Highlights:
Inoculation with ectomycorrhizal fungi (EMF) improved the growth of P. arizonica.
Plants inoculated with EMF had higher survival and higher mineral content.
EMF can establish symbiosis with P. arizonica at low inoculation volumes.
The smallest dose of EMF (10 mL: 106 spores∙mL-1) is recommended on P. arizonica.
Introduction
Most temperate forest trees form symbiotic mutualistic associations with diverse ectomycorrhizal fungi (EMF) (Marqués-Gálvez et al., 2022). The plant supplies carbon and energy to the fungal partner; at the same time, the fungal partner provides mineral nutrients that are difficult for the plant to assimilate, especially nitrogen and phosphorus (Ortega, 2015). Likewise, the associated fungus provides other benefits, such as increased tolerance to environmental stress, improved photosynthetic rate, osmotic resistance to pests and stimulation of growth regulating substances (Frank & García, 2021).
Inadequate forest reduction and management decreases or eliminates the mycorrhizal inoculum naturally found on the ground, resulting in a lower survival rate for fungal species and trees (Prado-Tarango et al., 2021). As an alternative to preserving fungal inoculum, measures have been taken to ensure the presence of EMF mycelium in plant roots. These include transporting large quantities of forest soil to the site where the seedlings are grown, but this practice is unsustainable (Sáenz-Romero, 2014). Another option is to grow EMF in vitro and perform mycorrhizal synthesis at the laboratory, greenhouses or field using aseptically germinated plants; however, it is necessary to determine the appropriate growth conditions, the fungal species to be used, as well as the cultivation techniques and the optimal amount of inoculum (Pérez-López et al., 2021; Walker & Jones, 2013).
Pinus arizonica Engelm. is one of the most commercially important pines in the state of Chihuahua, due to the quality of its wood, which has been affected by timber industry activities, forest fires, and agricultural clearing (Morales-Nieto et al., 2021; Silva-Flores et al., 2014). Despite their importance, no studies have been carried out to study the native fungal associates of P. arizonica in the Sierra Madre Occidental in Chihuahua; furthermore, no studies have reported the effects EMF may have on the growth of the species when inoculated in vitro or as spore suspension under greenhouse conditions. Therefore, the objective of this study was to evaluate the morphometric response of P. arizonica plants inoculated with spore preparations of Pisolithus tinctorius (Pers.) Coker & Couch and Astraeus hygrometricus (Pers.) Morgan. These EMF can live in poor soils with high acidity and withstand periods of drought stress (Sebastiana et al., 2020). The results of this research may be useful to improve the success of P. arizonica plants when established in the field during reforestation programs.
Materials and Methods
Seed preparation
P. arizonica seeds were provided by the Forest Management Unit (UMAFOR) in the village of San Juanito in the municipality of Bocoyna, Chihuahua. Seeds were placed in a container with tap water for 24 h to discard floating seeds that were considered non-viable (Monroy-Vazquez et al., 2017). The remaining seeds at the bottom of the container were placed on moist blotting paper in a sealable plastic bag until germination. Seedbeds (with 77 cavities) were used in the greenhouse and filled with a sterile peat moss substrate. Seeds were placed 0.5 cm deep in the substrate and moistened with deionized water every third day. The plants were maintained in the greenhouse at the Universidad Autónoma de Ciudad Juárez (UACJ) at an average temperature of 26.3 ± 1.06 °C and fertilized with Trifol 60 Plus® 20-20-20 once a month for nine months to ensure growth.
Field sampling of sporomes
P. tinctorius and A. hygrometricus sporomes (Figure 1) were harvested in August 2018 during the rainy season in temperate forests located in El Huérfano and San Juanito, Bocoyna, Chihuahua. Samples were taken to the Biodiversity Laboratory at the UACJ for spore extraction.
Preparation of fungal inoculum
A. hygrometricus and P. tinctorius sporomes were soaked with distilled water (to avoid possible contamination by bacteria or fungi) at a ratio of 1:50 for 2 h (Aguilar-Ulloa et al., 2016). The sporomes were then placed separately in a conventional blender and liquefied until homogeneous mixture, then sieved (1 mm mesh opening) and placed in glass vials. The inoculum was kept at 4 °C in the dark until use in the greenhouse. The number of spores per species was determined using a Neubauer chamber and the concentration was adjusted to 106 spores∙mL-1 (Konopická et al., 2021).
