Study contribution
Intestinal microbiota is an important topic in gastroenteric human and veterinary health. This study shows that high levels of digestible protein transiently promote beneficial bacteria in dog feces. It also demonstrated that, at least in healthy dogs, a low digestibility diet does not increase the abundance of pathogenic bacteria. Therefore, healthy dogs on either high or low protein content and digestibility diets can standardize and balance their microbiota.
Introduction
Dog and cat nutrition is an area that has gained interest recently. Nowadays, processed animal feed is designed not only to nourish but also to offer health benefits through the ingredients used in their formulation.1,2 Macronutrients, such as carbohydrates, fats, and protein, have a great impact on the intestinal microbiome in cats and dogs.3-4 Hence, intense research has focused on studying the interactions between microorganisms, food, and host, both in healthy and diseased animals.5
Part of the main associations found, include the fermentation of non-digestible carbohydrates, such as fiber. This dietary component favors greater production of volatile fatty acids, specifically butyrate, which is a nutritional source for enterocytes and has antineoplastic properties, thus being a fundamental part of gastrointestinal health.6,3 Conversely, proteins and amino acids used by proteolytic bacteria tend to increase fecal pH and stimulate the production of harmful metabolites for the intestine, such as ammonia, indoles and phenols.3,7
Key microorganisms that actively participate in fermentative processes, such as Eubacterium, Bacteroides, Clostridium, Peptococcus, Bifidobacterium, and Lactobacillus have also been identified.8 Some of these microorganisms interact with each other modify the microenvironment, prevent the colonization of pathogens, intervene in the digestion of some nutrients, and stimulate the immune system.9,10 Others, such as Clostridium perfringens, increase the concentration of putrefaction compounds,10,8 as well as biogenic amines that generate possible inflammatory effects, associated with chronic diseases.9 These predominant microorganisms can maintain stability, which is undoubtedly altered positively or negatively depending mainly on the diet consumed by the animal.11
Studies on how protein, fiber, carbohydrates, and other nutrients influence the microbiota have been increasing and have focused on comparing raw diets (BARF = Biologically Appropriate Raw Food) with commercial diets. It has been observed that food with high protein and fat, obtained from natural sources, reduce the proportion of genera such as Lactobacillus spp., Paralactobacillus spp., and Prevotella spp.,12 as well as Proteobacteria.5 Other studies that have evaluated the effect of protein and its sources (by-products) have determined that high concentrations of this macronutrient favor Fusobacteria.13 Other studies on functional fibers and prebiotics, such as inulin and fructans, have reported a reduction in the concentration of Fusobacteria as well as an increase in Firmicutes, although results may vary across individuals.14
The digestibility of the diet also plays an essential role in the microbiome. Everything that is not digested and absorbed in the small intestine by the animal represents a substrate available for bacteria in the colon.4 This study aimed to evaluate the effect of protein digestibility and the quality of two processed diets on the relative abundance of specific microorganisms.
Ethical statement
This study was evaluated and approved by the Institutional Subcommittee for the Care and Use of Experimental Animals (SICUAE), Faculty of Veterinary Medicine, National Autonomous University of Mexico, with protocol number DC-2018/2-10.
Animals
Twenty clinically healthy, non-sterilized adult dogs, 15 females and 5 males, different breeds between 2 and 6 years old, with an average weight of 7 kg, were used in a completely randomized design. Two groups were randomly formed; the food selection was made considering the parameters established by AFFCO (2011) for dogs in maintenance. The first group was fed with high protein kibble (>22 % CP in DM), high energy density (> 3 500 kcal/100 g) and protein digestibility greater than 75 % (high quality-digestibility (HD); the second group was fed a minimum level of protein (20 % CP in DM), low energy density (< 3 200 kcal/100 g) and protein digestibility less than 75 % low quality-low digestibility (LD).
A 3-day adaptation period to the new food was contemplated to avoid digestive problems. To determine the amounts to be offered, the energy requirement of the animals was calculated individually and based on the following formula:15
All animals were kept in house conditions, without cage confinement, with access to the yard to reduce stress, without additional food and evaluated for the presence of undesirable behaviors such as coprophagia. At the beginning, middle and end of the experimental period, the body condition, muscle mass and weight of the animals were evaluated.
Food
The food was weighed for individual administration to each experimental subject, offered in a daily intake in which total consumption was guaranteed. The offered food was subjected to a bromatological study to determine moisture, crude protein (CP), crude fat, crude fiber, ashes, carbohydrates, as well as true protein (TP), indigestible protein (IP), in vitro protein digestibility (IVPD), neutral detergent fiber (NDF) and acid detergent fiber (ADF). All these analyses were performed according to the reference methods of AOAC International,16 except for NDF and ADF17 (Table 1).
