INTRODUCTION
The mares reproduce in seasonal form with high photoperiod (many hours of light/day or spring-summer), and thus present their births in the season most suitable for the survival of their offspring (Bronson and Heideman, 1994, Escobar, 1997). They use the photoperiod to program their reproductive activity: ovulatory activity or estral cyclicity on the days with the greatest number of light hours and anestrus with the reduction of the photoperiod (Escobar, 1997). The effect of the photoperiod is carried out by means of the hormone melatonin, secreted in the pineal gland during the dark hours (Diekman et al., 2002). Therefore, different secretion patterns are established throughout the year, a period of greater and another with a shorter duration of melatonin, which determine the anestrus and ovulation seasons respectively (Guillaume et al., 1995). There are also two transition periods: spring and autumn (Donadeu and Ginther, 2002, Ginther., 2003a).
In the ovulatory season, the mare presents estrous cycles; they are repeated successively, while the mare does not conceive and the adequate photoperiod conditions remain (days with more light hours). To establish the interovulatory intervals, the hypothalamic-pituitary-gonadal axis is stimulated, with the additional participation of the uterus. This stimulus leads to follicular growth with estradiol production, ovulation, corpus luteum formation with progesterone production and regression of the corpus luteum.
Knowing the interovulatory intervals allows establishing the conditions to increase the fertility of the mares, by selecting the most appropriate time for natural mountaineering or artificial insemination; as well as the proper application of hormones, to manipulate the estrous cycle, when necessary. In addition, identify the alterations that occur in this part of the reproductive cycle, and apply the most appropriate treatments.
THE MELATONINE SECRETION
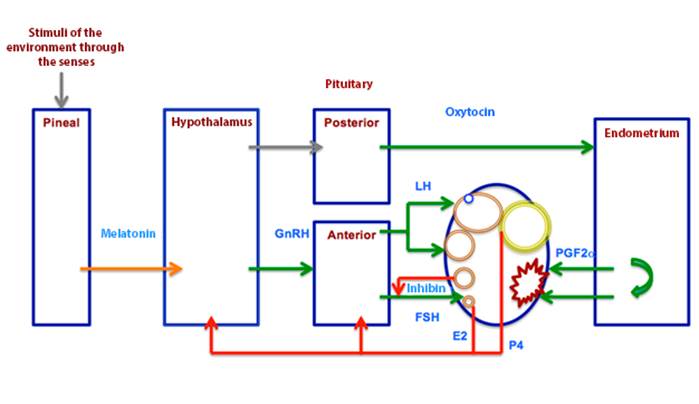
The photoperiod influences the secretion of melatonin via neuroendocrine. In the species where it has been studied, the stimulus is captured in the retina, and then it passes to the suprachiasmatic nucleus (NSQ) of the hypothalamus, superior cervical ganglion (GCS) and pineal gland (GP). The absence of stimulation of light in the pineal gland promotes the synthesis of the enzyme N-acetyl transferase, which influences serotonin to transform it into N-acetyl serotonin, which is converted into melatonin by the action of the hydroxy-indole enzyme-o-methyl transferase. Melatonin acts in the hypothalamus to regulate the secretion of gonadotropin-releasing hormone (GnRH). (Figure 1)
The retina acts as a photo-receptor, registers the presence or absence of light; the NSQ operates as an internal biological clock, regulates the endogenous circadian rhythm; and GP serves as a translator, converts neural information into a hormonal signal (Bittman et al., 1983, Lincoln, 1984b, Fitzgerald and McManus, 2000, Goldman, 2001)
The secretion of melatonin presents circadian rhythm, it is reduced during daylight hours and rises during darkness (Salazar-Ortiz et al., 2011); therefore, its secretion period varies according to the number of daylight hours and time of year. Figure 2 shows the concentration of melatonin in mares during two seasons of the year, with different ratio of light/dark hours. The concentration of the hormone increases in dark hours and it is reduced in hours with light.

