheology and thermal transfer properties are critical to determine its suitability for this technique8.
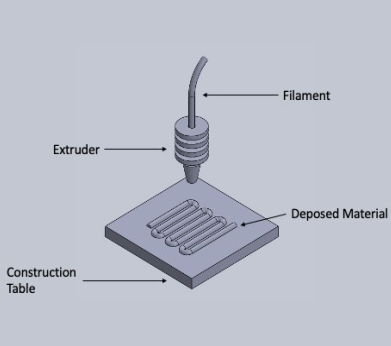
The scaffold is built layer by layer and it is important that the temperature of the material being worked is kept within a range where it can flow without dripping6. The temperature must also be suitable for melting and adhering to the deposited material, giving the scaffold rigidity and integrity8. To maintain the piece temperature at the appropriate level, it is common to use a heated construction bed. Figure 1 is a schematic representation of the operation of this technique.
Although there have been many advances in this technique, there are still problems to be solved, one of which involves the difficulty of incorporating cell deposition during the printing process, since the working temperature is high enough to cause cell damage and the extrusion can cause shear stresses which also impact in the cell viability6. Fahmy, et al. (2016), addressed this problem by means of a low melting point polymer, such as PCL, used as a thermal shield, and a high melting point polymer, such as PLA, as a mechanical support material. In the end, they concluded that PCL can completely block heat flow and increase cell viability.
A different approach consists of printing the scaffold without cells, then colonizing it in a bioreactor. However, in these cases, there is a cell concentration gradient with a high concentration on the scaffold surface and a decline in central areas. To avoid this situation, Ozbolat, et al. (2014)58 developed a double-nozzle bio-printer, in which one of the nozzles injects a biogel, and the other cell spheroids. This way, the cell distribution is uniform and can reach the central parts of the scaffold.
In principle, as many nozzles and materials can be incorporated as required, meaning there is no limitations to the composition gradients in any of the three axes8. Using this, Kang, et al. (2016)59 designed and built a printer with 4 nozzles, capable of depositing polycaprolactone, a hydrogel as a support and two different types of cells. The work mentioned stands out in its importance when it is considered that one of the challenges faced by additive manufacturing is the generation of hollow structures that emulate body structures, for example, capillary vessels, which are highly desirable structures. With aims of building these structures systems have been designed that deposit a sup-port material that acts as a support for the upper layers. Once the printing has finished, the support material can be removed. This material is known as sacrificial material or fugitive ink.
On the other hand, Adamkiewicz and Rubinsky (2015) modified a commercial printer to work with cryogenic materials. This work concludes that one of the advantages is the possibility of producing highly complex and detailed structures. These structures, in addition, are ready to be preserved for a long period of time.
Selective Laser Sintering
It is a technique in which the base material is in the form of powder, stored in a container with a moving bottom that rises to feed the system12. The building table has a second container, like the first, which moves vertically during the process. The powder is dispersed in a thin and uniform layer on the building table by means of a roller. Once in this form, a laser heats the material above the glass transition temperature in amorphous materials and just below the melting point in crystalline materials. This causes the powder particles to undergo a sintering process and maintain their shape.
Once a layer is completed, the building table drops by means of a piston and simultaneously a second piston rises loaded with new material to supply a new layer of powder. The process is repeated until the piece is completed. A diagram of this process is shown in Figure 2.
Unlike the others, in this technique it is not necessary to use support structures for the hollow channels, as the unsintered material serves as a support structure, although the disadvantage is that this material can be difficult to extract from small structures37. Another advantage is that it can be used practically any powder material whose degradation temperature is higher than the sintering temperature. Naing and colleagues (2006) designed a digital system responsible for creating the porosity of a piece by means of geometric shapes. After this, the piece was printed using polyether ether ketone, which is a biocompatible polymer with a high degradation temperature. In the end, they conclude that the system is capable of satisfactorily printing the desired figure.
It has been found that due to excessive material inside the pores of the piece, the actual porosity is 60% to 70% lower than the porosity of the piece when it was conceived37. In the work carried out, polycaprolactone scaffolds were manufactured using the previously described technique. In the end, it is concluded that this technique is appropriate for printing scaffolds that require supporting mechanical loads up to 27.4 MPa. However, it is mentioned that there is still work to be done in increasing the printing resolution, which is directly related to the laser focus and the size of the powder particle used. It is important to control the porosity of the piece because with this parameter the load supported by the bone can be controlled, which if not adequate can lead to the loss of normal functions of the bone, caused by the Wolff law also known as stress shielding.
Stereolithography
It was the first three-dimensional printing technique, invented by Charles Hull and patented in 198657. Although at that time it was not conceived for use with biocompatible materials.
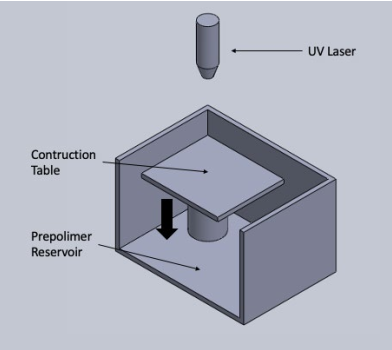
In this technique, photopolymerization is induced in the desired points, by means of a laser light source6)(11)(28. This causes a phase change in the material. Once a cross-section is finished, the bed lowers, and a new section is begun, and so on. A diagram with the principle of operation of the technique is shown in Figure 3.
It has the advantage of easy removal of unused material and the resolution that can be achieved, 1.2 μm, by focusing the laser beam11)(28 however, one of the disadvantages is the slow printing speed28).
One way to increase speed is to use ultraviolet light and a digital micro-mirror device (DDM); in this way, a cross section is worked on and speed increases. A deficiency of materials capable of being used with this technique has also been found11, as well as the photo-toxicity of the photoinitiator when polymerizing together with cells55. However, work has been done to develop photocurable, biocompatible compounds (Kweon, et al., 2003)61, where PCL was chemically modified to favor crosslinking in the presence of ultraviolet light. In the end, it is concluded that the scaffolds manufactured have a compression modulus of 6.9 MPa and a faster degradation rate, so they are suitable for use in certain tissue engineering applications.
conclusions
Regenerative medicine has widely benefited by the integration of additive manufacturing techniques, converging into a new medical field: tissue engineering. This new area of medicine opens the possibility to solve problems until now intractable: avoiding the need for immunosuppressants due to organ transplantation and shortening the waiting time for the procedure. One of the biggest challenges that still needs to be solved is the printing of hollow structures, this has become the biggest challenge of tissue engineering and it is expected that when achieved, access to the printing of more complex and functional parts will be obtained, which will bring us closer to the manufacture of complete organs.











 nueva página del texto (beta)
nueva página del texto (beta)