Plant inoculation
When pine plants reached an average height of 5 cm, the fungal inoculum was applied to the root system using 10, 25 and 50 mL volumes with 10 mL syringes; the spore inoculum (106 spores∙mL-1) was applied twice in one month (modified from Yin et al., 2018). Also, the commercial product Ectorrize® was used as a positive control, which contains spores of P. tinctorius and soil bacteria; similarly, the number of spores was adjusted to 106 spores∙mL-1. The negative control consisted only of non-inoculated plants. The number of inoculated plants in each treatment is shown in Table 1. The variability among the number of plants is explained by the fact that from the initial number (n = 40), there were elements below 5 cm in height to start the experiment.
Table 1 Volume of inoculum (106 spores∙mL-1) of Astraeus hygrometricus and Pisolithus tinctorius used on Pinus arizonica plants.
| Species | Volume of inoculum (mL) | Plants per treatment |
|---|---|---|
| A. hygrometricus | 10 | 29 |
| A. hygrometricus | 25 | 28 |
| A. hygrometricus | 50 | 23 |
| P. tinctorius | 10 | 30 |
| P. tinctorius | 25 | 33 |
| P. tinctorius | 50 | 33 |
| Ectorrize® | 10 | 31 |
| Ectorrize® | 25 | 32 |
| Ectorrize® | 50 | 24 |
| Negative control | - | 29 |
Evaluation of mycorrhizal efficiency
Aboveground plant growth (height, stem and foliage diameter) was measured monthly from the start of inoculation for nine months using a Neiko 01407A digital vernier. Biomass was recorded in fresh weight (g) for the aboveground part (leaves and stem) and root system; biomass in dry weight was measured by muffle drying.
Height measurements, root system diameter and biomass of aboveground part and root system (fresh and dry weight) were determined from a non-replaceable sampling method using five plants per treatment; the root system was washed with abundant tap water to remove the remaining substrate. Mycorrhizal colonization was determined with the equation used by Garza-Ocañas et al. (2018): colonization (%) = (number of mycorrhizal roots/ number of roots observed) * 100.
Characterization of ectomycorrhizae
The root systems of three randomly selected plants, per fungal species, were morphologically characterized nine months after inoculation. The external characteristics of fresh mycorrhizas were observed with the aid of a stereoscopic microscope and described in terms of branching type, root tip morphology, presence of rhizomorphs, mantle color and thickness, and mycorrhizal diameter (Agerer, 1991).
Histological characterization of the mycorrhizas began with a technique of fixing the samples with 1:1:1 formalin, alcohol, and acetic acid; they were then filtered with the aid of a vacuum pump followed by embedding in kerosene. Then, longitudinal and transverse sections of the mycorrhiza of 2 µm were placed on a rotating microtome. Finally, the sections were deparaffinized and stained with Loeffler's methylene blue (1 %) and brilliant green to observe and characterize the morphology of the fungal mantle and Hartig net (Prophet et al., 1995) using an optical microscope at 100x magnification.
Mineral content of the plant
Samples of the shoot and root system of the plants with each treatment were sent to the Laboratorio de Análisis de Suelos, Plantas y Aguas de la Facultad de Ciencias Agrotecnológicas de la Universidad Autónoma de Chihuahua, where a mineral composition analysis was performed to determine the percentages of total nitrogen (Nt), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg) and sodium (Na), as well as the amount in parts per million (ppm) of copper (Cu), iron (Fe), manganese (Mn) and zinc (Zn).
The micro-Kjeldahl technique was used to determine the amount of N, and the triazide digester mixture and the colorimetric vanadate technique for the percentage of P. The digester mixture is based on the extraction of P with the methodology of Tiessen and Moir (1993), and the colorimetric vanadate test is the one suggested by Murphy and Riley (1962). Quantification of K, Ca, Mg, Na, Cu, Fe, Mn and Zn was also carried out using a mixture of triazide digesters and atomic absorption spectrophotometry (GBC Avanta-Sigma) (Association of Official Analytical Chemists [AOAC], 1990).
Statistical analysis
Shapiro-Wilk (n ≤ 30) and Kolmogorov-Smirnov (n > 30) normality tests were carried out to determine whether the data conformed to a standard distribution; a Levene's test for homogeneity of variances (P ≥ 0.05) was also used.
