Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista ALCONPAT
versión On-line ISSN 2007-6835
Rev. ALCONPAT vol.7 no.2 Mérida may./ago. 2017
https://doi.org/10.21041/ra.v7i2.142
Applied research articles
Internal Sulphate Reaction (ISR) as degradation of the cement matrix: behavior of pastes dosed with different amounts of contamination by sulfate
1 PPGECC. Universidade Federal do Paraná (UFPR), Brasil.
2 CCET. Universidade Federal do Oeste da Bahia (UFOB), Brasil.
3 LACTEC. Institutos Lactec. Brasil.
4 COPEL. Companhia Paranaense de Energia Elétrica, Brasil.
The present work, developed in the scope of the R&D Program of ANEEL, project 6491-0301/2013, aims to evaluate how different levels of sulfide contamination interfere with the microstructural and mechanical characteristics of the cement pastes when they are submitted to two exposure conditions: wetting and drying cycle and aerated tank. For an analysis of the deteriorating mechanism, four series of cement pastes, one reference and three contaminated by sulfur, were evaluated by non-fresh tests (consistency and specific mass) and resistance (axial compression and flexural tensile strength, dimensional variation and XRD). At the end of the study period (84 days) it was noted that the auxiliary assays did understand the mechanism but that it would still be at an early stage.
Keywords: contamination by sulfides; deteriorating mechanism; environmental exposure; initial stage
El presente trabajo, desarrollado en el marco del Programa de P&D ANEEL, proyecto 6491-0301/2013, tiene por objetivo evaluar diferentes contenidos de contaminación por sulfatos que interfieren en las características microestructurales y mecánicas de mezclas, cuando estas son sometidas a dos diferentes condiciones de exposición: ciclo de mojado y secado y tanque aireado. Para el análisis del mecanismo deteriorante, se evaluaron cuatro series de mezclas, una referencia y tres contaminadas por azufre, por medio de ensayos en estado fresco (consistencia y peso específico) y endurecido (resistencia a la compresión axial y la tracción en la flexión, variación dimensional y DRX). Al final del período estudiado (84 días) se notó que los ensayos ayudaron en el entendimiento del mecanismo, pero que esta unión estaría en una etapa inicial.
Palabras clave: contaminación por sulfuros; mecanismo de deterioro; exposición ambiental; estado inicial
O presente trabalho, desenvolvido no âmbito do Programa de P&D ANEEL, projeto 6491-0301/2013, tem por objetivo avaliar como diferentes teores de contaminação por sulfetos interferem nas características microestruturais e mecânicas de pastas, quando estas são submetidas a duas diferentes condições de exposição: ciclo de molhagem e secagem e tanque aerado. Para a análise do mecanismo deteriorante foram avaliadas quatro séries de pastas, uma referência e três contaminadas por enxofre, por meio de ensaios no estado fresco (consistência e massa específica) e endurecido (resistência à compressão axial e a tração na flexão, variação dimensional e DRX). Ao final do período estudado (84 dias) notou-se que os ensaios auxiliaram no entendimento do mecanismo, mas que este ainda estaria em um estágio inicial.
Palavras-chave: contaminação por sulfetos; mecanismo deteriorante; exposição ambiental; estágio inicial
1. Introduction
The internal attack of sulfates in concrete, also known as internal sulfate reaction (ISR) is a relatively new field of study, particularly when compared to mechanisms of deterioration such as the alkali-aggregate reaction (AAR) and even to the external attack of sulfates (CASANOVA et al., 1997; CENTURIONE et al., 2003; PEREIRA et al., 2014). Specific studies in this area started to appear in the beginning of the 80’s in Europe and North America (KHELIL, 2014).
Degradation mechanisms in concrete structures, having contaminated aggregate, are initially caused by the phenomenon of oxidation of the mineral sulfide in the presence of humidity.
This process, in water, is responsible by the formation of soluble products, iron sulphate and sulfuric acid. For the formation of these products, the presence of oxygen and humidity in the environment is indispensable, as indicated by (1) (CZEREWKO e CRIPPS, 1999).
The oxidation reaction generates volumetric changes and fissures in concrete, precisely because of the internal pressure caused by the formation of the new products, which have a greater volume than the mineral sulfide that originated them. Besides, products generated by this phenomenon react with cement compounds, tricalcium aluminate (C3A), hydrated calcium (C-S-H) and calcium hydroxide (Ca(OH)2), forming new hydrated crystalline compounds, such as gypsum (CaSO4.2H2O) and (3CaO.Al2O3.3CaSO4.32H2O) (CENTURIONE et al., 2003; COUTINHO 2001; PEREIRA et al., 2014).
The main intervening factors in the oxidation process are: concentration of oxygen, presence of moisture, specific surface of the mineral sulfide, content of iron contained in the sulfide, presence of bacteria, ambient temperature, sulfide morphology system pH (GOMIDES, 2009).