Figure 2 Concentration of melatonin in mares with long and short periods of dark hours during the day (adapted from Salazar-Ortíz et al., 2011)
The reduced secretion of melatonin, as occurs in spring and summer, allows the hypothalamic-pituitary-gonad (HHG) axis function, and as a consequence the mare presents estrous cycles until it is able to conceive. In contrast, the longer period of melatonin secretion, as happens in autumn-winter, time of year with more dark hours, the follicles do not reach adequate growth to carry out follicular deviation or selection; this is due to the reduction in the secretion of luteinizing hormone (LH); hormone related to follicular deviation and maturation (Bergfeld et al., 2001; Collins et al., 2007). The ovarian follicles are squeezed and do not ovulate; therefore, the endocrinological concert that leads to ovulation is not presented and the mare remains in anoestrus (Guillaume et al., 1995).
There are also two transition periods: spring and autumn (Donadeu and Ginther, 2002, Ginther et al., 2003a). The spring transition period takes place from anoestrus to ovulation; it is characterized by increased follicular growth and culminates with the first ovulation of the year (Bergfelt et al., 2001, Donadeu and Ginther, 2002). The autumn season combines ovulatory and anestrus; it is characterized by reduced follicular growth (Ginther et al., 2003a).
Based on the above, seasonality should be increased in animals maintained in the hemispheres and reduced as it approaches Ecuador; and in fact this happens. Mares maintained in the hemispheres (Hughes et al., 1977, Sharp, 1980, Dowsett et al., 1993, Gentry et al., 2002) and around the Tropic of Cancer (Silva and Chávez, 1991, Orozco et al., 1992; Escobar, 1997); they have a more marked reproductive seasonality than at a lower latitude (González and Valencia, 1977, Saltiel et al., 1982) and in regions close to Ecuador (Quintero et al., 1995).
THE OVULATORY SEASON
In this season the hormonal concert that leads to ovulation is presented (Irvine and Alexander, 1994), to form the interovulatory interval. As can be seen in Figure 3, GnRH is produced in the hypothalamus, which stimulates the secretion of gonadotropins: follicle-stimulating hormones (FSH) and luteinizing hormones (LH), in the anterior lobe of the pituitary gland (Alexander and Irvine, 1987).
Gonadotropins promote follicular development, FSH up to deviation and LH up to the preovulatory level (Bergfelt et al., 2001). The follicles produce estradiol and inhibin. Estradiol exerts negative feedback on gonadotropins (Ginther et al., 2008a) and inhibin on FSH (Bergfelt and Ginther, 1985); in addition, LH is related to ovulation (Ginther, 1992). In non-pregnant mares maintained in the season with the greatest amount of light hours, ovulations (accompanied by estrus) are repeated every 21 days on average to constitute the estral cycle (Ginther and Pierson, 1989, Ginther, 1992, Ginther et al., 2008f).
Ovulation occurs and the corpus luteum develops, which produces progesterone; and exerts negative feedback on gonadotropins (Gastal et al., 1999). The hypothalamus also produces oxytocin, this hormone is stored and secreted in the posterior lobe of the pituitary (Lincoln, 1984a) and stimulates the endometrium for the production of prostaglandin F2α (Shand et al., 2000), which in turn responsible for the regression of the corpus luteum at the end of the cycle (Ginther and First, 1971, Stabenfelt et al., 1974, Ginther et al., 2008b, Ginther and Beg, 2009), with the subsequent reduction of progesterone and a new opportunity for that the mare conceives in the new estrous cycle (Neely et al., 1979).
Oxytocin is also produced in the uterus (Watson et al., 1997, Stout et al., 2000, Allen, 2001), which establishes its pulsatile secretion; important process in luteolysis. Based on the above, the ovarian follicles grow, mature and ovulate due to the effect of gonadotropins; and the mare has the opportunity to conceive and develop gestation (Gastal et al., 1997, Goudet et al., 1999, Crowell-Davis, 2007). The percentage of conceptions throughout the year is presented in Figure 4. As a consequence, deliveries occur in the spring, time of year with the appropriate conditions for the survival of their offspring.

