Introduction
lodine (I) is a trace element vital for the biosynthesis of thyroid hormones thyroxine and triiodothyronine, which are essential for the functioning of the nervous system and organs such as the liver, kidneys, muscles, and brain (Lee 2021). Worldwide, I deficiency affects approximately 35-45% of the population (Hatch-McChesney and Lieberman 2022, Aparo et al. 2023). The iodine recommended daily allowance is 90 ug for children aged 1 to 8 years, 120 μg for children aged 9 to 13 years, 150 μg for men and most women aged 14 years and older, 220 μg for pregnant women, and 290 ug for lactating women (Hatch-McChesney and Lieberman 2022).
Naturally, seafood, dairy products, eggs, and wheat derivatives are the main contributors of I; however, due to its low content in the soil and the fact that it is not considered an essential element for crops, its content in foods has decreased (Hatch-McChesney and Lieberman 2022, Zaremba et al. 2023). An alternative to increase the I content in plants and attenuate its deficiency in the population is the biofortification of crops, which, in addition to introducing I into the food chain, improves the plants defensive system by detoxifying free radicals, increasing the enzymatic and non-enzymatic compounds that can mitigate oxidative stress, generated by biotic and abiotic factors (Ramírez-Gottfried et al. 2023).
On the other hand, a characteristic of I is its great capacity to bind with polymers, forming complexes (Dávila-Rangel et al. 2020). In this sense, chitosan (poly β-(1,4)-N-acetyl-D-glucosamine, CS), is a trace element complexing biopolymer, which can be used to manufacture compounds with multiple advantages, including high biodegradability (Sangwan et al. 2023); therefore, if applied as a Cs-I complex, the uptake of I will be enhanced (Kanmani et al. 2017, Dohendou et al. 2021). It is important to mention that Cs and I are classified as biostimulants, which have the characteristic of improving nutrient absorption, nutraceutical quality and mitigating stress in crops, which translates into higher yields (Medrano-Macías et al. 2021, El-Amerany et al. 2022).
On the other hand, the fruits of the jalapeño pepper crop (Capsicum annuum L.) are a very important source of bioactive compounds (capsaicinoids, carotenoids, ascorbic acid, phenolic compounds, and glutathione), which provide antimicrobial, antiseptic, antioxidant and analgesic properties, as well as protection against cardiovascular diseases (Ao et al. 2022), so their consumption provides the human body with an important number of phytochemicals. Therefore, the objective of this study was to evaluate foliar spraying of a Cs-I complex on nutraceutical quality and I accumulation in jalapeño pepper fruits.
Materials and methods
Preparation of Solutions
The Cs-I complexes were prepared at the Center for Research in Applied Chemistry (CIQA). The chitosan used brand Marine Chemicals (Kerala, In-dia) had a viscometric molecular weight of 200,000 g mol-1 and a degree of deacetylation of 98%. First, a 1% solution of Cs in 1% acetic acid (AcOH) was pre-pared by adding the Cs little by little over 3 h, stirring at 300 rpm until completely dissolved, at a temperature of 60-65 °C. The resulting solution was filtered and diluted to volume of 1 L, for subsequent use as a control treatment. The Cs-I complexes were obtained according to the methodology described by Dávila-Rangel et al. (2020) by dissolving 0.1 M of KI in the 1% Cs solution to obtain complexes at concentrations of 5, 10, 15, 20, and 25 mg of iodine ion per liter.
Plant material and growing conditions
The present study was carried out in a shade net of the Instituto Tecnológico de Torreón, Mexico, 25° 32’ 38” N and 103° 25’ 08” W, 1 120 m. Jalapeño pepper plants cv. Odiseo (Ahern Seeds, San Diego, CA) was used. Seedlings were transplanted in black polyethylene pots 500 μm thick and 10 L capacity. Washed river sand and perlite (70:30 v/v) were used as the growing medium. Before transplanting, the sand was sterilized with 5% NaClO. The pots were arranged in rows, staggered, to obtain a density of four plants m2. Irrigations were carried out with Steiner nutrient solution (Steiner 1961), which was supplied manually, three times a day from transplanting to the beginning of flowering in such a way that each plant received 0.6 L per day, and from flowering to harvest received 1.8 to 2.0 L. At 60 days after sowing (DAS) the seedlings were transplanted, and 30 days after transplanting (DAT), the plants were trellised with raffia.
