Introduction
Worker ants follow paths towards the foraging site and return to their nest with the food collected after establishing bidirectional trails (StrÖmbom & Dussutour, 2018). Atta cephalotes ants bring leaves, buds and flowers back to their nest to cultivate symbiotic fungus, causing orchard losses of billions of dollars (Hölldobler & Wilson, 1990). Leaf-cutting Atta sexdens ants can occupy a 6-year-old nest that weighs 40,000 kg, and a colony with such dimensions can collect more than 5 892 kg of leaves (Wilson, 1971). Atta ants feed from plant biomass at rates up to 500 kg∙colony-1∙year-1 (Wirth et al., 2003), and defoliated 20 coffee bushes during one night, killing them at Costa Rica (Varón et al., 2007). An Atta colony produced sugar cane losses of 3.6 t∙year-1 in Brazil (Della-Lucia, 2003). Ants also feed from stem wounds at avocado trees causing infections (El-Hamalawi & Menge, 1996). A. cephalotes increased its foraging activity with temperatures up to 35 °C (Bustamante & Amarillo-Suárez, 2019), but climate changes killed the weeds in the soil. Therefore, to survive ants have to feed from green-leaves available at fruit trees.
In an experiment carried out at Cali, Colombia, there was a marked preference of the ants for foraging continuously of mango foliage being their herbivory rate above 90 % (Rodríguez et al., 2008). Young plants are usually totally defoliated, the damage resembling pruning, whereas in mature plants, often only young leaves are removed. Scars, resembling those made with a knife, are frequently left on green parts of the plant stem.
Traffic of Atta cephalotes workers is well described by the modified fluid-dynamic model (Burd et al., 2002). Forage ant rules optimize their movement, and their speed decreases by 21 % due to the number of collisions within the traffic lines (Burd et al., 2002). At night Myrmecia ant walking speed decreased by 55 %, as their visual landmarks became less reliable (Narendra et al., 2013). Environmental conditions such as temperature (Jayatilaka et al., 2011), rain (Farji-Brener et al., 2018) and wind (Alma et al., 2016) also affect ant walking speed.
Ant trails are marked chemically with pheromones secreted from ants’ glands and having different odor cues. Higher pheromone concentrations indicate higher quality of foraging patches (Wirth et al., 2003). Leaf fragments transported by the ants can help us find the main mound in one extreme and the tree where the leaves were cut in the other extreme. Atta ants have the habit of cutting and transporting various plant fragments to their underground nests for cultivating the fungus Leucoagaricus sp. (Valmir et al., 2004). As this fungus is the only food source for the larvae of cutting ants, they must cultivate it with leaves rich in water, nitrogen, and phosphorus but low in fiber and manganese (Rodríguez et al., 2008). Leaf transfer is a leafcutter ant task in which foragers return leaf fragments to the nest. This process can be direct when the leaf is passed from one ant to another immediately, or indirect when an ant drops a leaf along the trail for another ant to pick it up later (Kwaku et al., 2020). As leaves are transferred, ants deposit pheromones, preventing them from getting lost (Cevallos-Dupuis & Harrison, 2017). A bigger use of energy during trunk movement increases forage direct transfer avoiding that an ant falls down from an entire tree while carrying a leaf (Norton et al., 2014). A. cephalotes move faster over fallen branches than on ground, but more slowly ascending or descending a branch (Freeman & Chaves-Campos, 2016).
Atta cephalotes control has been studied for many years. The most efficient leaf-cutting ant control method are baits having sulfluramid presenting low toxicity to humans and other organisms (Britto et al., 2016). However, it has been considered as a Persistent Organic Pollutant (Mota-Filho et al., 2021). Filamentous fungus, virulent to the ant symbiotic fungus, can show synergistic effect on the sulfluramid (Carlos et al., 2010). Compostable material (leaf litter, poultry manure, molasses, and yeasts) was introduced to the ant nests and after three months the percentages of dead nests were 26.5 % (Armbrecht et al., 2013).
Ants active during day and night, require to adapt themselves to light intensity variations. Low sensitivity apposition eyes of nocturnal ants (Narendra et al., 2013) have larger lenses and wider photoreceptors than diurnal ants. Ant photoreceptors in day-active species have a diameter of 1.3 µm, four times smaller than photoreceptors in night-active species (Greiner et al., 2007). With night orientation pauses, more photons are captured through a larger integration time (Greiner et al., 2007). Each apposition eye, contains several ommatidial-lens units, which capture light, and a crystalline cone that funnels light onto a photosensitive structure called rhabdom (Narendra et al., 2017). Each rhabdom contains the pigment rhodopsin that absorbs light and converts it into an electrical signal (Warrant, 2017). Light-pulses reach ant eyes before being converted into membrane potential as bumps (Frolov, 2016). Photoreceptors in night-active ant M. vindex present three spectral sensitivities with peaks at 370, 430 and 550 nm (Ogawa et al., 2015). Red light applied with headlamps during nocturnal observation don’t disturb worker ant vision (Röschard & Roces, 2011).