Table 1 Means and standard deviations of proximate chemical composition (n = 3) of the experimental diets (wet basis)
| Analyte (%) | Diet1 | |
| HD | LD | |
| Moisture | 5.0 ± 0.87 | 7.5 ± 0.42 |
| Crude protein | 33.8 ± 0.28 | 20.1 ± 2.1 |
| Crude fat | 14.9 ± 0.01 | 7.1 ± 0.01 |
| Crude fiber | 1.5 ± 0.2 | 4.1 ± 0.01 |
| Ashes | 5.7 ± 0.01 | 9.0 ± 0.01 |
| Carbohydrates | 42.0 ± 1.25 | 53.0 ± 1.8 |
| True protein | 33.6 ± 0.07 | 19.1 ± 0.01 |
| Indigestible protein | 9.6 ± 0.01 | - |
| Protein digestibility | 71.8 ± 0.09 | 12.3 ± 0.28 |
| Neutral detergent fiber | 10.3 ± 0.32 | 8.5 ± 0.2 |
| Acid detergent fiber | 28.6 ± 0.36 | 31.5 ± 0.15 |
1 HD: high quality and digestibility; LD: low quality and digestibility
Stool samples
Samples were collected on the day the transition was completed (0, 15, and 30) of the experimental period. Excreta were collected immediately after defecation, ensuring that they did not touch the ground. Hermetic bags were used to preserve them and kept in deep-freezing conditions (-70 °C).
DNA extraction
For DNA extraction, the bacterial pellet was first obtained using 2 g of samples and 5 mL of 1× PBS (137-mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4). A modified centrifugation was performed at 200 × g for 5 min and 1 mL of the supernatant was recovered in a 2 mL Eppendorf tube. To obtain a cleaner pellet, two more centrifugation steps (200 × g for 5 min) were performed, decanting the liquid and recovering the pellet at each step, ending with a centrifugation at 10 000 rpm for 15 min and recovering the pellet in 1 mL of 1 × PBS.
In the final pellet, bacterial genomic (BG) DNA was extracted using a modified cetyl trimethyl ammonium bromide (CTAB, 10%) technique16,17 that includes precipitation in isopropanol leaving the sample under refrigeration for at least 12 h. We used the following modifications of the technique: A) Re-suspend the pellet obtained from washing in 570 µL of TE buffer + 50 µL of lysozyme (10 mg/mL) incubating at 37 °C for 30 min. B) Incubate at 56 °C for 1 h after the addition of 30 µL sodium dodecyl sulfate + 4 µL proteinase K.
The integrity of the DNA was confirmed by agarose gel electrophoresis (0.8 % agarose) run at 70 V and 300 mA for 45 min. The DNA was visualized using a gel photodocumentation system (Figure 1). Subsequently, quantifications were performed in a Nanodrop® spectrophotometer.
Real-time PCR
All samples were standardized at 4 ng/mL of DNA concentration, from which qPCR was performed using the KAPA SYBR® FAST for light cycler® 480 kit. The reaction volume was 12 µL (6µL KAPA SYBR® FAST + 3.4 µL nuclease-free molecular biology grade water + 0.6 µL forward and reverse primers + 2 µL sample). The following primers were used for detecting microorganisms targeting a fragment of the 16S gene. (Table 2) The samples were carried out in duplicate on the Rotor Gene® with an initial denaturation step (95° C/3 min), followed by 35 cycles of denaturation (95 °C/30 s), primer annealing (specific for each microorganism, see Table 2), amplification (72 °C/30 s), and final extension (72 °C/3 min).