Experiment design and application of treatments
A completely randomized experimental design was used, the treatments applied were: absolute control (distilled water), 1% Cs control, and 1% Cs and I complexes at concentrations of 5, 10, 15, 20, and 25 mg L-1. Each treatment was foliar sprayed on 10 plants at 15, 30, and 45 DAT. The application of the treatments was between 08:00 and 10:00 h with a manual sprinkler.
Biochemical variables evaluated
Four fruits were taken from each treatment, taken from four plants (first cut, which was at 55 DAT). The fruits were then stored at -20 °C until further analysis, where total phenols, flavonoids, vitamin C, capsaicin, antioxidant capacity, and iodine content were evaluated.
Extraction of phytochemicals
Two g of sample was placed in a Falcon tube of 15 mL capacity and 10 mL of absolute ethanol was added. It was vortexed for 1 min and allowed to stand in the dark for 24 h. The sample was decanted at 3,500 rpm for 15 min, the supernatant was separated and deposited in 15 mL falcon tubes. The samples were stored at -20 °C.
Total phenols
The content of total phenolic compounds was measured using a modification of the Folin-Ciocalteu method (Singleton et al. 1999). A extract volume of 30, uL was mixed with 270 μL of distilled water in a test tube, then 1.5 mL of diluted (1:15) Folin-Ciocalteu’s reagent (Sigma-Aldrich, St Louis.) was added and vortexed for 10 s. After 5 min, 1.2 mL of sodium carbonate (7.5% w/v) was added and vortexed for 10 s. The solution was placed in a bath and stirred for 10 min. The solution was placed in a water bath at 45 °C for 15 min and then allowed to cool to room temperature. The absorbance of the solution was read at a 750 nm wavelength in a UV-VIS spectrophotometer (Genesys 10). The calibration curve was prepared with gallic acid. The results were expressed in milligram gallic acid equivalents per 100 grams of fresh weight (mg GAE 100 g-1 FW).
Flavonoids
Total flavonoid content was quantified using the procedure described by Lamaison and Carnet (1990). For this, an aliquot of 250 μL of the ethanolic extract supernatant was taken, and then 1.25 mL of distilled water and 75 μL of 5% NaNO2 were added, vortexing the mixture and allowing it to react for 5 min. Subsequently, 150 μL of 10% AlCl3 was added, vortexing the mixture and allowing it to react for 6 min. Then, 500 μL NaOH 1 M and 275 μL of distilled water were added, vortexing. The calibration curve was prepared with quercetin. The absorbance was measured at 510 nm in a UV-VIS spectrophotometer, and the results were expressed in milligram quercetin equivalents per 100 g fresh weight (mg QE 100 g-1 FW).
Vitamin C
The vitamin C content in fruit was determined by the titration method of Padayatt et al. (2001). 10 g of fresh fruit were taken, crushed together with 10 mL of 2% hydrochloric acid, filtered, and made up to 100 mL with distilled water in an Erlenmeyer flask. With 10 mL of the dilute, it was titrated with 2,6 dichlorophenolindophenol (1 x10-3 N). The vitamin C content was calculated using the following formula:
Where: mL 2,6: volume of 2,6-dichlorophenolindophenol spent in the titration; TV: total volume of sample processed; VA: volume of aliquot taken for titration; WS: weight of sample. Results were expressed as milligrams per 100 grams of fresh weight (mg 100 g-1 FW).
Capsaicin
Total capsaicin content was determined by adapting the method of Cisneros-Pineda et al. (2007). The absorbance of the ethanolic extracts of the samples was read at 273 nm in a UV-VIS spectrophotometer. Total capsaicin content was calculated using a standard curve (Sigma, St. Louis, Missouri, USA). Results are reported in milligrams of capsaicin per kilogram of fresh weight (mg kg-1 FW).