Researchers analyze ant movement by filming sections of the principal trail with digital camcorders (Couzin & Franks, 2003; Narendra et al., 2013). Several monitoring systems and pest traps interrupt a LED or laser beam, and the detector signal triggers a time counter (Balla et al, 2020; Eliopoulos et al., 2018). When ants walk through two parallel lanes, the system requires of a laser reflective system with the sensor and detector in the same side (Brydegaard, 2015). The critical fusion frequency (CFF) is the threshold at which an insect cease to perceive a flickering light source as a series of flashes, but rather as a continuous stream of light (Inger et al., 2014). Most diurnal insects as honeybees and fruit flies have CFF values greater than 100 Hz (Barroso et al., 2015). Photoreceptors are damaged by high intensity flickering lights (Meyer-Rochow, 2002).
Regardless of the large amount of physiological research that has been done to understand how ants guide themselves towards their feeding site, this experimental work studies the possibility of using LED lightning as boundary control to divert ants from fruit trees. We suppose that ant speed changes under strobe-light LED stimulation at different frequencies. How can a control-illumination system apply pulse-beams to ant-eye with 100 % certainty and how will ants respond? A double trail having bifurcated lanes evaluated if ants changed their main traffic lane when pulsed illumination was applied at the trail end.
Materials and methods
Traffic of Atta cephalotes ants was studied at a mango orchard located at Loma Bonita, Guerrero (17° 25’ 47’’ LN y -101° 11’ 19’’ LW, 17 m a. s. l.). Experiments were carried out during October 2019 just after the raining season in this subtropical arid region. Mature ant colonies that contained 105 workers approximately, foraged nocturnally at mango trees located 30 m away from their nest.
A variable wide (10-60 cm) ant trail was installed beneath a tree trunk and was covered by a smooth soil surface. Four 30-cm long linear arrays (L300, SmartVisionLight, USA), with 12 high-intensity LEDS each, were used to illuminate the trail. L300 LED linear arrays were installed in the top of the artificial trail to study ant stimulation (Hahn & Bolaños, 2021). Each linear array illuminated an elliptic area at the trail surface where ants moved having a maximum illuminance of 19 000 lx. Continuous and pulsed light was supplied by an embedded system that could reduce the illuminance to 350 lx by an analog signal (Hahn & Bolaños, 2021).
Average continuous illumination is generated by pulse width modulation, and depends of the source illumination (SI). For example, an average illumination of 900 lx can be obtained by using a source illumination of 4 500 lx with a 20 % duty cycle. It can also be achieved from a SI of 2500 lx together with a duty cycle of 36 %.
In our precise eye stimulator, a pulse of 4 500 lx is much higher than 2 500 lx and will generate greater bumps through the photodetector membrane.
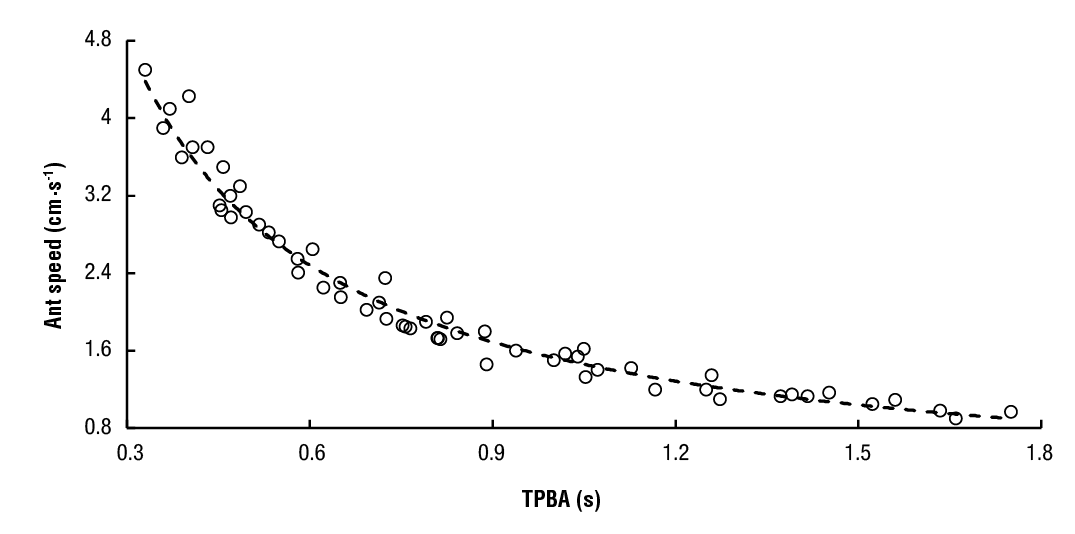
Ant speed was measured with laser-photodetector units using 5 mW red lasers (D650-51 USLaser, USA) to provide a constant non-dispersive beam. Small photodetectors (KDT00030, Fairchild) present linear current-illuminance response. The photodetector strip was fixed to one wall, meanwhile the lasers to the opposite wall. Laser-photodetector units determine ant speed by counting with a programmable counter array (PCA), the time period that the laser beam reflects on the ant body and the photodetector is off. TPA (time period ant) is obtained after counting 1-ms cycles during this period. Artificial ant speed was correlated against TPBA (time period between ants) with a controlled 30 cm-long plastic stripe trail. This stripe was displaced linearly with a high precision motor, meanwhile the photodetectors and lasers walls remained immobile. Wooden ants 25 mm long were replicated and glued to the plastic stripe letting a space between them of 12.5 mm. After taking more than fifty measurements, a potential regression (Equation 1) relates TPBA with ant speed (R2 = 0.9775, N = 56, P () 0.001). A smaller TPBA value is achieved for ants walking at higher speed (Figure 1).