Figure 4 Perception of conception throughout the year in mares maintained under natural photoperiod at 22° 58' (Escobar, 1997)
In relation to the behavior of the mare, the estrus cycle has been divided into estrus and right-handed; and in relation to physiology in two parts: follicular and luteal (Ginther et al., 1992, 1993). Estrus is the period of sexual receptivity of the mare, and the genital apparatus is able to receive and transport sperm, finally culminating in ovulation (Crowell-Davies, 2007, Ginther et al., 2008f). It is characterized by the presence of follicles in different development and the simultaneous secretion of estradiol, for which reason it is also known as the follicular phase. Its duration is from 5 to 7 days, with variation from 3 to 9 depending on the time of year; it is longer in autumn (7 to 10 days), and shorter in the beginning of summer (4-5 days). In the estrus, the mare looks for the stallion, with lateral displacement of the tail, urinates frequently in small quantities, with mucous secretion and eversion of the clitoris; also lowers the head, relaxes the facial muscles, inclines the pelvis and separates the hindquarters to allow the introduction of the penis at the time of intercourse (Crowell-Davis, 2007).
The deft understands the remaining part of the cycle, without altering the behavior of the animal; it remains in its daily activity. It is characterized by the presence of a corpus luteum with progesterone production, for which reason it is also known as the luteal part; its duration varies from 12 to 16 days. Progesterone exerts negative feedback on the hypothalamus to reduce GnRH secretion and as a consequence of LH (Irvine and Alexander, 1993). At the level of the ovaries, follicular growth with estradiol production, ovulation, corpus luteum formation and finally luteal regression occurs during the interval between ovulations or estrus cycle.
THE FOLICULAR GROWTH
Follicular growth is characterized by the proliferation, differentiation and secretion of follicular cells, in order to establish an appropriate environment for maturation and preparation to fertilize the ovocyte (Armstrong and Webb, 1997); it is done by means of waves and surges. In the mares, two types of waves have been found: ovulatory and anovulatory (Ginther, 1993). Ovulatory waves are the most frequent and begin their development in the middle part of the interovulatory interval and culminate with ovulation (Ginther et al., 2003). A detail of this information can be seen in Figure 5. The anovulators can be larger or smaller. The largest have been found in 24 to 25 % of the mares, with development of the ovulatory follicle (Ginther et al., 2004a) and develops during the first part of the interval between ovulations (Bergfelt and Ginther, 1993a). The minor anovulatory waves correspond to follicles that reach a size of 22 to 23 mm in diameter and become atretic. These waves can occur in less than 25 % of the cases and in any part of the interovulatory interval (Bergfelt and Ginther, 1993a, Ginther, 1993, Ginther et al., 2004a). The ovulatory waves, despite being a continuous process, can be divided for study in 4 phases or periods: common growth, deviation or selection, dominance and ovulation.

Figure 5 Follicular growth and blood concentration of progesterone during the estrus cycle of the mare (adapted from Gastal et al., 1997, Jacob et al., 2009a, Slough et al., 2011).
Common growth phase: includes the identification of the follicles by ultrasonography, usually 6 mm in diameter, until the deviation (period in which a follicle is selected to continue its growth and the rest suffer atresia (Ginther, 1993). Part of the process, the follicles increase their size uniformly, 2.8 mm/day, and none influences the growth of their partners (Gastal et al., 1997) All follicles have the ability to continue their growth and participate in the next phase of follicular development, however, only one (or occasionally two, Ginther et al., 2009a) will do so, the others lose this capacity approximately 48 hours after the deviation and suffer atresia (Gastal et al., 2004; Ginther et al., 2004a, 2004b).