The effects of the fungal species (factor A) and the volume of spore inoculum used (factor B) on the morphometric variables (response variables: plant height, foliage diameter and stem height) were determined using a completely randomized ANOVA. A 4 x 3 factorial arrangement was used, corresponding to the spore inoculum of the two fungal species, the commercial inoculum of Ectorrize®, the negative control and the three inoculum volumes. Also, an ANOVA with a randomized design was performed to evaluate the effect of the fungal species on the morphometric variables of height and diameter of the root system and biomass of the shoot and root system (fresh and dry weight). Data showing heterogeneity of variances were analyzed with a T3 multiple comparison of means by Dunnett. Statistical analysis was performed in the statistical program SPSS version 20 (IBM Corporation, 2011) and GraphPad Prism version 8.0 (GraphPad Software, Inc., 2018) was used.
Results
Efficiency and colonization
Table 2 shows that, at the end of the experiment, inoculated plants had higher survival (93.5 to 100 %) than non-inoculated plants (74.3 %).
Figures 2, 3 and 4 show that inoculation with P. tinctorius and A. hygrometricus had a positive effect (P ≤ 0.05) on all morphometric variables of P. arizonica. Average plant height (Figure 2), as well as diameter and length of foliage (Figure 4), was higher for inoculated treatments compared to the non-inoculated control, regardless of inoculum volume which had no statistically significant effect (P > 0.05). This indicates that EMF can establish symbiosis with P. arizonica plants at low inoculation volumes, generating effectiveness.
Table 2 Survival of Pinus arizonica plants after nine months of inoculation with Astraeus hygrometricus and Pisolithus tinctorius at a concentration of 106 spores∙mL-1 under greenhouse conditions.
| Treatment | Inoculum volume (mL) | Survival (%) |
|---|---|---|
| P. tinctorius | 10 | 100.0 |
| P. tinctorius | 25 | 96.9 |
| P. tinctorius | 50 | 100.0 |
| Ectorrize® | 10 | 93.5 |
| Ectorrize® | 25 | 90.6 |
| Ectorrize® | 50 | 100.0 |
| A. hygrometricus | 10 | 100.0 |
| A. hygrometricus | 25 | 100.0 |
| A. hygrometricus | 50 | 95.6 |
| Control | - | 74.3 |
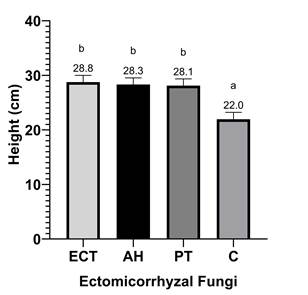
Figure 2 Height of Pinus arizonica plants under the effect of inoculation treatments with Pisolithus tinctorius (PT), Ectorrize® (ECT), Astraeus hygrometricus (AH) and a control treatment without inoculation (C). Different letters indicate significant differences between treatments according to Dunnett's multiple comparison of means (P ≤ 0.05). Variance bars represent standard deviation from the mean (n = 5).
Statistical analysis determined that inoculant volume had no significant effect (P > 0.05) on plant height growth (Figure 2), stem diameter (Figure 3), and shoot length and diameter (Figure 4); however, interaction or combination of EMF and inoculated volume had effect on the variables (Table 3). In the case of plant height, shoot length and shoot diameter, it was observed that P. tinctorius generated the greatest growth with doses of 10 and 50 mL, being similar with Ectorrize® at 10 mL; for stem diameter, the best response was generated with P. tinctorius at a dose of 10 mL.
Table 3 Interaction effect of ectomycorrhizal fungi and inoculum volume on growth variables of Pinus arizonica.
| Inoculum volume (mL) | Pisolithus tinctorius | Ectorrize® | Astraeus hygrometricus | Control (no inoculum) |
|---|---|---|---|---|
| Plant height | ||||
| 10 | 28.6 ± 2.4 f | 28.5 ± 3.4 f | 24.4 ± 2.9 de | 16.9 ± 3.4 a |
| 25 | 24.7 ± 2.3 e | 22.4 ± 3.4 bcd | 23.5 ± 3.4 cde | |
| 50 | 27.1 ± 4.2 f | 22.0 ± 2.4 bc | 25.0 ± 3.6 de | |
| Shoot length | ||||
| 10 | 26.1 ± 2.4 f | 25.7 ± 3.3 f | 21.3 ± 2.9 de | 14.8 ± 3.1 a |
| 25 | 22.3 ± 2.5 e | 19.7 ± 3.2 cd | 20.8 ± 3.2 cde | |
| 50 | 24.5 ± 4.1 f | 18.7 ± 2.3 bc | 21.4 ± 3.7 de | |
| Shoot diameter | ||||
| 10 | 21.7 ± 2.4 g | 21.5 ± 3.8 g | 17.6 ± 2.7 bcde | 12.9 ± 4.1 a |
| 25 | 16.5 ± 3.1 b | 18.6 ± 2.9 cdef | 18.1 ± 3.8 cdef | |
| 50 | 20.6 ± 4.0 g | 17.9 ± 3 bcdef | 17.6 ± 4.6 bcdef | |
| Stem diameter | ||||
| 10 | 5.7 ± 0.09 f | 5.3 ± 0.6 e | 4.3 ± 0.06 c | 3.2 ± 0.08 a |
| 25 | 5.3 ± 0.19 e | 4.6 ± 0.08 d | 4.1 ± 0.07 bc | |
| 50 | 5.1 ± 0.06 de | 4.6 ± 0.1 d | 3.9 ± 0.06 b | |
± standard deviation of the mean. For each variable, different letters indicate significant differences between means according to Dunnett's test (P ≤ 0.05).