Data collection
Once the trail was installed, several experiments were carried out analyzing TPBA, collision number and recovery time under different lightning schemes. Different lightning systems and intensities were provided for each experiment (Table 1).
Three levels within the experiment divide the type of lightning, the trail width and the illumination intensity. It is important to mention that ants laid pheromones through the trail before the experiments were carried out. The experimental setup monitors:
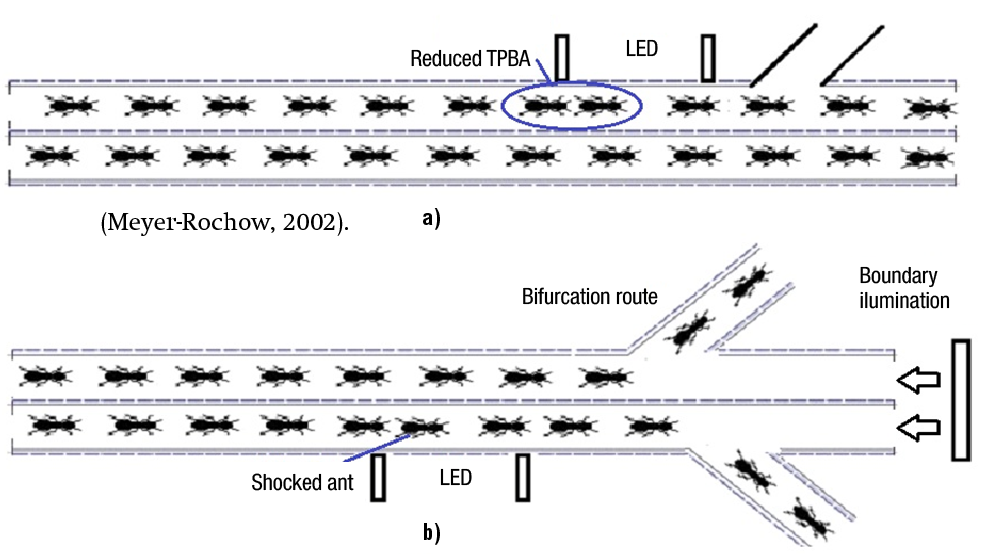
a: Ant TPBA and number of ants impacts within the narrow trail under different white strobe illuminations (STI). In the first experiment, light-pulses were applied vertically towards the top surface of the ant head (Figure 2a). In the second experiment, a wall-mirror reflected the light from a 45° rotated L300 array (Figure 2b). The mirror was deflected in order to reach the ant head, and if the height of the mirror was higher, its slope should be stepper. To optimize the slope, a precision bolt pushed the mirror until the light-spot arrived to the desired place (ant face), simulated by a 3 cm wooden piece. Each experiment was carried out with 100 ants and was repeated three times.
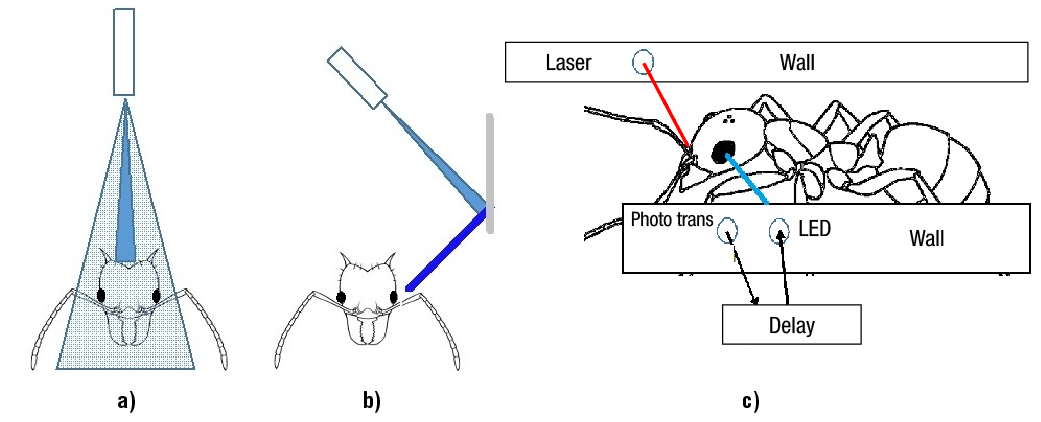
Figure 2. Ant head illuminated (a) from the top with a L300 array, (b) after mirror reflection, and (c) with LED lateral control.