The follicles of greater diameter, reach the size before the deviation (Gastal et al., 1997); therefore, they are more likely to continue their development; the probability increases as the expected diameter approaches for the beginning of the deviation. In 60 % of the waves, the larger follicle continues its growth; in the remaining cases, the major follicle stops (or slightly reduces) its increase in size during the common growth phase and is replaced by the second. FSH stimulates follicular growth during the common phase in all waves; in the ovulatory waves, the blood concentration of FSH increases gradually from the previous period, to the identification of the follicles, by ultrasonography up to 13 mm in diameter; what happens 3 days before the expected date for the deviation (Gastal et al., 1997, Donadeu and Ginther, 2001, Bergfeld et al., 2001, Ginther et al., 2003a, 2003b). Subsequently the blood concentration of FSH decreases, but with sufficient level to promote the development of the future dominant follicle up to 48 hours after the expected diameter for deviation (22 mm), but unable to promote the development of others, which suffer atresia due to the lack of hormonal support (Ginther et al., 2003a, 2003b, Checura et al., 2009). The detailed information of this process is shown in Figure 6.
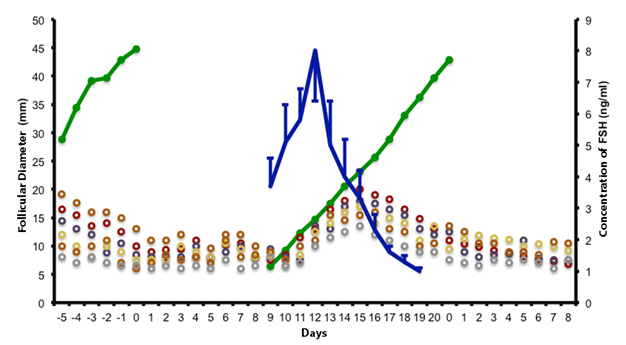
Figure 6 Follicular development during the common phase of growth (different colors), deviation and dominant follicle (green line); as well as means of FSH (blue line) during the estrus cycle of the mare (adapted from Gastal et al., 1997, Jacob et al., 2009a).
The effect of FSH is done through the IGF-I (Ginther et al., 2004c; 2004d; 2008c; 2008d; Checura et al., 2010a; 2010b), and its reduction is due to the negative feedback effect that the estradiol and inhibin; hormones produced in granulosa cells (Miller et al., 1979, 1981, Bergfelt and Ginther, 1985, Bergfelt et al., 2001, Watson et al., 2002, Donadeu and Ginther, 2003, Ginther et al., 2005a; 2008a). The level of FSH returns to its basal concentration 2 to 3 days after the deviation (Checura et al., 2009).
Follicular deviation or selection: in follicular deviation or selection one follicle of each wave (occasionally two) continues its production of estradiol and as a consequence maintains its growth, the others suffer atresia (Gastal et al., 1997, 1999; Ginther et al, 2003a, 2003b, 2004b). The continuous increase in the synthesis of estradiol in this follicle promotes the development of receptors for LH in its granulosa cells, receptors that increase as the follicular diameter increases (Goudet et al., 1999); with which it can increase its production of estradiol (despite the reduction of FSH) and become the dominant follicle (FD) (Goudet et al., 1999, Gastal et al., 1999a, 1999b, Ginther et al., 2004a); This is not the case in subordinate follicles, which is why they suffer atresia (Ginther and Bergfelt, 1993, Ginther et al., 2004a, Gastal et al., 2006a, 2006b, Claes et al., 2017).
The reduction in LH concentration leads to a decrease in follicular diameter (Gastal et al., 1999b, 2000). The continuous decrease in FSH, as in this part of the process, leads to the morphological and functional damage of the subordinate follicles (Gastal et al., 1999a, Gastal et al., 1999b, Donadeu and Ginther, 2001); therefore, the FSH supports follicular development up to deviation. The task of promoting the growth of the dominant follicle corresponds to LH (Gastal et al., 1997, Bergfelt et al., 2001).
The blood concentration of LH increases before the deviation (Berfelt et al., 2001); in addition to the hormonal changes discussed above, blood flow in the future dominant follicle is increased two days before it acquires the expected diameter for the deviation (Acosta et al., 2004b).