The use of aggregates contaminated by sulfides in concrete structures is not recommended. Hasparyk et al. (2003), for instance, consider aggregates containing sulfides as materials unsuitable to be used in concrete structures. However, in some cases the use of these materials is necessary, either by the lack of resources considered as more proper ones or because of cost. Thus, there are standards establishing the maximum limits of contamination by SO3 to be used in concrete structures. Two internationally disseminated standards are:
American Concrete Institute - ACI 201 (1991) - establishes a limit of 0.5% of SO3 regarding the total weight of the aggregate;
French standard AFNOR NF P 18-541 (1997) - establishes, for hydraulic concrete, the maximum amount of sulfur (S) expressed in SO3 of 1% or 0.75% of pyrite (FeS2) regarding the aggregate mass.
Brazilian standard NBR 7211 (2009), establishes a limit of SO42-, of all constituents of concrete (water, aggregates, cements, additions and chemical additives), regarding the total mass of the mixture, equal to 0.2%
Based on information available in the technical environment, an experimental study was used to evaluate how the application of different amounts of contamination by sulfides interferes in the microstructural, physical and mechanical characteristics of cement pastes.
2. Experimental program
Considering the factors mentioned by Gomides (2009) for the occurrence of ISR, the option was to expose the materials to two different conditions of environmental exposition. The objective was to evaluate whither the attack is influenced by the presence of oxygen and humidity.
The two exposition conditions used in the study were:
Tank with continuously aerated aqueous solution: materials were submitted to a total and continuous submersion in a tank with continuously aerated water.
Wetting and drying cycles: the test specimens had their conditions alternated weekly between a dry chamber (R.H 50±5% and 20 ± 2 ºC), and an aerated tank.
In order to evaluate the amounts of contaminant and exposition conditions, pastes were submitted to assays, both in their fresh as well as in the hardened state.
The assays performed in fresh state, which wore used for characterizing the material, were: specific mass and consistence by means of Kantro’s mini cone trunk assay.
The assays selected for evaluating the evolution of the attack were performed in different ages of pastes, being them: dimensional variation, resistance to compression, traction in flexion and X-ray diffraction.
2.1 Materials
The materials used in the assay were: cement CP V - ARI, fly ash, pyrite and limestone filler.
The composition of the cementitious system was performed in laboratory, by replacing 35% of the CP V - ARI, in paste, by fly ash, this amount between 15% and 50%, in paste, established by NBR 5.736 (1991), in order to classify the composition as a typical type CP IV cement.
The CP V - ARI cement used had specific mass of 3.13 g/cm3 and specific BET area of 1.07 m2/g. Fly ash, from the combustion of mineral coal from the reservoir of the Valley of the river Tubarão, had specific mass of 1.95 g/cm3 and BET specific area of 1,09 m2/g.
Contaminant added to paste was pyrite (FeS2), resulting from the beneficiation of mineral coal from the region of Figueira - Paraná. This material, also studied by Pereira (2015), has, in its chemical composition, a purity of 94%. The material has specific mass of 4.95 g/cm³ and, after grinding, was classified as a material passing through the sieve with mesh openings of 2.4 mm.
Limestone filler, inert material, was used to compensate the contaminant mass in the pastes, its percentage variable according to the amount of pyrite added to the mixture. The specific mass of carbonaceous material was 2.84 g/cm3.
2.2 Pastes with mineral addictions
The follow-up of reactions due to ISR and consequent implications in the mechanical properties of the hydrated matrix were evaluated by means of study in Portland cement paste. The reason for executing the study in paste was to facilitate the identification of products formed by the degradation mechanism, since the presence of sand and gravel would generate the occurrence of crystalline peaks, of higher intensity, in the X-ray diffractograms, making the identification of compounds formed by ISR difficult.
2.2.1 Contamination
For the contamination of the pastes, three amounts of SO3 were adopted, besides the reference (0% contamination), two amounts mentioned as limits by standards, 0.5% and 1.0% and one over the limit allowed by them (amount of 5.0%).
For adjust the amounts (SO3xFeS2) it was necessary a standardization of the amount of sulfur present in the elements. Considering the atomic masses of the studied elements (S=32.06 u.m.a; O=15.99 u.m.a; Fe=55.85 u.m.a), the molar masses of the studied molecules (SO3=80.03 g/mol and FeS2=119.97 g/mol) and the compositional amounts of sulfuric anhydride, obtained by means of (2)-(3), it was possible to get the factor between pyrite and SO3 (4).
In Table 1 are presented the amounts used in this research, in pyrite and SO3.
Table 1 Proportional contamination levels in SO3 and pyrite.
| Content in SO 3 | Content in pyrite |
|---|---|
| 0.00% | 0.00% |
| 0.50% | 0.38% |
| 1.00% | 0.75% |
| 5.00% | 3.75% |
The previously mentioned standards limit the amount of the utilization of materials containing sulfides regarding the total mass of aggregates. Since the pastes have no aggregate for applying the amounts, a trace of base concrete was used, for then determinate the amounts regarding the mass of cement.