b: Ant adaptation time after flashing directly her eye with the lateral LED system (Figure 2c). Ten experiments were carried out with 50 ants each. Five experiments used light intensity pulses of 300 lx and the remaining 900 lx. The five 300 lx experiments used single pulses of 1, 4, 8, 10 and 60 ms. The same periods were used for the 900 lx experiments, being 250 ants evaluated. Each light-stimulated ant that stopped, was lifted carefully with forceps and placed inside a plastic container within a dark space (Farji-Brener et al., 2010). The time before the ant moved its head corresponds to the dark adaptation period and was counted with a precise chronometer. Half an hour later, each ant was inserted into the dark trail evaluating its proper orientation.
c: Ants that left the 60-mm wide trail at the end, after being flashed by mirror-reflected white-pulses. Twelve experiments with 10 repetitions each, were carried out to determine the quantity of ants that left the trail (ALT). One hundred ants entered the trail during each experiment, and after 25 % of them left, light-pulses were applied. Laser-photodetector units counted the number of ants at the entrance and exit of each trial-lane. Four different number of 10-ms light-pulses (3, 6, 9 and 12) with a frequency of 90 pulses per minute were applied along the trail at three different light intensities (300, 900 and 1 800 lx). The second L300 array provided three or six strobes, meanwhile for 12 pulses, the second and third array applied six pulses each. Visual observation helped to understand why ants returned back to the entrance.
d: Ant status and trajectory followed after lateral LED flashing. Two different experiments were conducted being repeated 5 times with 100 ants each. In the first experiment, light pulses were applied to 33 % of the ants that passed trough the trail; the quantity of ALT immediately and after 7 min were counted. Ant recovery or dark-adaption time determined ant-status. In the second experiment, lateral pulses of light were applied to five consecutive ants, counting the number of ALT exit.
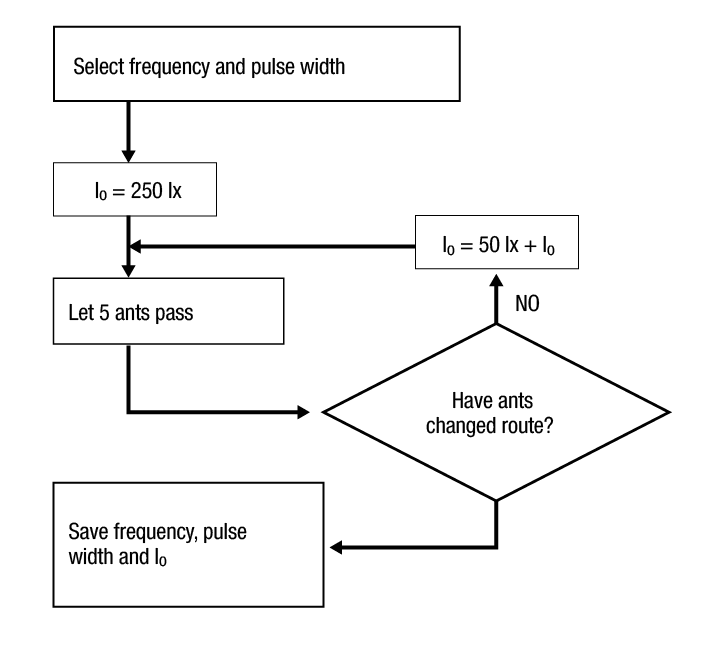
e: Ant status and trajectory followed after L300 boundary lightning. The boundary lightning system at the trail end, consisted of a horizontal L300 fixed 3-cm over the soil. Ants entered in both lanes and an alternative route was built. The L300 boundary system provided 20 % duty cycle pulses at different frequencies for 12 s to frontrunner ants. The light intensity threshold that forced ants to change of route using light pulses of 10 Hz-20 ms, 30 Hz- 6.6 ms, 100 Hz-2 ms and 200 Hz-1 ms was obtained. For each ant group a frequency-pulsewidth was selected starting with a L300 light source of 250 lx. The intensity was incremented by 50 lx, until ants changed of route obtaining the light intensity threshold (Figure 3). The experiment was repeated five times with the given threshold to ensure its effectiveness.
Results and discussion
Twenty-seven ants followed each other within the 100 cm trail, being ants 2.5-cm long and the space between them 1.25 cm. Although mobility changes in artificially illuminated traffic lanes were expected, it was found from our experiments that ants rarely modify their speed with continuous-light changes. A. cephalotes foragers advanced in night trails nearby the mango trunk with speed varying between 1.6 and 1.9 cm∙s-1, corresponding to TPBA of 0.95 and 0.83 s, respectively. Ants walking during the day at 2.3 cm∙s-1 crossed the 100-cm trail in 43.48 s. A longer time of 62.5 s was found at night speed of 1.6 cm∙s-1. When the light-adapted leader changed its speed, workers followed her (Hahn & Bolaños, 2021). The number of collisions showed that it was easier for ants to adapt to dark pulses in the day, than to intense light pulses in the night (Hahn & Bolaños, 2021). Light pulses in the day scatter through natural illuminance and dark pulses do not appear within the trail.
Although researchers use camcorders to study ant movement, we did not use them as the L300 arrays blocked the camera field of view. Instead laser-photodetector units fixed to the trail walls counted ant speed, being red lasers insensitive to ant vision (Ogawa et al., 2015; Röschard & Roces, 2011). Photodetector response variations caused by the L300 arrays were negible after being filtered. The L300 was cleaned-up continuously to reduce lightning-loss (Harley & Ritzmann, 2010). Only 20 % of the L300 radiation reached the ant-eye, after being absorbed by the trail plastic base or the soil organic matter.