Figure 5 shows the dominant follicle that maintains its constant growth until one or two days before ovulation (Jacob et al., 2009a), and ovulates (ovulatory surge) or suffers atresia (major anovulatory surge). The dominant follicle increases its size by 2.5 to 3 mm in diameter per day after luteolysis. Consequently, the follicle reaches a diameter of 40 to 45 mm on the day before ovulation (Ginther, 1993, Ginther et al., 2003), and pear-shaped (Kimura et al., 2005). The growth rate of the ovulatory follicle decreases on the eve of ovulation in mares with one and two ovulations (Gastal et al., 2006a, Gastal et al., 2006b, Ginther et al., 2008c). The beginning of the reduction of the follicular diameter coincides with the higher level of LH of the ovulatory secretion (Gastal et al., 2006a, Gastal et al., 2006b, Gastal et al., 2006c) (Figure 7).
Growth factors: similar to insulin-I (IGF-I) and vascular endothelial (VEGF), also participate in follicular deviation. IGF-I stimulates proliferation in granulosa cells (CG) and performs synergy with gonadotropins to promote the differentiation of follicular cells (Spicer and Echternkamps, 1995). The concentration of free IGF-I increases differentially in the future dominant follicle before the onset of deviation (Donadeu and Ginther, 2002), and even stimulates its development in animals with low gonadotropin levels (Checura et al., 2010a). Estradiol increases the synthesis of IGF-I and potentiates the expression of gonadotropin receptors in granulosa cells. IGF-I increases the sensitivity of granulosa cells to gonadotropins.
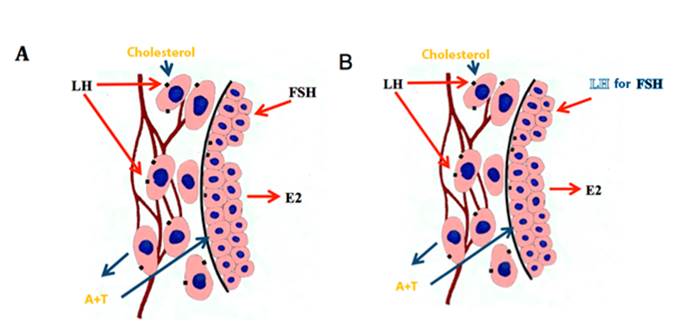
Figure 7 Synthesis of estradiol in the follicles before (A) and after (B) of the deviation (several authors, see text).
VEGF is increased in the dominant follicle and its increase appears to be partly mediated by IGF-I (Ginther et al., 2004d). It is believed that VEGF is involved in the increase of vascularization of the future dominant follicle before deviation, which presumably increases the availability of circulating gonadotropins to the follicle (Acosta et al., 2004a). The dominant follicle increases blood flow before deviation, greater flow than subordinate follicles (Acosta et al., 2004b). The increase of the vasculature in the wall of the dominant follicle is maintained during its maturation and as it approaches ovulation (Gastal et al., 2006a, 2007, Ginther et al., 2009d), but with drastic reduction of blood perfusion hours before ovulation (Gastal et al., 2006a, Ginther et al., 2007b).
THE OVULATION
Ovulation is the process by which the follicular wall disintegrates to release the ovocyte and follicular fluid in the ovulatory fossa. The oocyte and the radiated crown are deposited in the oviduct and the follicular fluid in the abdominal cavity. The hormones contained in the follicular fluid are absorbed and exert their action in the reproductive process (Bergfelt et al., 1991). Mares with two dominant follicles in a cycle have double ovulation, with 24 hours of interval, which happens in approximately 40 % of cases, and could lead to double gestation (Ginther et al., 2008e).
Luteolysis and progesterone reduction are presented, as well as the decrease of estradiol in the days before ovulation. Negative feedback is removed on the hypothalamus and, consequently, the release of GnRH is increased; which, in turn, stimulates the secretion of LH that is related to ovulation (Gastal et al., 1999b, Ginther et al., 2006). The concentration of LH increases gradually in the last days of the interovulatory interval, its increase in this part of the cycle is very slight; later it registers a considerable increase in the period between 48 hours before a day after ovulation; the highest level occurs the day after ovulation (Ginther et al., 2005, Ginther et al., 2006, Jacob et al., 2009a). See Figures 8 and 9.