Table 2 Forward (F) and reverse (R) primers used to amplify a fragment of the 16S gene
| Microorganism | Sequence | Amplicon size (bp) | Annealing temperature (°C) |
| Bacteroides fragilis | F: CAGTTCGCCATACAA R: GGATTCTCTTTCCGCTTTGAC |
131 | 57 |
| Clostridium perfringens | F: TGA AAC TGG GAG ACT TGA GTG C R: CTT AGG TAA GGT TCT TCG CGT TGC |
100 | 55 |
| Enterococcus faecium | F: GCATAGCCCGCACCTG R: GTTACTCTCATCCTTGTTCTTCTC |
160 | 54 |
| Fusobacterium varium | F: GGGATGTCAAACGCTGG R: GGCGCTGAGGTTCGAG |
143 | 57 |
| Lactobacillus salivarius | F: GTTCTCCTACGGCTACCTTGTTACG R: TTCTCAGTTCGGATTGTAGGCTG |
225 | 57 |
| Universal | F: ACTCCTACGGGAGGCAGCAG R: ATTACCGCGGCTGG |
136 | 60 |
The results were analyzed with the Qrex® program. The obtained cycle time (Ct) threshold values were considered acceptable in replicates that differed by a maximum of one cycle from each other. Relative abundance was determined (Microsoft® Excel) by the ΔΔCt method.18 The universal primer was used as reference Ct and the day zero sampling Ct as controls. We used the following formula to calculate the ΔΔCt:
Statistical analysis
The experiment had a completely randomized design with two treatments, each one with ten replicates. The results of relative abundance were analyzed with the IBM® SPSS® Statistics program, version 23. Normality (Shapiro-Wilk test) and homogeneity of variances (Levene’s test) were evaluated19; by not complying with the assumptions for parametric tests, non-parametric tests were used, working with ranges for the results.
We compared the bacterial abundance between diets over time by using the Mann Whitney U test (rank-sum). The Kruskall Wallis test was used to evaluate the differences between the relative abundance of each microorganism. Finally, to determine wether there was a difference in the relative bacterial abundance of the diets over time, the Friedman test was used as an alternative to ANOVA for paired samples. A P-value (P < 0.05) was considered a statistically significant difference. All the original values (relative abundance) were transformed into ranks for analysis.
Results
Main rank of relative abundance between diets
Table 3 shows the difference in the main rank of relative abundance of each microorganism between diets over time. We observed a higher abundance of the microorganisms Fusobacterium varium and Enterococcus faecium in the first 15 days of consumption and Lactobacillus salivarius and Bifidobacterium animalis after 30 days of consumption of the HD diet.
Table 3 Main rank of relative abundance of microorganisms in the feces of dogs*
| Microorganism | Day 15 | P-value | Day 30 | P-value | ||
| 1Diet | 1Diet | |||||
| HD | LD | HD | LD | |||
| C. perfringens | 12.3 (4-20) |
8.7 (2-16) |
0.1900 | 10.3 (6-18) |
10.7 (2-20) |
0.9120 |
| F. varium | 14.7a (10-20) |
6.3b (3-14) |
0.0010 | 13.1 (8-20) |
7.9 (2-16) |
0.0520 |
| L. salivarius | 15.1a (10-20) |
5.9b (2-12) |
< 0.0001 | 13.4a (8-20) |
7.6b (2-16) |
0.0280 |
| E. faecium | 14.7a (10-20) |
6.3b (3-14) |
0.0010 | 11.5 (4-20) |
9.5 (2-18) |
0.4810 |
| B. fragilis | 13.9a (8-20) |
7.1b (4-16) |
0.0090 | 13.5a (10-18) |
7.5b (2-20) |
0.0230 |
* Diets at 15 and 30 days post administration (n = 10)
1 HD: high quality and digestibility; LD: low quality and digestibility
a,b Different superscripts within the same row denote a statistically significant difference (P < 0.05) according to a Mann Whitney U test.
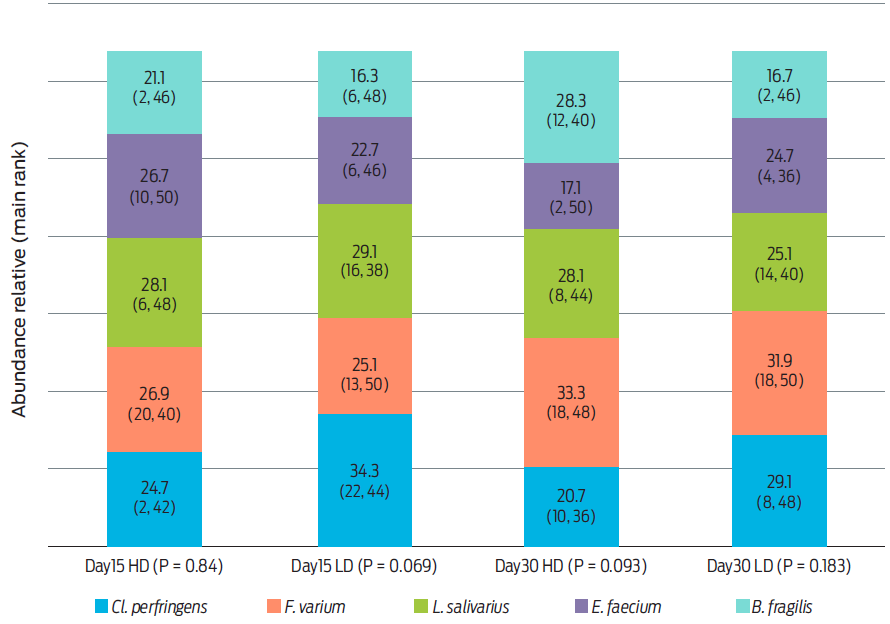
Main rank of relative abundance of populations
The main rank of relative abundances of the populations of microorganisms did not change significantly during the study period per diet offered (Figure 2).