Lateral L300 and LED illumination
Light applied vertically from the L300 array, reflected on the ant head but never reached their eyes (Figure 2a). Ant collisions (22.3 ± 0.29; mean ± SE) caused by mirror-reflection increased during the night as 75 % of the L300 illumination reach their eyes. In the mirror reflected trail, ant size and speed did not assure perfect ant-eye lightning. After reflecting the beam in the mirror system, it increased coverage area, but reduced converging power. Also, the L300 array filter smooths the LED beam reducing its intensity. Beam mirror reflection can be useful at laboratory level, but it would be difficult to implement at the field.
The probability that the LED beam flashed the ant-eye, depended on its size, speed and strobe frequency, so a controlled lightning system was designed to cope with these variables. After the ant head blocks for first time the red-laser beam, the photodetector turns-on a time delay (Figure 2c). After the delay is over, a voltage turns-on the white LED, that will accurately irradiate the ant-eye. With an eye 0.69 mm long (Moser et al., 2004), and a walking speed of 1.6 cm∙s-1, the lateral LED system has to deliver a 43-ms light pulse. It could be implemented by nine 1-ms pulses at 200 Hz (20 % duty cycle), or two 6.6-ms at 30 Hz (20 % duty cycle). The illumination system provides ON periods of 13.2 ms at 30 Hz or 9 ms at 200 Hz, representing less than 30 % of the required 43 ms light pulse. A lower frequency of 1.5 Hz (20 % duty cycle ) will provide a 133-ms pulse.
As light pulses penetrated only one ant eye in the lateral precise LED system, ants never lost their visual system. After exposing ants to the precise LED stimulator within single-line trails, stunned ants stopped for 2-3 s. This precise stimulator allowed us to determine the effect of light pulses on ant vision adaptation and walking recovery time. Due to the short period of flashing (43 ms) at different frequencies, ants did not become blind and even if they lost some receptors, ants reached their final path (Schwarz & Wystrach, 2011).
The results obtained after applying light pulses at night with intensities of 300 and 900 lx for different time periods during “experiment b” are shown in Table 2. The light pulse applied to the ant-eye upset it, requiring a recovery time to walk normally again. The flash pulse-width affects adaptation time, which increases as light intensity becomes brighter. With a 50-ms pulse of 900 lx, dark adaptation increased to 1169 ± 6.05 s (mean ± SE). An average of 90 % ants survived with the 1.5 Hz-50 ms light-pulse, and only 11.2 % lost their good orientation. Ants flashed for more than 5 ms presented orientation problems (Table 2).
Table 2. Dark adaptation recovery time, survival rate and good orientation after applying different strobe pulses and intensities with the precise lightning system.
| Pulses | Light intensity | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 300 lx | 900 lx | ||||||||
| SR/GO (%) | MDA (s) | SEDA | SR /GO (%) | MDA (s) | SEDA | ||||
| 200 Hz-4 ms | 100/100 | 47.42 | 0.22 | 100/100 | 140.3 | 0.67 | |||
| 200 Hz-1 ms | 100/100 | 28.78 | 0.22 | 100/100 | 44 | 0.41 | |||
| 60 Hz-8 ms | 100/90 | 79.7 | 0.31 | 100/90 | 212.8 | 0.88 | |||
| 60 Hz-1 ms | 100/100 | 26 | 0.2 | 100/100 | 53.7 | 0.36 | |||
| 1.5 Hz-10 ms | 100/90 | 92.42 | 0.45 | 90/93.3 | 272.6 | 0.64 | |||
| 1.5 Hz-50 ms | 90/88.8 | 401.78 | 2.21 | 88.8/88.6 | 1169 | 6.05 | |||
SR = survival rate; GO = good orientation; MDA = mean dark adaptation; SEDA = standard error dark adaptation.
Wide trail experiments
A wider trail of 60 mm between walls was used for experimental setups c, d and e. After the fifth light-pulse, foragers collide within the traffic line (Figure 4a). The new forerunner moves to another lane, being followed by the other ants. Ants return to the trail entrance, after two collisions took place and were unable to advance (Figure 4b). The minimum ALT that can be obtained is of 25 % as white-pulses started flashing after 25 ants left the trail. ALT average never decreased below 95.3 ± 0.82 % for pulse-intensities of 300 lx. Ants flashed by three 10-ms pulses with an intensity of 1800 lx had a minimum ALT value of 99.3 ± 0.67 %. Many ants left the trail where they entered with 12 pulses, being the ALT average of 62.33 ± 1.26 % and 32.9 ± 2.33 % for light intensities of 900 and 1 800 lx, respectively (Figure 4c). For ALT=32.9 %, only 33 % of the ants left the trail at the end and the other 67 % returned back to the entrance. Ants that received nine pulses with an intensity of 1800 lx also returned back (ALT = 61.2 ± 2.53 %). In both cases, the red ellipses indicate ALT at the entrance (Figure 4c).