The concrete trace adopted was: 1.0 kg cement, 2.7 kg fine aggregate, 2.7 kg coarse aggregate and 0.6 kg water. The choice of this proportion comes from the application of a medium trace in the execution of conventional concrete in a hydroelectric plant. Contamination percentages were applied over the total mass of aggregates (2.7 kg fine aggregate + 2.7 kg coarse aggregate) and determined the proportional contamination regarding the cement mass, according to results presented in Table 2.
Table 2 Proportional contamination levels of the reference concrete.
| Series studied | Pyrite content in relation to the total mass of aggregates | Pyrite content in relation to the mass cement |
|---|---|---|
| Ref. (0.0%) | 0.00% | 0.00% |
| SO3 (0.5%) | 0.38% | 2.02% |
| SO3 (1.0%) | 0.75% | 4.05% |
| SO3 (5.0%) | 3.75% | 20.25% |
Reference paste was prepared with pozzolanic cement with the addition of 20.25% of limestone filler over the mass of binding material (CP V cement + fly ash). The ratio water/cement used in the mixture of the paste was 0.6, and it was kept constant in all of the studied systems.
In contaminated pastes, the limestone filler was gradually replaced, in mass, by pyrite, due to the different amounts of contamination, until, in the paste of the group with 3.75% in pyrite mass, all limestone filler was replaced by the contaminating agent.
2.2.2 Molding
For the assays of compressive strength and flexion traction strength, prismatic test specimens were molded with dimensions of (4 x 4 x 16) cm. The test specimens for the assay of dimensional variation, also prismatic, were molded with dimensions of (2.5 x 2.5 x 28.5) cm. Finally, samples of approximately 10g were collected and conditioned, in hermetically sealed vials, for the assay of X-ray diffraction (XRD). All materials were submitted to underwater curing, saturated with lime, until 28 days of age.
Pastes were mixed in mechanical mixer, as described in NBR 13.276 (2005). Densification, to remove the imprisoned air, was performed with spatula strokes and, at the end of filling the molds, the upper section was leveled.
After 48hs, according to recommendations from NBR 13279 (2005), the test specimens and XRD samples were taken from the molds and submitted to humid healing, in water saturated with lime, until 28 days of age. In the same day, materials were submitted to the exposition conditions, wetting and drying cycle and aerated tank.
For tests of resistance to compression and traction in flexion, test specimens were molded for rupture ate 28 and 84 days of age. For X-rays diffractometry, samples for assays were collected at ages of 28 and 56 days. For the assay of dimensional variations, bars were molded for readings in previously defined dates.
2.2.3 Assays in fresh state
After finishing the mixture of pastes, the determination of their consistencies was performed by means of Kantro’s mini cone trunk assay (KANTRO, 1980).
Posteriorly, the assay of the specific mass of the material in fresh state was executed, according to recommendations from NBR 13278 (2005).
2.2.4 Assays in hardened state
For determining the mechanical properties of the pastes, assays according to standard NBR 13.279 (2005) were executed, first the traction test in flexion and then the one of axial compression. Test specimens proposed by the standard are prisms of (4 x 4 x 16) cm.
For analyzing the dimensional variation of the prismatic test specimens (2.5 x 2.5 x 28.5) cm, measurements were executed in a standard gantry to which a comparing watch with a precision of 0.001mm is coupled. As there is no normative recommendation of this test for bars submitted to internal attack from sulfides, provisions of standard NBR 13583 (2014), were used. It standardizes tests of dimensional variation in bars of Portland cement mortar, exposed to solution of sodium sulfate. Due to the sensibility of the test, it was opted by daily readings during the first 28 days of age and, after that, by weekly readings.
The main objective of performing the XRD assay was the identification of chemical phases, ettringite and gypsum, coming from ISR. At the assay ages, samples were fragmented in pieces with a maximum dimension of 5.0mm, conditioned in polymeric recipients and immersed in ethyl alcohol p.a. during 24 hours. After this period, samples were removed from alcohol, dried in kiln at 40 ºC and conditioned again in polymeric recipient with cover, which were stored in desiccator containing silica gel until the moment of executing the assay (PAN et al., 2002). Equipment used was a RIGAKU Ultima IV X-ray Diffractometer, under the following assay parameters: step of 0.02 º2θ and step time of 1 second, using tube with copper anode, 40 KV / 30 mA and divergent slit of 1º. Analysis was executed from 5º 2θ until 75º 2θ.
3. Results and discussion
3.1 Consistency of pastes by Kantro’s mini cone assay
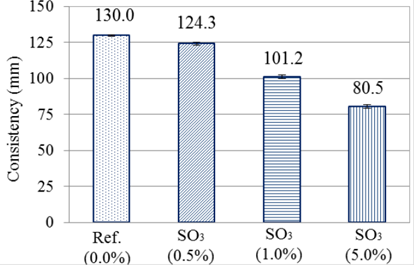
The reference group, without the addiction of pyrite, had the greater spreading among the other groups. Observing Figure 1 it is possible to see that the spread was decreasing with the increase of the addition of contaminating material.
This is according to what was exposed by Katsiadramis et al. (2010), because materials of greater granulometry, in this case pyrite (Dmax equal to 2.4mm), act as obstacles, increasing the viscosity of the compound. This is contrary to the fine grains, in this case the filler, which act as lubricants increasing the fluidity of the material.