Main rank of relative abundance between days 15 and 30
Fecal samples from animals on the HD diet had higher overall bacterial relative abundance (P = 0.025) on day 15 than on day 30 post administration: main rank 2 (range 1-2) and 1 (range 1-2), respectively. Conversely, overall bacterial relative abundance was similar (P = 0.180) at 15 and 30 days post administration in the feces of dogs fed the LD diet: main ranks 1.2 (range 1-2) and 1.8 (range 1-2) respectively.
Discussion
Current nutrition in dogs seeks to meet the physiological requirements of the animals and generate greater intestinal integrity and health, which is reflected in the health of the individual, ranging from the immune system to possible repercussions on mental health.20 It is becoming increasingly evident how diet affects the intestinal microbiota, not only within an individual, since it differs between individuals.3
Given these facts, it is evident that in many cases it has not been possible to establish clear patterns for an ideal dietary profile. Still, it allows us to think of tailor-made or individual diet profiles that lead to this “healthy gut” balance. Several studies on microbiome, have established that the intestinal anatomophysiology differs between species or breeds as there is an evident individual variability.21 This situation is usually reflected in the results that show that even with changes in the diet, the microbiome tends to return to a balance.3
This “dietary” balance depends on multiple factors such as the use of antibiotics, stress, infectious processes, and chronic diseases. When the individual homeostasis is broken, generating alterations such as colitis, malabsorption syndromes, or persistent diarrhea can lead to the individual´s death.10 The microorganism associated with this type of digestive disorder is Clostridium perfringens. Experimental evidence6,12,13 has shown that environments rich in protein and with high amounts of amino acids promote the growth of both Clostridium difficile and Clostridium perfringens, microorganisms related to inflammatory processes.22 For instance, a study reported an increase in the concentration of C. perfringens (from 3.3 to > 8 logCFU/g feces) in dogs under high-protein diets (> 40 % CP) with low quality and digestibility.22
Despite expecting a similar change, in this study, it was impossible to associate the protein digestibility of the diet with an increase in the relative abundance of C. perfringens. Thus, it appears that even if the diet has low digestibility and allows a colonic environment rich in amino acids, the microbiome can stabilize the growth of possible pathogens in response to the presence of other nutrients or intrinsic factors of a healthy dog.23 In our study, the stool consistency was not evaluated, nor was the diet evaluated for more than 30 days.
The results of Fusobacterium varium obtained in our study are similar to those reported by Mori,24 who compared the microbiome of healthy dogs fed with four prescription diets. In that study, high relative abundance of Fusobacterium was found when the animals consumed a diet with the highest level of crude protein (30 %) (abundances: 7.6 vs. 2.9, 1.4, and 0). Here, the higher crude protein diet (33.8 vs. 20.1 %) resulted in a higher relative abundance of Fusobacterium (14.7 vs. 6.3). However this effect was only significant for the first 15 days.
Moinard also reported an increase in Fusobacterium when the diet was high in protein (29.3 % CP).25Fusobacterium spp. is considered an amino acid fermenter, which is sometimes related to the presentation of digestive disorders. Hang13 observed a high relative abundance of this type when a very high protein diet (60 % CP) was administered. In that study, diarrhea and changes in the consistency of the feces were also reported. Nevertheless, the tendency of Fusobacterium spp. to increase its relative abundance in high protein diets could also have a beneficial effect as this microorganism produces butyrate.6 This volatile fatty acid is used as an energy source for the intestine and is associated with antineoplastic properties.
The genus Enterococcus presents great ecological diversity. While Enterococcus faecium is a microorganism widely distributed in nature, some strains are considered causative of nosocomial diseases in humans and zoonotic.26 Conversely, other strains have a probiotic potential effect.27,28 The identification and quantification of this microorganism has been documented in both healthy and diseased dogs.29,30
In this study it was possible to identify a higher relative abundance of E. faecium in the high protein and high-quality diets (14.7 vs 6.3), the associations between the type of diet or substrates with the growth of this microorganism are few. However, according to previous studies, it has a high capacity to use carbohydrates.28 In the case of the HD diet, it presented a lower concentration of carbohydrates than the LD (42 vs 53 %), which could explain the reported abundance of this microorganism. It is essential to consider that the primers used for the molecular identification of E. faecium¸ did not contemplate any particular strain. Therefore, it would be interesting to conduct further studies to determine wether the abundance associated with the diet belongs to potential zoonotic or innocuous strains.