Antioxidant capacity
The antioxidant capacity was determined with the in vitro DPPH method (Brand-Williams et al. 1995). A solution of DPPH (Aldrich, St. Louis, Missouri, USA) in ethanol at 0.1 M was prepared. For the determination of the antioxidant capacity, 0.5 mL of the sample and 0.5 mL of the 0.1 M DPPH were mixed. It was then allowed to stand for 15 min and the absorbance was read at 530 nm. Results were reported in the micromolar equivalent of trolox per 100 g fresh weight (jM TE 100 g-1 FW).
Determination of iodine in fruit
For this determination, the fruit was dried in a drying oven at 70 °C for 72 h. Iodine content was determined using the alkaline digestion technique (Medrano Macías et al. 2021). All reagents were prepared, and all materials were cleaned using deionized water. The iodine concentration was measured using Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-OES). The results were expressed in micrograms per kilogram of dry weight (μg kg-1 DW).
Statistical analysis
The normality and homogeneity of the variances of the data obtained were verified using the Kolmogorov-Smirnov and Bartlett tests, respectively. Subsequently, the analysis of variance of simple classification and multiple comparisons of means was performed through the Tukey test at a significance of 5%, with SAS v 9.0 software (SAS 2004).
Results and discussion
Phytochemicals in fruits
In addition to being a biofortifying element, I in adequate concentrations is a biostimulant because its application promotes the synthesis of enzymatic and non-enzymatic antioxidants in plants (Riyazuddin et al. 2023, Ramírez-Gottfried et al. 2023). In this study, the addition of the Cs-I complex acted in this way by substantially enhancing the biosynthesis of bioactive compounds in jalapeño pepper fruits. The results show that the concentration of 15 mg L-1 of the Cs-I complex increased the content of non-enzymatic antioxidants such as phenols, flavonoids, vitamin C, and capsaicin by 18, 78, 17 and 11%, respectively, concerning the fruits of control plants (Table 1). Regarding the antioxidant capacity (Figure 1), the treatment of 15 mg L-1 of Cs-I was the one that increased it the most, exceeding the control by 23%, which shows a positive correlation between the bioactive compounds and the antioxidant capacity of this treatment. Above this concentration, a decrease in the values obtained was observed. In contrast, the use of 1% CS caused a decrease of 4, 15, 9, 2, and 4% in the quantification of phenols, flavonoids, vitamin C, capsaicin, and antioxidant capacity, respectively, concerning the control.
Table 1 Effect of foliar application of Cs-I on phytochemicals in jalapeño pepper fruits.
| Treatments | TF (mg GAE 100 g-1 FW) | FL (mg QE 100 g-1 FW) | VC (mg 100 g-1 FW) | CAP (mg kg-1 FW) |
|---|---|---|---|---|
| Control | 258.7 f | 131.36 f | 107.50 c | 8.96 b |
| Cs (1%) Cs-I (mg L-1) | 248.7 g | 111.17 g | 97.53 d | 8.79 b |
| 5 | 266.44 e | 149.42 e | 109.56 c | 8.86 b |
| 10 | 274.17 d | 166.21 d | 117.55 b | 9.04 b |
| 15 | 305.21 a | 233.37 a | 126.40 a | 9.97 a |
| 20 | 292.05 b | 204.89 b | 122.27 ab | 9.50 ab |
| 25 | 282.03c | 183.22 c | 117.67 b | 9.18 ab |
Different letters within each column indicate significant differences between treatments (Tukey, p ≤ 0.05). Cs: Chitosan; I: Iodine; TF: Total phenols; GAE: Gallic acid equivalents; FL: Flavonoids; QE: Quercetin equivalents; VC: Vitamin C; CAP: Capsaicin; FW: Fresh weight; n = 4.
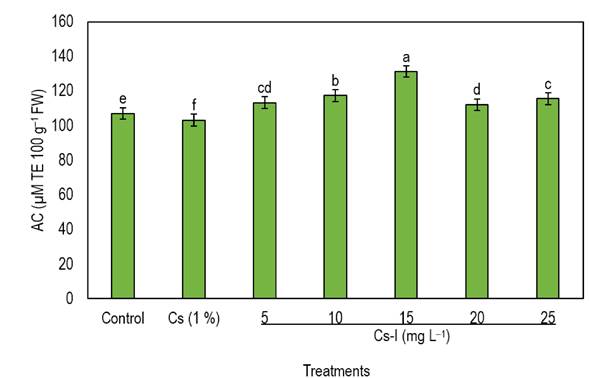
Figure 1 Effect of foliar application of Cs-I on the antioxidant capacity in jalapeño pepper fruits. Different letters indicate significant differences between treatments (Tukey, p ≤ 0.05). AC: Antioxidant capacity; TE: Trolox equivalent; FW: Fresh weight; Cs: Chitosan; I: Iodine; n = 4; Bars represent the standard error.