Besides the mentioned physical effect, there is the possibility of a chemical effect in consequence of the interactions of cement particles with pyrite’s ions.
3.2 Specific mass in fresh state
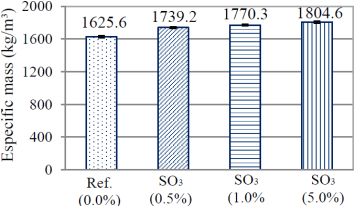
Figure 2 indicates the growth of specific mass during the replacement of the filler by pyrite.
In this case a behavior opposite to the one presented in the consistency assay was perceived. As the replacement of the filler by pyrite was being made, the specific mass in the fresh state of the series was increasing. This behavior is due to the fact that the replacement happens between two materials of different specific masses: filler (2.84 g/cm3) and pyrite (4.95 g/cm3). Since a material of lower specific mass is removed and one of greater specific mass is added, the specific mass of the compound is increased. The gradual substitution of filler also collaborated with an increase of the consumption of cement in the paste, in volume. It was perceived that the consumption for the series Ref. (0.0%) was 943.162 kg/m3, while series SO3 (0.5%), SO3 (1.0%) and SO3 (5.0%) have consumptions equal to 945.857 kg/m3,948.588 kg/m3 and 971.820 kg/m3, respectively.
3.3 Resistance to axial compression and to traction in flexion
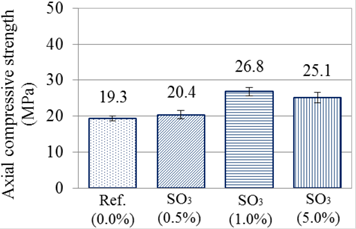
In Figures 3 and 4 are presented the values of resistance to axial compression for all of the analyzed groups (28 and 84 days).
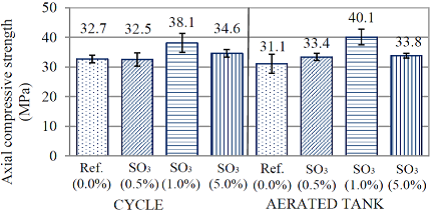
Figure 4 Axial compressive strength of the cement pastes at 84 days of age (28 days in submerged cure + 56 days of exposure).
With values obtained at 28 days, it was possible to observe that group reference and 0.5% of SO3 have resistances to compression of 20 MPa, considered as equal, according to Tukey’s test with 95% of trust. Series 1% and 5% of SO3, despite the proximity of values and overlap of the error bar, could not be considered as statistically equivalent by the test used. Thus, at the age of 28 days, the series having more resistance was the one of 1% of contamination with SO3.
The greater values of resistance to axial compression of the series with higher amounts of contamination, SO3(1.0%) and SO3(5.0%), may be explained by different factors. First, as previously exposed, those two traces have more consumption of cement (3.03%). This may had contributed for the resistance at the age of 28 days being higher than the others. Another factor that could explain this increase of resistance is the filling of voids in the pastes, by products formed by the internal attack of sulfates. This is according to Araújo (2008), because the formation of products coming from the internal attack of sulfates in the voids, fills the spaces, generating gains of resistance in an initial stage. Biczók (1972) and Ouyang et al. (2014), studying the external attack of sodium sulfate, also reported that with the increase of compactness, there is a gain of resistance at an initial stage.
Besides the analysis between groups, it was possible, for the age of 84 days, to evaluate the influence of exposition. Again, with the application of the test, it was observed that both exposition conditions had statistically equivalent resistances.
Regarding the series, it was noted that, at this age, series Ref. (0.0%) and SO3(0.5%) continued indicating statistical similarity, in both conditions of exposition. However, it was also noted statistical equivalence of series SO3(0.5%) with SO3(5.0%), in both studied conditions. This similitude between the group of lower contamination and the group of greater contamination does not indicate fall of resistance by the last one, but an increase of resistance that is inferior to the others. During the condition of wetting and drying, for instance, group Ref.(0.0%) had, at 84 days, a gain of approximately 69%, while group SO3(5.0%), under the same condition, had increase of 38.11%. As much as there is no fall in the obtained value, a change in the behavior of groups is perceived already at the age of 84 days. Series SO3(1.0%) was the one, again, with the greater value of resistance.
The delay in resistance gain observed to series SO3(5.0%) is according to results from Pereira (2015) and Oliveira (2013). Thus, it is supposed that the sulfate provided by the initial reactions of oxidation of pyrite changed the hydration kinetics of the cement, having differences in resistances already at 84 days.
In Figure 5 are displayed the values of flexion strength obtained for pastes at 28 days of age. In this case, it was perceived that the reference paste and the pastes with amounts of contamination by SO3 had statistically equal resistances, between 5 and 6 MPa.
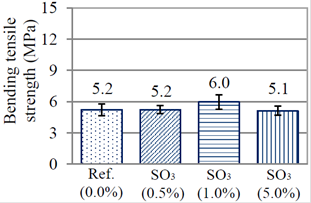
Figure 5 Bending tensile strength of the cement pastes at 28 days of age (submerged curing condition).