For stem diameter (Figure 3), plants inoculated with Ectorrize® and P. tinctorius spores showed greater growth, followed by A. hygrometricus, compared to the control (3.36 cm).
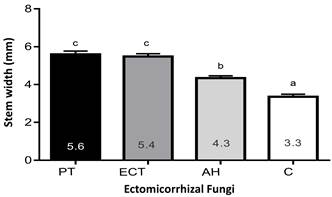
Figure 3 Stem diameter of Pinus arizonica under inoculation treatments with Pisolithus tinctorius (PT), Ectorrize® (ECT), Astraeus hygrometricus (AH) and a control treatment without inoculation (C). Different letters indicate significant differences between treatments according to Dunnett's multiple comparison of means (P ≤ 0.05). Variation bars represent standard deviation from the mean (n = 5).
Length and diameter of the shoot in the inoculated treatments were higher than in the non-inoculated control (Figure 4A). With respect to the root system, the inoculant treatments had a statistically similar effect on the length (P > 0.05) compared to the control (Figure 4B); it ranged from 14.04 to 15.26 cm. In contrast, the results for root system diameter (Figure 4B) showed differences among treatments (P < 0.05), with the control being smaller (3.72 cm); the highest values were reported with the Ectorrize® and A. hygrometricus treatments, with 4.60 and 4.62 cm, respectively.
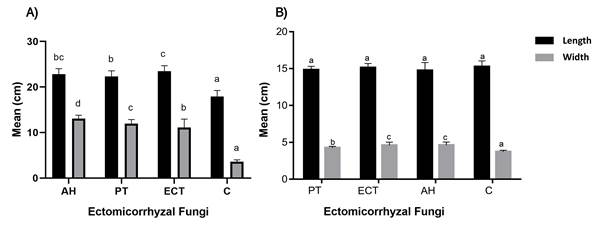
Figure 4 A) Average length and diameter of the shoot (A) and root system (B) of Pinus arizonica under inoculation treatments with Pisolithus tinctorius (PT), Ectorrize® (ECT), Astraeus hygrometricus (AH) and a control treatment without inoculation (C). Different letters indicate significant differences between treatments according to Dunnett's multiple comparison of means (P ≤ 0.05). Variance bars represent standard deviation from the mean (n = 5).
The presence of inocula in the early stages of plant development has been reported to reduce the incidence of root pathogens (Sharma et al., 2014) because natural competition for space occurs between them and the EMF (Jung et al., 2012). In this study, no diseases caused by pathogenic fungal species were detected in inoculated P. arizonica plants or in the control.
In terms of biomass production, both shoot and root system (Figure 5), showed positive effects on P. arizonica plants compared to the control. Treatment with P. tinctorius spores stimulated higher shoot growth of plants (8.08 g) which differed significantly (P < 0.05) with the non-inoculated control treatment (2.40 g). The highest fresh weight biomass for the root system was induced by P. tinctorius spore and Ectorrize® treatments with values of 7.92 and 7.80 g, respectively, and these differed significantly with those obtained by the control. The P. tinctorius spore treatments and the commercial product Ectorrize® also generated higher biomass in dry weight in the root system with 3.12 and 3.22 g, respectively.
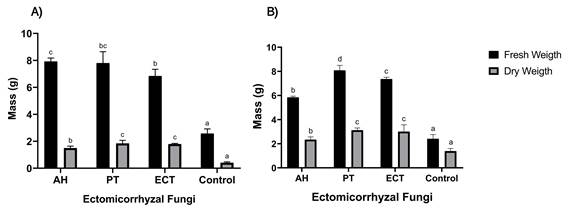
Figure 5 Biomass production of shoot (A) and root system (B) of Pinus arizonica under inoculation treatments with Pisolithus tinctorius (PT), Ectorrize® (ECT), Astraeus hygrometricus (AH) and a control treatment without inoculation (C). Different letters indicate significant differences between treatments according to Dunnett's multiple comparison of means (P ≤ 0.05). Variance bars represent standard deviation from the mean (n = 5).