The improvement in bioactive compounds by the use of Cs-I is in agreement with previous studies reporting increases in the biosynthesis of non-enzymatic antioxidants (Consentino et al. 2022, Krzepitko et al. 2023). The use of Cs-I as a biostimulant agent has been previously reported in Lactuca sativa L., where it is mentioned that it increased the concentration of total phenols, reduced glutathione, and antioxidant capacity (Dávila-Rangel et al. 2020). The complex was also tested in Solanum lycopersicum L and increases in vitamin C and carotenes were reported (Mageshen et al. 2022). El-Amerany et al. (2022) evaluated the application of Cs individually in Solanum lycopersicum L, and showed increases in α-tocopherol, lycopene, and flavonoids.
The response of plants in any of its forms depends on the concentration and chemical species used and can range from a biostimulant or toxicity effect (Riyazuddin et al. 2023), in our case we believe that it was both conditions since the bioactive compounds increased and decreased with the application of the treatments. Added to this, there are studies where it is indicated that I was one of the first inorganic antioxidants, which allowed organisms to mitigate oxidative stress, by neutralizing reactive oxygen species, which are reduced thanks to its electronic configuration, which gives it a great reducing power (Medrano-Macías et al. 2016). When applied, it exerts an important effect as a biostimulant, since it can be detected by membrane receptors and trigger a cascade of cell signaling that ends in the activation of the antioxidant system (Riyazuddin et al. 2023, Zhang et al. 2023). In addition, I can also enter at the cellular level via membrane transporters and directly activate the antioxidant system by producing bioactive compounds (Riyazuddin et al. 2023).
Now, Cs is a biopolymer that is classified as a biostimulant, and in adequate concentrations can stimulate the synthesis of different antioxidant compounds, however, the biological model of study and environmental conditions also influence the results (Dávila-Rangel et al. 2020, El-Amerany et al. 2022). In this study, the use of 1% Cs individually caused a decrease in all bioactive compounds evaluated. There is research that reports that Cs does not cause toxicity in plants, due to its biodegradability; however, high concentrations of Cs increase the degree of polymerization, which increases the permeability of cell membranes, to the point of causing cell death (Chirkov 2002, Hidangmayum et al. 2019). For such reason, it is believed that this was one of the reasons why 1% Cs treatments individually decreased the concentration of bioactive compounds, since possibly, jalapeño pepper is sensitive to this biopolymer. In the case of the treatments of 5, 10, and 15 mg L-1 of Cs-I, surely the I helped to attenuate the effect of Cs (1%) and became a positive effect when acting in complex (Dávila-Rangel et al. 2020). In this sense, with concentrations of 20 and 25 mg L-1 of Cs-I, the concentrations of all the bioactive compounds evaluated began to decrease, which can be explained by the effect of Cs in causing cell death due to the high permeability of cell membranes and by the high concentrations of I that can be toxic (Chirkov 2002, Medrano-Macías et al. 2016).
lodine content in fruit
Apart from salt iodization, the natural way in which the population acquires I is through the consumption of seafood, dairy, egg, and wheat derivatives, which are the main contributors to I, however, the change in agricultural and industrial practices has contributed to the decrease of its content in food (Hatch-McChesney and Lieberman 2022). Therefore, agricultural practices such as biofortification provide the opportunity to obtain vegetables enriched with trace elements such as I that would also be accessible to the world population (Golubkina et al. 2021). It is important to have an adequate intake of I, as it is needed for the production of thyroid hormones, which help proper bone and brain development during pregnancy and childhood (Mégier et al. 2023).