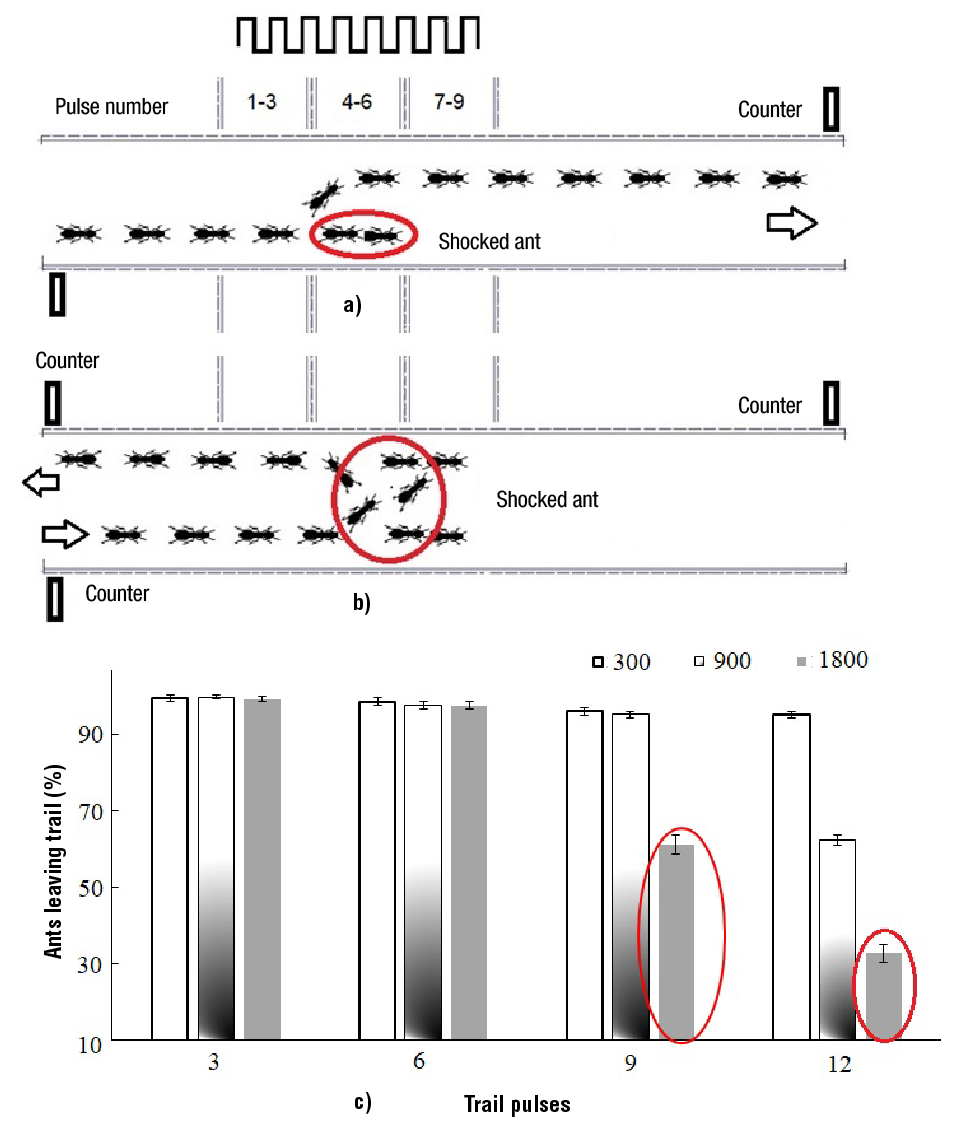
Figure 4. Ants leaving the trail: a) after one collision, b) after two collisions, and c) in function of the number of 10 ms light-pulses and light intensity.
Workers walking in parallel lines (Figure 4) received lateral LED light pulses in their eyes. When ants were moving over trail and following each other, the irradiation applied arrived to their faces. Nevertheless, as ants have not the same size, and the exposure is not continuous some specimens were not struck by the lightning at all. As the ant head received the irradiation, it slowed down and lost its way. An ant that receives a 300 lx-1.5 Hz-50 ms light-pulse requires 6.7 min to recover (Table 2). From every 100 ants, 33 were flashed by the lateral LED system. After 33 % of the ants were stimulated by 900 lx-1.5 Hz-35 ms light-pulses, only 66.8 ± 0.49 % ALT at the end. A higher percentage of ants (82.4 %) left immediately at the exit, when flashed by nine 1-ms light pulses at 200 Hz and 300 lx (Table 3). Another 15.4 % ants walked out through the trail exit between the first and the seventh minute. The last 2.2 % were still in recovery mode, but none died.
Table 3. Percentage of ants leaving the trail immediately after being irradiated by high and low frequency pulses with intensities of 300 or 900 lx and between the first and 7th minute.
| Frequency | Pulse width | Light intensity | ||||
|---|---|---|---|---|---|---|
| 300 lx | 900 lx | |||||
| MALT (%) | SE | MAlT (%) | SE | |||
| Immediately | 200 / 9-1 ms | 82.4 | 0.68 | 70.6 | 0.68 | |
| Between 1 and 7 min | 200 / 9-1 ms | 15.4 | 0.24 | 26.8 | 0.37 | |
| Immediately | 1.5 / 1-35 ms | 69.8 | 0.58 | 66.8 | 0.49 | |
| Between 1 and 7 min | 1.5 / 1-35 ms | 24.8 | 0.37 | 1.4 | 0.24 | |
MALT = mean ants leaving trail; SE = standard error. PHAC = promedio de hormigas que abandonaron el camino; EE = error estándar.