In Figure 6 are displayed results of flexion strength at 84 days of age. It was observed that the standard deviation between samples was high, with coefficients of variation near 20%, which collaborated for Tukey’s test not indicating statistical difference between all samples. Analyzing exposition conditions, at 84 days, it is noted that only series SO3(1.0%) had statistical difference regarding itself, the lower value being obtained in the cycle condition.
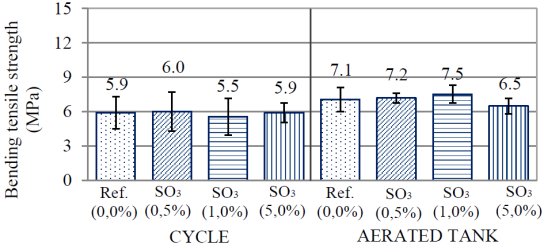
Figure 6 Bending tensile strength of the cement pastes at 84 days of age (28 days submerged cure + 56 days of exposure).
Besides the statistical equivalence observed between series at the age of 84 days, it was perceived that there was no significant difference between the age of 28 days and the age of 84 days, from all of the studied series. Thus, it is not possible to declare that there was gain or loss of resistance between both ages (28 and 84 days) for all groups.
3.4 Dimensional Variation
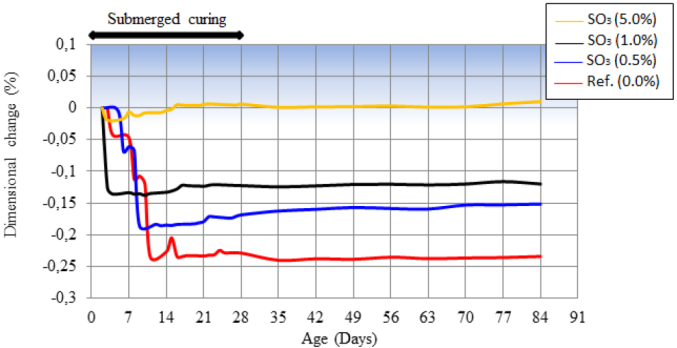
In Figure 7 is presented the dimensional variation of the pastes, when submitted to weekly cycles of drying and wetting. The reference paste had the greater retraction between the studied pastes. Retraction happened progressively until the age of 11 days, moment when it stabilized and started to fluctuate as a function of the type of exposition to which it was submitted. The partial substitution of the limestone filler by pyrite, in different amounts, caused the reduction of the retraction of pastes. The use of contaminated material, in different amounts, caused the reduction of the retraction of pastes. The use of contaminated material in amount of 1.0% SO3 reduced retraction to, approximately, half of the retraction observed in the reference paste. With the amount of 5% of SO3 practically no retraction was observed, which may be verified at the age of 28 days, moment before the exposition to the drying and wetting cycles. This behavior indicates the beginning of the oxidation of pyrite and the formation of expansive hydrated compounds, to be analyzed in the presentation of the X-ray diffractograms. From 28 days of age, the exposition to the cycles of drying and wetting cycles enabled the dimensional variation (retraction/expansion) of the paste that, in average, was similar to the dimension observed at 28 days, the moment before the procedures of drying and wetting. This behavior indicated that the oxidation reaction of pyrite was not significantly influenced by the variation of exposition condition (until 84 days of age of the tested compounds), which aimed to provide, mainly, oxygen for its occurrence.
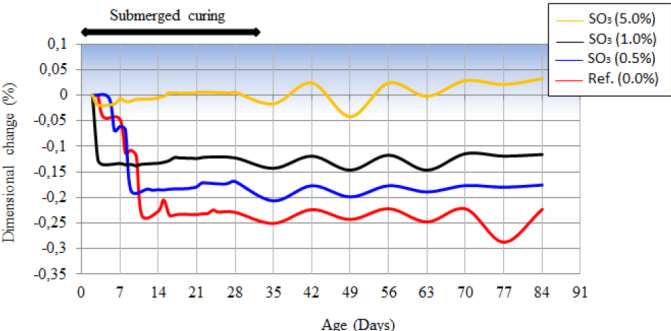
Figure 7 Dimensional variation of the test specimens subjected to the wetting and drying cycle at 84 days of age.
In Figure 8 are presented the results of dimensional variation of pastes when submitted to the initial underwater curing of 28 days, at the tank with continuously aerated water. Dimensional variation until the age of 28 days was equivalent to the one observed in Figure 7. In this case no variations (oscillations) were noted, as in the previous exposition condition. It was noted that, from 28 days of age, there was no significant variation in any of the studied series.
Generally, there was a tendency of forming expansive compounds due to the internal attack of sulfates when the contaminated cementitious material was exposed to drying and wetting cycles.