Regarding Lactobacillus salivarius, its relative abundance remained high in the feces of dogs on HD diet during the 30 days of experimentation. These findings are consistent with observations of Middelboss,31 who found a higher relative abundance of this microorganism in the feces of dogs fed a diet supplemented with prebiotic fiber compared with controls fed unsupplemented feed (12.2 vs. 10). This effect is associated with the fact that Lactobacillus genera are microorganisms associated with the presence of functional fibers such as inulin, fructooligosaccharides, mannanoligosaccharides and beet pulp.
High quality and highly digestible feeds contain inulin among its ingredients, which could explain the behavior of this microorganism. In turn, Bermingham et al., 2017 dietinduced changes in faecal microbiota observed in humans and rodents have been extrapolated to pets in spite of their very different dietary and metabolic requirements. This lack of direct evidence means that the mechanisms by which microbiota influences health in dogs are poorly understood. We hypothesised that changes in faecal microbiota correlate with physiological parameters including apparent macronutrient digestibility. Methods. Fifteen adult dogs were assigned to two diet groups, exclusively fed either a premium kibbled diet (kibble; K; n = 8, also reported a correlation between the level of digestibility of the diet, and the growth of Lactobacillary, similar to the results of this.32
Bacteroides is a gender in which important discrepancies have been detected. While some authors have reported high concentrations in patients with diarrhea,33,34 others have reported it in lower concentrations when the patient presents with inflammatory bowel disease.35 These possible differences are associated with issues such as the extraction technique used, the type of sample, species (dog or cat), as well as the type of diet.(34) Regarding this last factor, it has been possible to associate Bacteroides with diets high in protein and fat.24,36 Deusch et al.37 found that cats fed a high-protein, low-carbohydrate diet (110 g crude protein and 49 g crude fat/1 000 kcal ME) had a higher relative abundance of this microorganism compared to those fed moderate protein and carbohydrate diets (78 g crude protein and 44 g crude fat/1 000 kcal). This result is similar to that obtained in this experimental, considering that the high-quality diet has a profile of 91.2 g crude protein and 40.15 g crude fat/1 000 kcal. Swanson34 also found a relationship between the type of fibers contained in the feed and growth of species belonging to the genre Bacteroides. Similar to what was mentioned with L. salivarius, the high quality-digestibility diet contains inulin among its ingredients, a component that could favor these two microorganisms during the 30 days of the experimental period.
Finally, it is possible to observe that the general relative abundance of microorganisms was modified over time in the HD diet. This effect may be associated with the ability of the intestinal microbiome to adapt quickly and regulate itself to transient changes intrinsically.23 They offered two intercalated diets to Labrador dogs, and observed that after some time, the microorganisms recovered their initial stability or profile, with significant changes in certain bacteria, such as Fusobacterium, Bacteroides and Bifidobacterium. This effect has also been reported in humans,38 where evaluating the microbiome of 98 individuals fed with two types of diets (with different levels of fat and fiber), significant changes were detected during the first 24 h, and a stabilization of the population after 10 days.
Conclusions
This study demonstrated that the diet’s protein content, energy density, and digestibility significantly modify the relative abundance of some microorganisms commonly present in the dog’s fecal microbiome, except for the pathogen C. perfringens. Apparently, this effect does not persist over time for some of the bacterial species under study.
Data availability
The data sets used and analyzed in this experiment are available from the corresponding author on reasonable request.
Acknowledgments
The authors thank the support of Animal Nutrition and Biochemistry Department, Faculty of Veterinary Medicine, National Autonomous University of Mexico (UNAM).
Conflicts of interest
The authors have declared no conflict of interest.
Author contributions
Conceptualization: L Gutiérrez, C Gutiérrez
Data curation: KE Cosío
Formal analysis: MG Sánchez
Funding acquisition: L Gutiérrez, C Gutiérrez
Investigation: KE Cosío, CC Márquez
Methodology: CC Márquez
Writing original draft: KE Cosío
Writing-review and editing: KE Cosío, L Gutiérrez, C Gutiérrez, CC Márquez, ME Ortega











 nueva página del texto (beta)
nueva página del texto (beta)