Ectomycorrhizal colonization of Pinus arizonica roots
Table 4 indicates that P. tinctorius spores produced the highest percentage of ectomycorrhizal colonization on P. arizonica plants, followed by A. hygrometricus and Ectorrize®. The degree of mycorrhizal formation was classified according to Tateishi et al. (2003); based on the number of mycorrhizal roots, inoculated plants had a medium to high level (level IV).
Table 4 Ectomycorrhizal colonization per inoculation treatment in Pinus arizonica plants.
| Species | Colonization (%) |
|---|---|
| Pisolithus tinctorius | 67.6 ± 1.1 d |
| Ectorrize® | 56.8 ± 27 b |
| Astraeus hygrometricus | 63.4 ± 2.6 c |
| Control | 0.0 a |
± standard deviation of the mean. Different letters indicate significant differences between species according to Dunnett's test (P ≤ 0.05).
Morphological characterization of ectomycorrhizas
Mycorrhizal characteristics, such as mantle color and branching type, helped to distinguish the two fungal species, and cross sections of histological sections were useful to identify the Hartig network and mantle thickness. Dichotomous mycorrhizas were the most abundant.
Figure 6 shows the light brown ectomycorrhizas of P. tinctorius, with darkening at the base, mainly dichotomous type. Some coralloid formations were observed but were not frequent; rhizomorphs were also identified. The length of the mycorrhizal system ranged from 1.3 to 2.5 mm and the diameter from 0.4 to 0.7 mm. Root tips measured 0.7 mm in length and 0.3 mm in diameter. The texture of the fungal mantle varied from granular to smooth, and the coloration was light brown to hyaline. Longitudinal sections of the mycorrhizas showed the mantle to be of plectenchymatous type H (Agerer, 1991); these are characterized by 15 to 20 μm diameter irregularly shaped hyphae. No clamp connections were observed. The Hartig network was composed of 2 to 3 μm diameter ovoid cells.
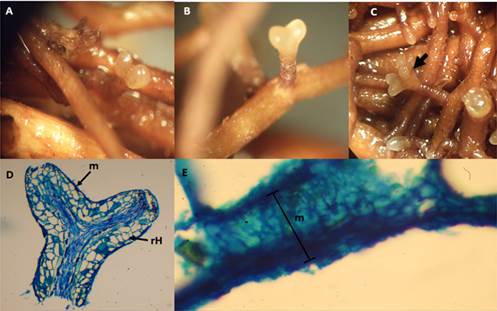
Figure 6 Ectomycorrhizae of Pisolithus tinctorius on Pinus arizonica plants: A) initial formation, B) dichotomous morphotype, C) choraloid morphotype, D) longitudinal section of ectomycorrhiza indicating the mantle (m) and Hartig network (rH), E) 20 μm thick mantle.
The morphological variability of the ectomycorrhizas produced by the Ectorrize® treatment is observed in Figure 7. The mycorrhizal system is dense with dichotomous type, with main branches and straight tips in the initial stages, although slightly sinuous at mature stage. The presence of rhizomorphs was not observed. The color of the mycorrhizas varied from dark brown to golden brown and the tips were lighter in color than the rest. The mycorrhizal system measured, on average, 3.1 to 5.4 mm in length and 1.5 mm in diameter, and the tips were 0.9 to 2.3 mm in length and 0.2 to 0.5 mm in diameter. The texture of the mantle varied from smooth to granular with a light brown to hyaline coloration. Transverse sections showed an N-type plectenchymatous mantle (Agerer, 1991), characterized by irregular hyphae. The mantle measured 10 to 15 μm thick and the Hartig network had semicircular shaped cells.
Morphology and color of mycorrhizas formed by the Ectorrize® treatment were similar to P. tinctorius but only at the initial stages, while the length of the mycorrhizal system was higher with the positive control. Changes in both treatments may be due to the addition of different bacterial strains used in the Ectorrize® product.