A total of 26.8 ± 0.37 % ants, hit by 900 lx high frequency pulses, recovered between the first and seventh minute. In a similar way, 24.8 ± 0.37 % ants recovered from 300 lx low-frequency stimulation. All these values make sense after checking the dark adaptation times of Table 3. When five consecutive ants were flashed by lateral LED’s, all of them came to a halt, forcing the followers to change of route.
Precise light-pulses hit ant-eyes and brought them to standstill in wider trails. With more space in the wide trail, ants could turn, and were not forced to stop after finding an immobile ant. Ants that turned, avoided collisions and continued its way straight ahead. After two or three ants were shocked and the trail blocked, ants communicated between them by means of antennal contact (Gordon, 2019). Ants came back to the entrance without confusion and remained together. Without pheromones laid by motionless ants (Franks et al., 1991), other ants occasionally circulated through the trail. Some stunned ants were pushed to the exit by their companions, but some of them remained within the trail, taking up to 20 min to recover and adapt to the darkness (Table 2). Ants moved within the trail without proper orientation and hit other ants.
Ant route deviation with illumination at the trail end
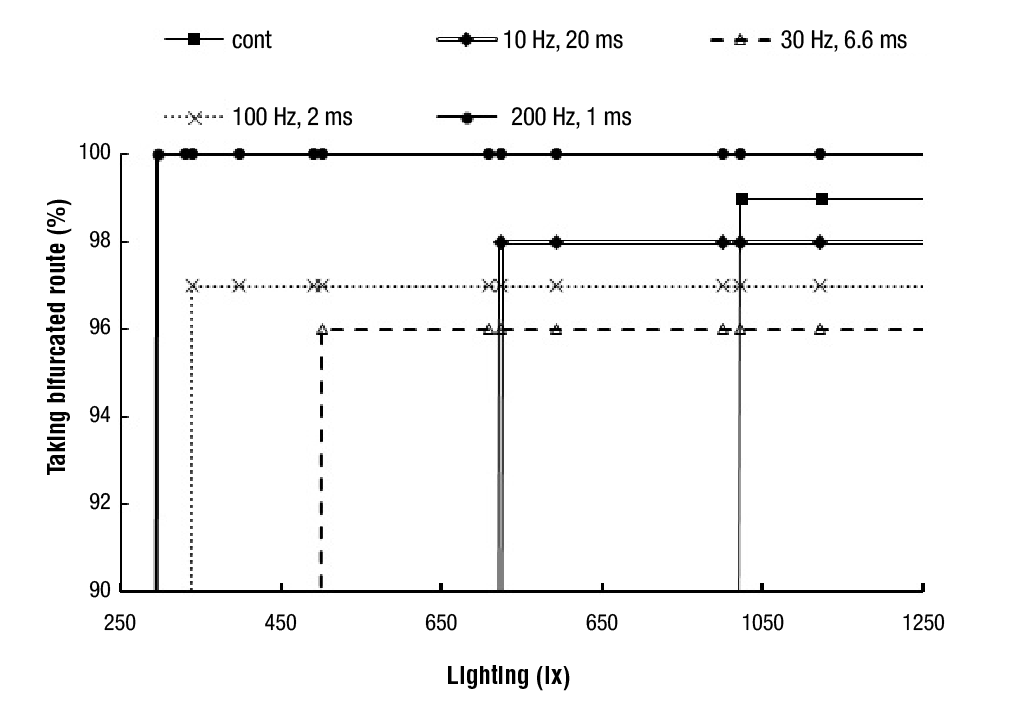
Without turning-on the horizontal L300 array fixed at the end of the trail, ants never changed of route. A change in the path where ants travel can be forced by exceeding a given eye-flashing threshold, being of 1 000 lx for continuous illumination (Figure 5). The percentage of ants taking the bifurcated route was always 100 %, but for distinguishing the different thresholds it was varied between 96 and 100 % (Figure 6). The amount of light received by the ants during 12 s was the same for all frequencies since they used the same 20 % duty cycle. If the distance from the L300 array to the turnover point (bifurcated route) increases, a higher light intensity is required to change ants route. A 724 lx threshold was required with 10 Hz-20 ms flashing, and only 500 lx with 30 Hz- 6.6 ms pulses. The 30 Hz dark-adaptation pulse was of 26.7 ms for each 6.6 ms light-pulse. This period does not allow eye photodetectors bumps to return to 0 V. In the 200 Hz-1 ms flashing, route switching occurred at a lightning of 300 lx, but the 4-ms dark pulse was also too short for the eye photodetector to get dark-adapted. If a shorter timing than 5 s was applied at the boundary end, ants continued their path normally. Pulses sent from the trail end for a period of 12 s, caused ants to change of route without impacts and bottlenecks. L300 lightning intensity was controlled by the applied voltage and when it was over 75 % of its maximum value, ants moved away and did not climb the tree. A control turned-on the lamps during sunset when ants climbed the trees (Figure 7). All the ants changed their route when the light intensity was maintained, laying pheromones over their new path. Under environmental conditions proper illumination handling is required. Movement changes during an event may be an adaptive feature of ant brain (Hunt et al., 2016), being these responses found during rainstorms (Farji-Brener et al., 2018), predator attacks or under these powerful light-intense stimuli. Gallotti and Chialvo (2017) showed that ants can determine their next move on their way and do not require to be at rest.