Figure 8 Follicular development during its growth phase (several colors), deviation and dominant follicle (green line); in addition, LH averages during the estrus cycle of the mare (adapted from Gastal et al., 1997, Ginther et al., 2006).

Figure 9 Hormone concentration in relation to ovulation in the mare (adapted from Jacob et al., 2009a).
FSH shows a slight increase that coincides with a considerable increase in LH and estradiol reduction, two days prior to ovulation (Jacob et al., 2009a). Estradiol exerts negative feedback on the secretion of gonadotropins; this is due to the coincidence between estradiol reduction and gonadotropin increase (Miller et al., 1981, Donadeu and Ginther, 2003, Gastal et al., 2006a, Ginther et al., 2007th, Ginther et al., 2008a, Ginther et al., 2008c, Ginther et al., 2009b, Ginther et al., 2010). Estradiol presents its highest concentration two days prior to ovulation and then decreases to register its basal level, 7 days later; this is equivalent to 5 days after ovulation (Ginther et al., 2007a, Jacob et al., 2009a), see Figure 9.
Inhibin exerts negative feedback on FSH, its release in the abdominal cavity, with its corresponding absorption, interrupts the previously initiated FSH increase; this is done 12 hours before 12 hours after ovulation (Nambo et al., 2002, Ginther et al., 2008a). As shown in Figure 9, after this slight interruption, the concentration of FSH continues to increase (Jacob et al., 2009a). The highest level of inhibin coincides with ovulation (Bergfelt et al., 1991, Rosser et al., 1994, Nambo et al 2002, Ginther et al., 2008a). Estradiol and inhibin have a synergistic effect on the suppression of FSH (Miller et al., 1981, Donadeu and Ginther, 2003, Ginther et al., 2008a).
Progesterone increases gradually after ovulation and exerts negative feedback on LH (Gastal et al., 1999b, Ginther et al., 2006, Ginther et al., 2007a, Ginther et al., 2007b), therefore, the LH level is reduced after the day after ovulation (Jacob et al., 2009a); therefore, as the level of progesterone increases, LH secretion is reduced (Jacob et al., 2009b). This information can be seen in Figure 9. The same happens during follicular development; treatment with progesterone during the first part of the follicular growth wave reduces the circulating LH concentration (Gastal et al., 1999b, Gastal et al., 2000; Bergfelt et al., 2001). LH, in addition to participating in follicular maturation and ovulation, is also responsible for the development and maintenance of the corpus luteum (Ginther et al., 2004a, Ginther et al., 2005b, Ginther et al., 2008f).
The mare ovulates from 24 to 48 h before the end of estrus, with variation of the follicular diameter between 35 and 55 mm (Ginther, 1993, Ginther and Bergfelt, 1993), and the pear-shaped follicle (Kimura et al., 2005). For this, the follicle migrates to the ovulatory fossa, the only site in which the ovary releases the ovocyte. The change in shape from spherical to non-spherical (Gastal et al., 1998) and reduction of its turgor (Gastal et al., 2006c) occurs 24 to 12 hours before ovulation. Mares ovulate consistently at the same follicular diameter in consecutive cycles (Cuervo-Arango and Newcombe, 2008).
The information available in the ovulation process in the mare indicates that the increase in LH, during the ovulatory pulse, activates the matrix of metalloproteinases (Li et al., 2006). The proteolytic enzymes are responsible for the tissue remodeling that extends to the apex of the follicle, which culminates with the disintegration of the follicular wall and, as a consequence, ovulation (Song et al., 1999; Robker et al., 2000; et al., 2004; Sessions et al., 2009).