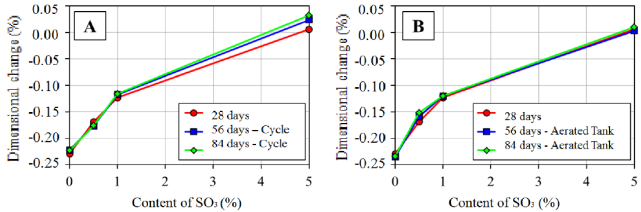
In Figure 9 is presented, comparatively, the dimensional variation of the pastes, at ages of 28, 56 and 84 days, as a function of the SO3 amount and of the exposition condition after the initial underwater curing in water saturated with lime.
Exposition of the pastes to the drying and wetting cycles (Figure 9-A) evidenced that, for amounts of SO3 until 1.0%, no influence was verified, even at the same ages, in the dimensional variation observed at 28 days. For the amount of SO3 of 5.0%, the exposition to the drying and wetting cycles tended to cause expansion in the paste. In the case of exposition to the tank with constantly aerated water (Figure 9-B), independently of the SO3 amount, there was no dimensional variation regarding the age of 28 days. Those data are indications of the importance of continuing the monitoring of the dimensional variation over time, being possible to generate greater difference among the series of assays at most advanced ages due to the progress of ISR.
The linearization of results presented in Figure 9, by means of the square root of the SO3 amount, enabled to establish the slope of trend lines for ages 28, 56 and 84 days, representing the coefficient of internal attack by sulfates. In Figure 10 is presented the dimensional variation as a function of the amount of contaminant contained in the pastes and of the exposition condition. Coefficients of determination (R2) of the trend lines were over 0.979, indicating that the linear model used properly represented the behavior observed in the pastes.
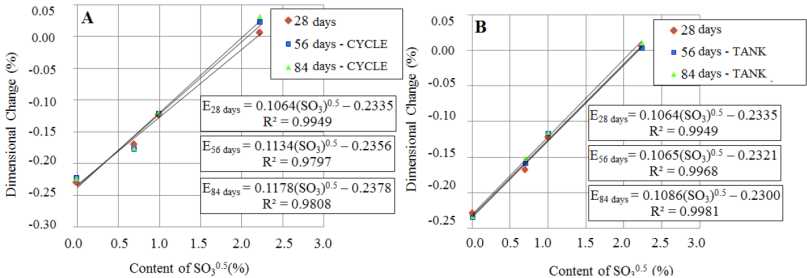
Figure 10 Dimensional variation as a function of the contaminant content (SO3) contained in the cement pastes and the exposure conditions: A - drying and wetting cycles; B - tank with aerated water.
In Figure 10-A is evidenced that the exposition condition of drying and wetting tended to potentiate the internal attack by sulfates, because there was expansion of pastes over time in contamination of 5.0% SO3. This behavior was not observed during the exposition to the aerated water tank (Figure 10-B). Besides all, the differences presented are small and the monitoring must continue in order to get information from a large time of exposition to ISR.
3.5 X-ray diffractometry
The analysis of hydrated compounds formed after the different types of exposition, at the age of 56 days, was performed comparatively to the respective samples with 28 days, age when started the exposition of the pastes to the drying and wetting and the submersion in continuously aerated water, with the aim of verifying if those conditions influenced the reaction of pyrite, responsible by the formation of deleterious hydrated compounds in the hydrated matrix.
In Figure 11 are presented the diffractograms of the four cementitious compositions studied at the 28th day of age. Analyzing the diffractograms, it is possible to say that the products formed, independently of the composition of the different pastes, were the same ones, with some differences regarding to calcite, pyrite and ettringite. Calcite (CaCO3), a constituent of the limestone filler, has main peak (of greater intensity) in the region of 29º 2θ and, thus, as this material was replaced by pyrite (FeS2), it started to stand out jointly with the peaks of pyrite, located at 33, 37 and 56º 2θ. Phases identified in diffractograms were the mullite, quartz, calcite, ettringite, portlandite, the hydrated calcium monocarboaluminate and the pyrite, in pastes with addiction, as identified by the ICDD.
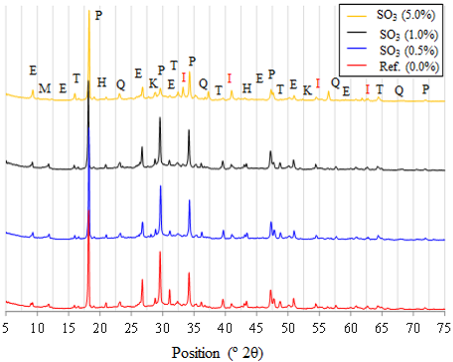
Figure 11 Diffractograms of cement pastes at 28 days of age in submerged curing (T - Mulite, P - Portlandite, E - Etringite, K - Calcite, M - Calcium monocarboaluminate hydrate, Q - Quartz, I - Pyrite).