Application as pest control
This research provides basic illumination concepts for pest control by deflecting ants before reaching the tree trunk. Lightning system installation plays a very important role, being essential that light-pulses reach forager eyes. Mango fruit production on the following season depends on successful pruning, to stimulate branching flushes of lateral shoots to form a dense canopy that flowers (Davenport, 2006). This chemical-free and ecological boundary lightning system can avoid tender leaf foraging by Atta cephalotes after pruning. Boundary lightning showed excellent results at soil level, as it is aggressive against forerunners and produces changes of route. LEDS at soil level are very susceptible and risky to environmental conditions as rain, wind, organic matter, soil discontinuity and passage of other animals. Therefore, different LED arrangements are being tested. High 200 Hz flickering frequency or low frequency of 90 pulses per minute can change ant traffic paths if properly used.
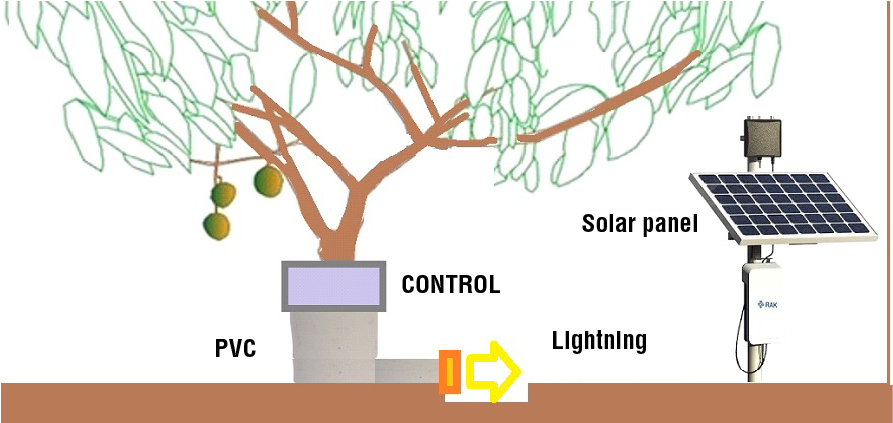
If the system wants to be used by an entire orchard, 100 systems should be installed per hectare (one per tree). The energy required per tree can be harvested from the sun with a 40W solar panel (Figure 7) and saved in a battery so that it can be used at night. At floor level a PVC tube allows the ants walk to the tree trunk and a circuit will detect when ants climb. In one side of the PVC (Figure 7-orange block) the LEDS are fixed and will turn-on when a phototransistor (TEPT5700, Vishay, India) is activated by a low power emitting diode (XZCDGK53W-8VF, SunLED Company Limited, Hong Kong). This action takes place when ants circulate. Hahn and Martínez (2023) published the entire circuit, where two low power MOSFET¨s were used together with a CC2541 (Texas Instruments, USA) microcontroller. The cost per tree is depicted in Table 4, being of $34.20 US dollars. If a 30 kg box can be paid at $10.00 US, and one solar panel can be used by two trees, the system can become very-cost effective. The system is protected against water erosion caused by rainstorms by closing the PVC tube, avoiding LEDS and circuits damage.
In 2018, also 60 % of the trees were attacked and the trees production decreased by 35%. Very mature mango trees 30 years old were less prone to ants-attack as young leaves were very high and during 2019 only two trees were ant-pruned. Ten-year mango trees with lower tender leaves were attacked more and trails covered with leaf sections were found in 55% of the trees. In 2019, mango production decreased by 28 %. The use of the system in thirty 10-year trees during the night avoided ants foraging their foliage.
Conclusions
As top L300 lighting operated poorly over the trail, other illumination alternatives were tested. With top lightning only a small percentage of the L300 incident light was reflected from the soil to the ants-face. Although this paper continuously mentions that light impacted ant eyes, its damage was never tested with a microscope, and only indirect effects (speed, collisions, orientation loss) were noted. It impacted directly to their heads and bodies.
As trail light intensity became brighter ant walking speed increased and average TPBA decreased. When the visual system was impacted by strong lateral pulses, ants presented collisions. Meanwhile ants were beaten by followers they tried to adapt to light flickering. The LED system was very precise and photoreceptor cells were unable to switch quickly. The LED intensity that reaches the ant eye can cause damage to some photodetectors, creating adaptation problems and orientation loss.
Atta cephalotes can cope with continuous lightning, but suffer adaptation problems under intermittent light pulses. High 200 Hz flickering frequency can change ant traffic paths optimizing power usage.











 texto en
texto en