The action of LH is probably done through prostaglandins. In the mare, the enzyme prostaglandin G/H synthase-2 (PGHS-2), also called cyclooxygenase-2 (COX-2), it is expressed in granulosa cells, 30 h after the start of the ovulatory increase in LH, or after the application of hCG. PGHS-2 is the first limiting enzyme in the biosynthesis of prostanoids from arachidonic acid (Sirois and Dore, 1997). Ovulation has been prevented in mares treated experimentally with inhibitors of prostaglandin synthesis, such as flunixin meglumine (Cuervo-Arango and Domingo-Ortiz, 2011) even in mares with a high concentration of LH (Cuervo-Arango et al., 2011).
THE FORMATION OF THE CORPUS LUTEUM
The ovulatory secretion of LH, in addition to causing follicular rupture, luteinizes the granulosa cells to constitute the corpus luteum. In each place where the follicular rupture is performed during ovulation, a corpus luteum is formed. The corpus luteum retains the same shape as the follicle before ovulation, in the form of a pear (Kimura et al., 2005). The corpus luteum in the mare is formed from the granulosa cells of the ovulatory follicle, and it is constituted by large and small luteal cells (Van Niekerk et al., 1975). Large cells produce progesterone, under the influence of LH and progesterone; these hormones act through their specific cellular receptors for the production of progesterone (Roberto da Costa et al., 2005; Galvao et al., 2010).
The luteotropic effect of LH is carried out through the path of the signal-transduction PKA and MAPK with the increase in the phosphorylation of the StAR protein. The phosphorylated StAR protein acutely increases the transport of cholesterol through the membrane of the mitochondria, so that the cytochrome P450 side chain cleavage enzyme acts on it; the limiting enzyme in the synthesis of progesterone. The secretion of this hormone begins at the time of ovulation (Roberto da Costa et al., 2005), gradually increases until reaching its highest concentration in the blood circulation (12.8 ng/ml) on day 8 of the interval, subsequently it is slightly reduced until the regression of the corpus luteum or luteolysis, this last happens around day 14 (Ginther et al., 2007c).
The period of slight reduction of the hormone, that is to say, between days 8 and 14, it is known as preluteolytic, and it is due to the reduction of the hormonal support of the LH (Ginther et al., 2007c) and to the secretion of prostaglandin F2α (Ginther et al., 2011b). In most of the mares (67v %) there are 2 to 3 pulses of low amplitude (≈45 pg/ml), at 8-hour intervals of prostaglandin F2α in the preluteolytic period (Ginther et al., 2011b). See Figure 9. The concentration of progesterone is very variable, some studies have published different concentrations, but with a level higher than 4 ng/ml (Evans and Irvine, 1975, Beules and Holdworth, 1978, Hunt et al., 1978, Nagy et al., 2004; Honnens et al., 2011; Slough et al., 2011), values considered adequate to maintain pregnancy (Ginther, 1992). The area of the corpus luteum and the blood concentration of progesterone present the same tendency, they are reduced in parallel on day 8 of the cycle at the beginning of luteolysis (Ginther et al., 2007c).
Progesterone promotes the secretion of the endometrium, which prepares the uterus for pregnancy, inhibits the contraction of the myometrium and presents negative feedback on GnRH (Irvine and Alexander, 1993). As a consequence, it inhibits the behavior of estrus.
THE PRODUCTION OF PROGESTERONE
Progesterone is synthesized from the cholesterol precursor, which in the luteal cell passes into the mitochondria to transform into pregnenolone, under the influence of the cleavage enzyme of the cytochrome P450 side chain. The steroid acute regulator protein (StAR) participates in the entry of cholesterol into the mitochondria. This process is the limiting step for the synthesis of steroids (Stocco and Clark, 1996a,b, Watson et al., 2000, Slough et al., 2011). Pregnenolone leaves the mitochondria and goes to the smooth endoplasmic reticulum, where the enzyme 3β-dehydrogenase hydroxysteroid transforms it into progesterone (Slough et al., 2011). It has been suggested that LH simultaneously increases the expression of the coding genes for StAR protein and the cleavage enzymes of the side chain P450 and 3β-hydroxysteroid dehydrogenase (Beg et al., 2005; Slough et al., 2011; Kozai et al. ., 2012).