Ettringite, with characteristic peak of higher intensity located at position 9.1º 2θ, was identified in all pastes, however with different intensities. Despite the technique of X-ray diffractometry being qualitative/comparative, the variation of intensity of the characteristic peek may indicate the presence of this phase in greater of smaller amounts. The analysis of this peek evidences that the contamination of the paste with pyrite intensified the formation of ettringite which demands, necessarily, an extra source of sulfate ions besides those made available by the gypsum part of the Portland cement. This behavior constitutes evidence that the pyrite contained in the pastes is taking part in the reactions, in other words, there was oxidation of iron sulphide for the formation of the intermediary phases. The paste with amount of 5.0% of SO3 had a more pronounced peek of ettringite than the other pastes. These results corroborate with the behavior observed in the dimensional variation of pastes at the age of 28 days. The paste with the higher amount of contaminant (5.0% SO3) had greater relative dimensional variation, justified by the possible formation of a greater amount of ettringite, being this an expansive phase.
In Figure 12 are presented the diffractograms of the four cementitious compositions, comparing the chemical phases formed at 28 days of age, after underwater curing, with those ones formed at 56 days, for both conditions of exposition (drying and wetting and aerated water tank).
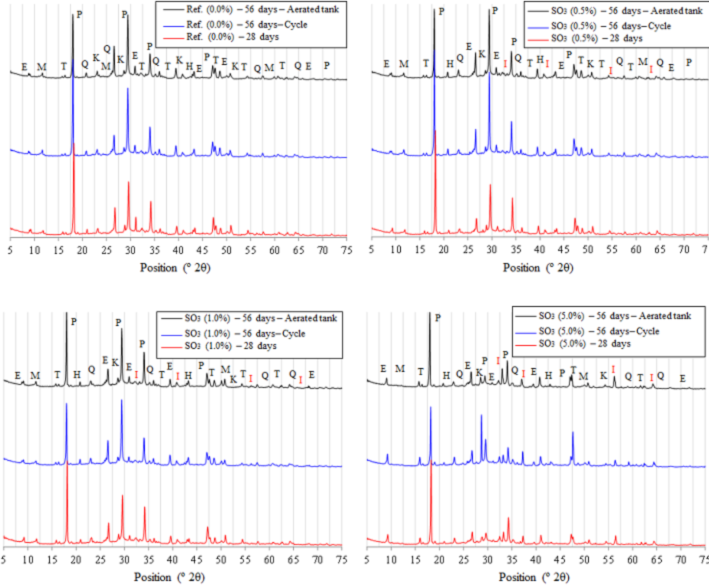
Figure 12 X - ray diffractograms for the analysis of the evolution of RSI comparing the 28 - day cure and the changes from the types of exposure (T - Mulite, P - Portlandite, E - Etringite, K - Calcite, M - Calcium monocarboaluminate hydrate; Q-Quartz, I-Pyrite).
Phases indicated were the same commented for Figure 12, with the variation of intensity of the peek of ettringite being highlighted, more pronounced for the amount of 5.0% of SO3.
4. Conclusion
The results of all assays used in this study helped the process of understanding the mechanism of degradation of the internal attack by sulfates. It was noted that there are many variables involved and that the time of occurrence of the attack is a determining factor.
For the materials and times tested, the analysis of the mechanical properties of the pastes, by means of resistance to axial compressions and to traction in flexion, showed to be satisfactory tools, but they would need to be executed in more advanced ages of the material, in order to be possible to make an analysis regarding the durability of them over their lifespan.
The assay of resistance to axial compression did not indicate influence of the exposition conditions used in the materials. The one of resistance to traction in flexion indicated, for the SO3 (1.0%) series, smaller results for the drying and wetting cycle.
Dimensional variation of bars indicated a behavioral difference already at early ages, the paste with contaminant amount of 5.0% of SO3 being the one with greater expansion, totally compensating the chemical retraction of the Portland cement. Again, for this assay, the drying and wetting cycle was pointed as the cause of greater expansion, therefore being the worse condition.
The analysis of chemical phases by means of the technique of X-ray diffraction evidenced that the contamination of the paste by pyrite potentialized the formation of ettringite, the hydrated phase responsible by the expansion of the hydrated matrix.
5. Acknowledgments
The authors thank the infrastructure and support in human resources and funding to COPEL GeT project R&D 6491-0301/2013, to ANEEL, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação Araucária, LACTEC institute, to CNPq Lei 8010/90 (LI 15/2187214-1; LI 14/4695814-5; LI14/3410726-9) and the Federal University of Paraná (PPGECC/UFPR) - Brazil.