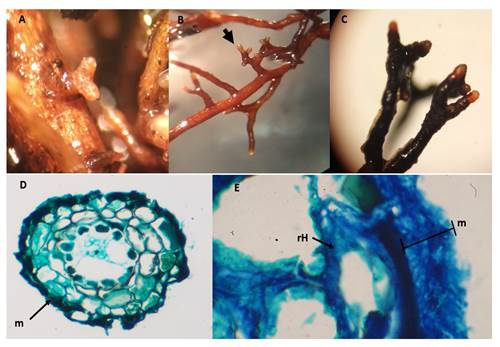
Figure 7 Ectomycorrhizae in Pinus arizonica plants with Ectorrize® inoculation treatment. A) Initial stage, B) dichotomous morphotype, C) mature ectomycorrhizas, D) cross section of ectomycorrhiza indicated in the mantle (m), E) plectenchymatous mantle (m) 15 μm thick N-type (Agerer, 1991) and Hartig network (rH), magnification 100x.
Figure 8 shows the morphology of A. hygrometricus mycorrhizas. It is important to mention that the study presents for the first time the morphological description of the mycorrhizal association of A. hygrometricus with P. arizonica. Formations were dichotomous and choraloid, the latter being dominant at the mature stages. The presence of dense rhizomorphs was observed but restricted to one point. Mycorrhizas had white colorations, and some had slightly hyaline root tips; older mycorrhizas had a grayish color. The dichotomous type mycorrhizal system measured 2.1 mm in length by 1.4 mm in diameter and the tips averaged 1.1 mm in length by 0.4 mm in diameter. The coralloid morphotype ranged from 2 to 2.5 mm long by 2 mm in diameter and the tips measured 1.3 µm long by 0.5 µm in diameter. The texture of the mantle was granular to smooth at the tips. The histological cross section showed that the mantle morphology is plectenchymatous type B which is characterized by irregular hyphae on the mantle surface, arranged mainly longitudinally (Agerer, 1991). The thickness of the mantle was 70 µm. The Hartig network showed 3- to 5-µm long, semicircular, and elongated cells.
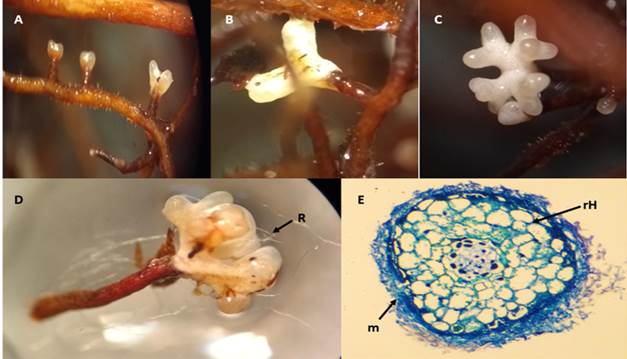
Figure 8 Ectomycorrhizas of Astraeus hygrometricus on Pinus arizonica plants: A) initial stage, B) dichotomous mycorrhizas, C) mature ectomycorrhizas with coralloid branching, D) rhizomorphs (R) and coralloid ectomycorrhizas, E) cross section of ectomycorrhizas showing the mantle (m) and Hartig network (rH).
Mineral analysis in Pinus arizonica plants
High macronutrient content was observed in both shoot and root parts of Pinus arizonica plants inoculated with the commercial product Ectorrize® and with the fungi A. hygrometricus and P. tinctorius (Table 5). For the negative control, root system analyses could not be carried out, since the amount of biomass was insufficient.
The results support that pointed out by Ortega (2015), Smits and Wallander (2017), and Chen et al. (2018), who mention that mycorrhizae absorb sugars from plant roots and introduce nutrients such as P, N, K, Ca, S and Zn, among others, into their vascular systems.
Table 5 Mineral composition of Pinus arizonica plants inoculated with ectomycorrhizal fungi.