THE REGRESSION OF THE CORPUS LUTEUM
In the absence of a viable embryo, the structural and functional regression of the corpus luteum, also known as luteolysis, occurs with a drastic reduction in the blood concentration of progesterone to less than 1 ng / ml. Previously, pre-luteolysis (from day 8 to day 14 of the cycle), and the transition period from pre-luteolysis to luteolysis; the latter is manifested by a pulse of prostaglandin F2α of ≈45 pg / ml and corresponds to the moment when the drastic reduction of progesterone begins (Ginther and Beg, 2012a); then comes postluteolysis and corresponds to the period with the lowest concentration of progesterone (≤0.9 ng/ml).
Luteolysis in the mare begins on the 14th day of the cycle, equivalent to 9 days before the next ovulation, lasting approximately 23 hours (Ginther et al., 2011a, Ginther et al., 2011b, Ginther and Beg, 2012a ; Ginther and Beg, 2012b). The prostaglandin F2α produced in the endometrium promotes regression of the corpus luteum (Ginther and First, 1971, Stabenfeldt et al., 1974). The mare is very sensitive to the action of prostaglandin F2α (Kimball and Wyngarden, 1977); this hormone is segregated in pulses, the average interval between them is 9 hours (Ginther et al., 2011b); The amplitude of the pulses of this hormone increases as luteolysis progresses. At the beginning, a pulse of ≈78 pg / ml was presented, followed by another of greater amplitude, ≈193 pg / ml (Ginther et al., 2011a). Luteolysis begins with the first pulses of secretion of prostaglandin F2α (Ginther et al., 2009c) and it is induced with 2 to 4 sequential pulses (Ginther et al., 2008f, Ginther et al., 2009a).
The secretion of cortisol, estradiol and oxytocin, associated with the pulsatile secretion of prostaglandin F2α during luteolysis have been identified (Ginther and Beg, 2011a, Ginther and Beg, 2011b, Ginther and Beg, 2012a, Ginther and Beg, 2012b). From these, oxytocin is secreted simultaneously at each pulse of luteolysis; therefore, its secretion is also pulsatile. In fact, the secretion of prostaglandin F2α has been stimulated by pulsatile application of oxytocin. Therefore, oxytocin is considered, as has been done for other species, to stimulate the secretion of prostaglandin F2α and as a consequence participates in luteolysis (Penord et al., 2013; Santos et al., 2015). Oxytocin is synthesized in the hypothalamus and stored in the posterior lobe of the pituitary or posterior pituitary. It does not occur in the corpus luteum (Stevenson et al., 1991, Stout and Allen, 1999) as it does in ruminants (Wathes and Swann, 1982, Ivell and Richter, 1984, Swann et al., 1984). But in the mare it is also synthesized in the endometrium. See Figure 3. In regression of the corpus luteum, blood supply is reduced, leukocyte infiltration occurs, cell disruption and loss of steroidogenic capacity of the luteal cells to disintegrate the corpus luteum, and as a consequence the secretion of progesterone (Ginther and Beg, 2011).
CONCLUSIONS
The estrous cycle in the mare occurs in spring and summer, during the period of greatest number of light hours / day (high photoperiod); which is equivalent to a shorter period of secretion of the hormone melatonin. Melatonin is produced in the pineal gland. The hypothalamus secretes GnRH (gonadotropin-releasing hormone), with characteristics to promote the secretion of follicle-stimulating hormones (FSH) and luteinizing hormone (LH), from the anterior lobe of the pituitary gland or anterior pituitary, in an adequate way to stimulate ovulatory function. FSH promotes follicular growth and LH is responsible for follicular maturation and ovulation; its coordinated action is related to the production of estradiol, a hormone related to the manifestation of heat. After ovulation the corpus luteum develops which produces progesterone, a hormone that exerts negative feedback on the secretion of gonadotropins. At the end of the cycle, oxytocin promotes the endometrial secretion of prostaglandin F2α, which is responsible for the regression of the corpus luteum. With this another cycle begins, with a new opportunity for the mare to conceive.











 texto en
texto en