In this study, foliar spraying of Cs-I complexes significantly increased the I content in jalapeño pepper fruits (Figure 2). The higher the concentration of the complex, the greater the accumulation of I in the jalapeño pepper fruits. The 25 mg L-1 treatment of the Cs-I complex increased the I concentration in the fruit by 70% compared to the control. Ramírez-Gottfried et al. (2023) report an increase of I in grapevine fruit as KI concentration increased. Similarly, García-Fuentes et al. (2022) report that the application of KIO3 on tomato plants caused an increase of I in fruits compared to untreated plants. Dávila-Rangel et al. (2020) report that the use of I in ionic form (KI and KIO3) in complex with 1% Cs increased the content of the element in leaves of Lactuca sativa L. Mageshen and Santhy (2023) mention that the use of the Cs-I complex increased the accumulation of the element in tomato fruits, compared to the application of individual I.
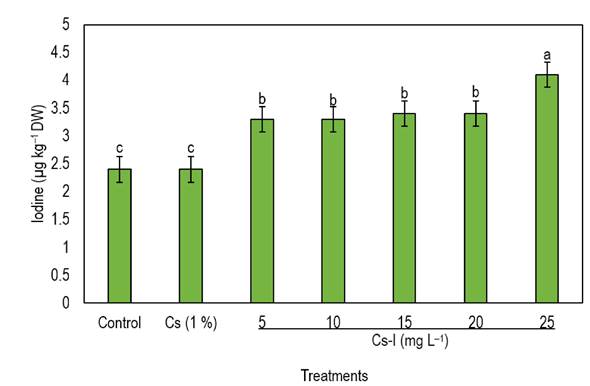
Figure 2 Effect of foliar application of Cs-I on the accumulation of I in jalapeño pepper fruits. Different letters indicate significant differences between treatments (Tukey, p ≤ 0.05). DW: Dry weight; Cs: Chitosan; I: Iodine; n = 4; Bar represent the standard error.
The I, when applied foliarly, is absorbed by the stomata and by cuticular waxes with a high degree of unsaturation, which facilitates its entry and transport to the fruits (Zhang et al. 2023). This is important to consider, as foliar applications may be more effective than soil application when it comes to biofortification of fruits or leaf vegetables (Lawson et al. 2015). If the application is to the soil, the transport to the fruits and leaves for consumption is more complicated and the accumulation of the element is less (Dávila-Rangel et al. 2020). Plants can absorb I in the form of iodate (IO3 -) and iodide (I-), however, in soils IO3 - is absorbed more efficiently than I-, due to its thermodynamic stability (Lawson et al. 2015, Germ et al. 2020). That said, IO3 - is the form in which I is most available to plants (Medrano-Macías et al. 2016).
It has been shown that the use of the Cs-I complex significantly increases the I content in the edible parts of the plant, due to the ability of Cs to chelate these trace elements (Dávila-Rangel et al. 2020, Mageshen et al. 2022). I, being a halogen, has the ability to volatilize easily, so in iodized salt, it is easily lost (20% during storage and another 20% during cooking in food) (Duborská et al. 2022). In plants, I is unstable, and they volatilize it as methyl iodide (CH31) using the enzymes ion halide methyltransferase and halide/thiol methyltransferase (Medrano-Macías et al. 2016). For this reason, the use of Cs is a promising option that allows to form an electrostatic interaction with I, avoiding volatilization and gradually increasing the bioavailability in planttissues (Gonzali et al. 2017, Mageshen et al. 2022).
According to the results obtained, the application of the 15 mg L-1 complex of Cs-I improved the nutraceutical quality of jalapeño pepper fruits, in contrast to the application of 1% Cs individually. With the application of the 25 mg L-1 complex of Cs-I, the highest accumulation of I in fruits was achieved. In this sense, foliar spraying of Cs-I can improve the synthesis of bioactive compounds and accumulate the element in the edible parts of vegetables, thus improving the health of consumers. It is worth mentioning that the concentration used, the biological model, the environmental conditions, and the form of application will be determinants in the stimulation of the antioxidant system and the accumulation of I in plant tissues, in addition to the fact that the age of humans requires different concentrations of the element in question.











 nueva página del texto (beta)
nueva página del texto (beta)