| Variables | Astraeus hygrometricus | Pisolithus tinctorius | Ectorrize® | Control | ||||
|---|---|---|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | Shoot | Root | Shoot | Root | |
| Fresh weight (g) | 5.94 | 8.00 | 8.74 | 6.69 | 7.45 | 7.10 | 2.64 | 2.86 |
| Dry weight (g) | 2.58 | 1.67 | 3.34 | 1.45 | 3.32 | 1.85 | 1.53 | 0.39 |
| Nt (%) | 1.26 | 1.26 | 1.20 | 1.20 | 1.26 | 1.13 | 1.32 | 1.13 |
| P (%) | 0.19 | 0.27 | 0.16 | 0.14 | 0.16 | 0.15 | 0.11 | NE |
| K (%) | 1.04 | 0.63 | 0.56 | 0.43 | 0.45 | 0.49 | 0.30 | NE |
| Ca (%) | 4.14 | 2.32 | 1.97 | 2.83 | 2.32 | 1.72 | 2.68 | NE |
| Mg (%) | 0.19 | 0.22 | 0.17 | 0.19 | 0.17 | 0.16 | 0.15 | NE |
| Na (%) | 0.12 | 0.13 | 0.11 | 0.12 | 0.12 | 0.12 | 0.13 | NE |
| Cu (ppm) | 4.00 | 6.00 | 4.00 | 4.50 | 4.00 | 8.00 | 3.50 | NE |
| Fe (ppm) | 78.00 | 165.00 | 48.00 | 107.50 | 50.50 | 88.00 | 37.50 | NE |
| Mn (ppm) | 86.50 | 32.50 | 88.50 | 34.00 | 81.00 | 39.00 | 40.00 | NE |
| Zn (ppm) | 43.00 | 68.50 | 41.50 | 63.00 | 32.50 | 45.00 | 30.00 | NE |
NE = Not enough for analysis.
Discussion
A favorable effect on the growth of P. arizonica species was recorded with any of the inoculated EMF, and also with the commercial product Ectorrize®. These results show the great mycorrhizal potential of P. tinctorius or A. hygrometricus spore inocula.
Wang (2017) suggested that the use of plants inoculated with mycorrhizal fungi plays an important role in reforestation of heavily disturbed areas. In the present study, inoculation of P. arizonica plants with mycorrhizal fungi caused substantial increase in growth and survival variables. P. tinctorius and A. hygrometricus have been reported to have high capacity to form associations with a wide range of angiosperms and gymnosperms (Kayama & Yamanaka, 2014). This potential may be attributed to the ability of spores to germinate over a wide pH range (4 to 6.5) compared to other temperate forest EMF, which are limited to pH 5 or so; in addition, they could withstand higher temperatures than other species such as Rhizopogon roseolus (Corda) Th. Fr. (Maltz et al., 2019).
Sebastiana et al. (2018) noted that inoculation with P. tinctorius had positive effect on plant height, shoot biomass, shoot basal diameter, and root growth of Quercus suber under field conditions, also increased nitrogen concentration and drought tolerance compared to non-inoculated plants.
Regarding morphological aspects, Kayama and Yamanaka (2014) found that the development of leaves, stems, branches and roots of Quercus glauca Thunb., Q. salicina Blume and Castanopsis cuspidata Thunb. inoculated with A. hygrometricus was higher than when no EMF were inoculated. Adeleke et al. (2015) inoculated Pinus patula Schl. et Cham. plants with P. tinctorius, Paxillus involutus (Batsch ex Fr.) Fr., Laccaria bicolor (Maire) P. D. Orton and Suillus tomentosus (Kauffman) Singer under in vitro conditions. These fungi induced higher root and shoot biomass production and improved the physiological status of the plants compared to non-inoculated plants.
Wen et al. (2017) inoculated Chinese red pine (Pinus tabuliformis Carr.) plants with P. tinctorius and Cenococcum geophilum Fr. and showed that the level of mycorrhization depended on the fungal species used. The inoculum of P. tinctorius gave the best results in nutrient accumulation and pine shoot growth; the authors concluded that these fungi can benefit plant establishment in contaminated areas. Finally, Gómez et al. (2013) evaluated the establishment of Pinus pseudostrobus Lindl. in the field with plants inoculated and not inoculated with P. tinctorius; the survival rate of inoculated pines was higher (86 %) compared to those with no inoculum (62 %).
Regarding colonization, the results of the present study were consistent with those reported by Garza-Ocañas et al. (2018) and Garza et al. (2022) with values between 60 and 80 % in roots of forest species under greenhouse conditions.
Stem diameter is one of the morphological attributes widely used to characterize the quality of nursery plants and is believed to be related to water and nutrient transfer, mechanical strength, and robustness. It is also a proxy measure of resistance to climatic and biological factors (Sanchez-Costa et al., 2015). In the present study, stem diameter developed appropriately in all treatments except the control.