REFERENCES
AFNOR. NF P 541: Granulats - Granulats Pour Beton Hydrauliques - Specifications 2Eme Tirage. França, 1994. [ Links ]
American Concrete Institute. Guide to Durable Concrete. Detroit - USA, 1991. (ACI 201). [ Links ]
Associação Brasileira de Normas Técnicas. NBR 5736: Cimento Portland pozolânico. Rio de Janeiro, 1991. [ Links ]
Associação Brasileira de Normas Técnicas. NBR 13276: Argamassa para assentamento e revestimento de paredes e tetos - Preparo da mistura e determinação do índice de consistência. Rio de Janeiro, 2005. [ Links ]
Associação Brasileira de Normas Técnicas. NBR 13278: Argamassa para assentamento de paredes e revestimento de paredes e tetos - Determinação da densidade de massa e teor de ar incorporado. Rio de Janeiro, 2005. [ Links ]
Associação Brasileira de Normas Técnicas. NBR 13279: Argamassa para assentamento e revestimento de paredes e tetos - Determinação da resistência à tração na flexão e à compressão. Rio de Janeiro, 2005. [ Links ]
Associação Brasileira de Normas Técnicas. NBR 7211: Agregados para concreto - Especificação. Rio de Janeiro, 2005. [ Links ]
Associação Brasileira de Normas Técnicas. NBR 13583: Cimento Portland - Determinação da variação dimensional de barras de argamassa de cimento Portland expostas à solução de sulfato de Sódio. Rio de Janeiro, 2014. [ Links ]
Araújo, G. S. (2008) “La reacción sulfática de origen interno em presas de hormigón. Propuesta metodológica de análisis ”. Tesis Doctoral Universitat Politácnica de Catalunya. Departament d´Enginyeria de la Construcción. Barcelona. [ Links ]
Biczok, I. (1972) “Concrete Corrosion and Concrete Protection .” Chemical Publishing Company, Inc., New York. [ Links ]
Casanova, I.; Aguado, A.; Agulló, L. (1997) “Aggregate Expansivity due to sulfite oxidation. Physico-chemical modeling of sulfate attack .” Cement and Concrete Research. Vol 27. Nº 11. p.1627-1632. [ Links ]
Centurione, S. L., Kihara, Y., Battagin, A. F. (2003) “Durabilidade de concreto submetidoa ataques de íons sulfato ”. Anais do 47º Congresso Brasileiro de Cerâmica. João Pessoa. [ Links ]
Chinchón-Payá , Aguado, A., Chinchón, S. (2012) “A comparative investigation of the degradation of pyrite and pyrrhotite under simulated laboratory conditions .” Engineering Geology 127. pp 75-80. [ Links ]
Czerewko, M. A., Cripps, J. C. (1999) “Sources of sulfur species - Identification and quantification. Thaumasite and other forms of concrete deterioration and protection .” Halifax Hall: University of Sheffield. [ Links ]
Coutinho, J. S. (2001) “Ataque por Sulfatos ”. Faculdade de Engenharia do Porto (FEUP). Portugal. [ Links ]
Gomides, M. J. (2009) “Investigação de agregados contendo sulfetos e seus efeitos sobre a durabilidade do concreto” Tese de Doutorado Universidade Federal do Rio Grande do Sul. Porto Alegre. [ Links ]
Hasparik, N. P., Nascimento, J. F. F., Andrade, M. A. S., et al. (2003) “Estudos de laboratório com concretos contendo agregados obtidos a partir de rocha com sulfetos ”. In: Reunión Técnica de la AATH - Seminario sobre Hormigones Especiales, 15ª, Argentina. [ Links ]
Kantro, D. L. (1980) “Influence of water reducing admixtures on properties of cement pastes - A miniauture slump test .” Cement Concrete Aggregates. [ Links ]
Katsiadramis, N. J., Sotiropoulou, A. B., Pandermarakis, Z. G. (2010) “Rheological and mechanical response modifications for a self-leveling mortar .” 14th International Conference on Experimental Mechanics. France. [ Links ]
Khelil, N. et al. (2014) “Development of an accelerated test for Internal sulfate attack study” Université de Toulouse. France. [ Links ]
Oliveira, I. C. (2013) “Análises de dados para a elaboração de diretrizes visando à detecção de sulfetos e sulfatos na composição de CCR”. Dissertação mestrado, Universidade Federal do Paraná. Curitiba. [ Links ]
Ouyang, W., Chen, J., Jiang, M. (2014) “Evolution of surface hardness of concrete under sulfate attack .” Construction and Building Materials, Vol. 53, p. 419 - 424. [ Links ]
Pan, Z. et al. (2002) “Hydration products of alkali-activated slag-red mud cementitious material.” Cement and concrete research. Volume 32, pp. 357-362. [ Links ]
Pereira, E., Bragança, M. O. G. P., Oliveira, I. C, Godke, B., Portella, K.F. (2014) “Ataque interno por sulfatos em estruturas de concreto contaminadas por pirita: Uma revisão da literatura”. 1º Congresso Brasileiro de Patologias das Construções. Foz do Iguaçu, Paraná. [ Links ]
Pereira, E. (2015) “Investigação e monitoramento do ataque por sulfatos de origem interna em concretos nas primeiras idades ”. Tese de Doutorado apresentada a Universidade Federal do Paraná. Curitiba. [ Links ]
*Citation: A. P. Brandão Capraro, M. H. F. de Medeiros, J. H. Filho, M. O. G. P. Bragança, K. F. Portella, I. C. de Oliveira (2017). “Internal Sulphate Reaction (ISR) as degradation of the cement matrix: behavior of pastes dosed with different amounts of contamination by sulfate”, Revista ALCONPAT, 7 (2), pp. 119-134, DOI: http://dx.doi.org/10.21041/ra.v7i1.142
Received: June 30, 2016; Accepted: February 16, 2017











 texto en
texto en