Root system length is related to the ability of plants to explore the soil for nutrients and water; the content of this element in the substrate will influence root development. If water is abundant, root growth is not stimulated, but if water is scarce, a wide root system will be necessary to survive (Yang et al., 2017). Another important aspect is the capacity that the hyphal system has to explore the substrate and establish connections with tree roots; according to Agerer (2001), P. tinctorius and A. hygrometricus have long-distance exploratory capacity, which allows them to extend their hyphae a few decimeters and generate ectomycorrhizas more easily. On the other hand, the biomass of the root system of inoculated plants is related to an increase in the absorption surface, and to a higher volume of soil used by mycorrhizal plants (Halifu et al., 2019). Although root length was similar in all treatments, inoculated plants had better root biomass production than the control. Wang et al. (2021) reported higher biomass production of both shoot and root system of P. tabuliformis when inoculated with Suillus variegatus (Sw.) Kuntze. According to Arteaga-León et al. (2018), shoot biomass is related to higher photosynthetic and transpiration activity, implying good health and, consequently, a strong root system. Again, the highest values in shoot and root biomass production of Pinus arizonica plants were found in those inoculated with EMF.
The relationship between dry weight of shoot and root system reflects the degree of development of the plant in the greenhouse. According to the measurements, plants inoculated with EMF had ratio values higher than 1, indicating that shoot biomass was higher than in the root system; these values are within the optimal range (1.5 to 2.5) (Subira et al., 2016). In contrast, non-inoculated control plants had a ratio of 3.44 was obtained, indicating a disproportion between both parts. This reveals an insufficient root system to provide the nutrients the plant needs.
In the identification of symbiont fungi, mycorrhizal morphology is considered valuable, because this is characteristic for each host; the dominant branching type in pine ectomycorrhiza is usually bifurcate, dichotomous, coraloid or tuberculate. In other studies, identification is carried out using molecular methods (Agerer, 1991; Sulzbacher et al., 2016); however, because no EMH experiments had been performed in the greenhouse and treatments were separated from each other, cross-contaminations with other species did not occur and, therefore, molecular analysis of the ectomycorrhizas formed was not necessary.
The morphology and color of the mycorrhizas of P. tinctorius agree with that reported by García et al. (2006) in Pinus greggii Engelm. ex Parl. plants; however, the morphology of the mantle reported by these authors was of the prosenchymatous type. On the other hand, the coloration was different from that reported by Valdés et al. (2010), who mentioned a whitish to slightly yellowish color in P. devoniana plants. In this regard, the same authors point out that the mycorrhizas of P. tinctorius have a whitish color when they are young and then change from beige to yellowish brown as they mature. It is inferred that after nine months of inoculation, P. arizonica plants would have mycorrhizae in the mature phase, which may also affect the organization of the mycelium and mantle.
Commercial inoculum has been used by authors such as Gómez et al. (2013) for restoration of P. pseudostrobus plantations. These authors did not characterize the mycorrhizae but observed significant changes in plant morphometric variables compared to the control. Several commercial inoculants based on EMF and used in combination with bacteria are beneficial to plants; however, inoculation studies have been conducted mainly with wild fungi (Gómez et al., 2013).
The morphology and color of the ectomycorrhizas formed by A. hygrometricus were similar to those reported by Fangfuk et al. (2010) on Pinus densiflora Siebold & Zucc.; but are different from those reported by Kaewgrajang et al. (2013) using Astraeus odoratus Phosri, Watling, M. P. Martín & Whalley on Dipterocarpus alatus Roxb. ex G. Don plants. It is important to mention that this tree is from tropical climates, therefore its nutritional and environmental needs are different from those of temperate forests.
The present study showed a favorable effect on growth and nutritional content of P. arizonica. It is possible to confirm the assimilation of N, P, K, Ca, Mg, Na, Cu, Fe, Mn and Zn in shoot and root system. Plants grown with fungal inoculation had, in general, higher shoot and root biomass, as well as higher macro- and micronutrient contents compared to plants grown without the addition of mycorrhizae. This agrees with that reported by Rentería-Chávez et al. (2017), who found higher uptake, growth and mineral biomass in P. greggi plants inoculated with EMF.
Conclusions
Pinus arizonica plants inoculated with spores of Astraeus hygrometricus and Pisolithus tinctorius at various concentrations successfully formed mycorrhizae, making both ectomycorrhizal fungi (EMF) effective in establishing symbiosis. Plants inoculated with these fungi had higher height, stem diameter, and shoot and root biomass compared to non-inoculated plants. A similar trend was observed in mineral uptake and incorporation, mainly for N, Zn, Mn and Fe. The use of high doses of spores for the inoculation of P. arizonica plants is not necessary to obtain a good mycorrhizal colonization. Therefore, the use of a small dose of spore inoculum (10 mL; 106 spores∙mL-1) is suggested for the production of P. arizonica plants in greenhouses; in particular, P. tinctorius is recommended for having the best response in the evaluated variables.











 texto en
texto en 




