Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias forestales
versión impresa ISSN 2007-1132
Rev. mex. de cienc. forestales vol.13 no.69 México ene./feb. 2022 Epub 09-Mayo-2022
https://doi.org/10.29298/rmcf.v13i69.1176
Scientific article
Reproductive phenology of trees in a mountainous area of western Mexico
1Departamento de Ecología y Recursos Naturales, Centro Universitario de la Costa Sur, Universidad de Guadalajara. México.
Knowledge of the flowering and fruiting patterns of tree species is important, given their relationship with pollinators and dispersers and because they are indicative of the best dates for collecting flowers and fruits for taxonomic and reproductive purposes. Therefore, the flowering and fruiting patterns of the tree species of the Las Joyas Scientific Station and adjacent areas in the Sierra of Manantlán in the state of Jalisco were analyzed, and their correlation with precipitation, temperature, insolation, relative humidity and evaporation was researched. Herbarium specimens with flowers and fruits collected from 1985 and deposited in the ZEA Herbarium of the University of Guadalajara were reviewed. The descriptive statistics of the data were performed using histograms. One way variance analysis was performed in search of differences between flowering and fruiting periods and Pearson's correlations in order to determine the relationships between flowering and fruiting and environmental variables. The highest richness of flowering and fruiting species was found to occur during the dry season of the year. The type of vegetation that registered the most species was the cloud forest, a plant community whose flowering and fruiting pattern conforms to the general pattern of all taxa. The richness of flowering and fruiting tree species exhibited a negative and significant correlation with relative humidity and precipitation, the most important environmental variables.
Key words Cloud forest; relative humidity; Jalisco; precipitation; Sierra of Manantlán; vegetation
El conocimiento de los patrones de floración y fructificación de las especies arbóreas es de importancia por la relación que presentan con polinizadores, dispersores y su contribución para conocer las mejores fechas de recolecta de flores y frutos con propósitos taxonómicos y reproductivos. Por lo anterior, se analizaron los patrones de floración y fructificación de las especies arbóreas de la Estación Científica Las Joyas y áreas aledañas en la sierra de Manantlán en el estado de Jalisco; y se indagó su correlación con la precipitación, temperatura, insolación, humedad relativa y evaporación. Se revisaron ejemplares de herbario con flores y frutos recolectados a partir de 1985 que forman parte del acervo del Herbario ZEA de la Universidad de Guadalajara. Se realizó la estadística descriptiva de los datos mediante histogramas; además se hicieron análisis de varianza de una vía en busca de diferencias entre los períodos de floración y fructificación, y correlaciones de Pearson para determinar las relaciones entre la floración y fructificación con las variables ambientales. Se registró que la mayor riqueza de especies en floración y fructificación se presentó en la estación seca del año. El tipo de vegetación con más especies fue el bosque mesófilo de montaña y el patrón de floración y fructificación en esta comunidad vegetal se ajustó al patrón general de todos los taxones. La riqueza de especies arbóreas en floración y fructificación tuvo correlación negativa y significativa con la humedad relativa y la precipitación, las variables ambientales de mayor importancia.
Palabras clave Bosque mesófilo; humedad relativa; Jalisco; precipitación; sierra de Manantlán; vegetación
Introduction
Some authors document that plant populations and species have evolved response mechanisms to environmental signals that stimulate the development of vegetative and reproductive structures (Hamann, 2004; Elzinga et al., 2007), which together generate the behavior at the community level. The environmental triggers to which the flowering and fruiting patterns of tree taxa respond are varied and differ depending on the ecosystem in question: while in temperate zones, the species respond to obvious factors such as photoperiod and temperature (Tooke and Battey, 2010; Diez et al., 2012), in tropical regions, they react to the variation of atmospheric and soil humidity, as well as to the incidence of solar radiation (Hamann, 2004; Ochoa et al., 2008).
The presence of water, through rainfall, exerts a strong influence on phenology in tropical ecosystems, but its effect has a different degree of importance in those with high moisture content and in dry ecosystems. Furthermore, it is relevant how humidity is distributed throughout the year, generating differentiated phenological behaviors in ecosystems (Acevedo and Hernández, 2013).
Researchers agree that complex evolutionary processes have led species to coincide in their flowering times, in response to environmental factors that increase the probability of their reproduction; among them, abiotic factors such as precipitation, temperature, photoperiod, and biotic factors such as the presence of pollinators, herbivores, etc. (Janzen, 1967).
In dry and humid seasonal neotropical forests, there is a tendency for the richness and abundance of flowering tree species to be concentrated in the dry season or periods of lower humidity (Janzen, 1967; Williams and Meave, 2002; Cortés-Flores et al., 2019), a pattern that is registered also for some mesophilic mountain forests in Mexico (Hernández and Carreón, 1987; Solórzano et al., 2010); however, for some neotropical temperate forests, photoperiod is one of the most influential elements in the floral phenology of woody taxa (Cortés-Flores et al., 2015).
Fruit production is expected to occur there where the best soil moisture conditions for the diaspores prevail, allowing the seeds to germinate and grow sufficiently in their underground and aerial parts to enable them to survive during the first dry season of the year (van Schaik et al., 1993; Hamann, 2004; Zimmerman et al., 2007). Among the patterns observed in fruiting at the community level in dry tropical forests, certain species bear fruit at the dry-rainy season interface (Smythe, 1970), and the fruits germinating in the immediately subsequent rainy season (recalcitrant seeded taxa); while in other species, fruiting occurs at the end of the rainy season, and their diaspores, which usually have dormancy mechanisms of months (species with orthodox seeds), germinate until the next wet season or in the following wet seasons (González et al., 2016; Topete et al., 2020).
In tropical forests, with little or no seasonality, the flowering and fruiting patterns of tree species seem to respond to the incidence of solar radiation (Hamann, 2004; Zimmerman et al., 2007); while, in Mexican temperate forests, day length has a positive effect on floral phenology (Cortés-Flores et al., 2015).
Understanding flowering and fruiting behavior in different ecosystems helps to explain the patterns that govern the distribution and abundance of animal groups (Newstom et al., 1994); is also relevant for determining the optimal time for collecting diaspores for propagation purposes (Brennan, 1996; Ochoa et al., 2008).
The purpose of this research was to describe the reproductive phenology, flowering and fruiting, at the community level, of tree species existing in a mountainous area of Western Mexico with high environmental heterogeneity. For this purpose, the following questions were addressed: How does the flowering and fruiting of tree species vary within the annual cycle at the community level? Are there differences in flowering and fruiting patterns between existing plant communities and with terrain altitude? Is there any correlation between flowering and fruiting patterns with temperature, precipitation, evaporation, relative humidity and solar radiation?
Materials and Methods
Study area
The research was carried out in a mountainous area of the Sierra Madre del Sur, an area comprising a 6 × 6 km quadrangle, with an area of 3 600 ha and an altitudinal gradient of 1 500 to 2 250 masl. The quadrangle is located between the coordinates 19°34'14"-19°37'30" N and 104°14'49"-104°18'16" W, in the municipalities of Autlán de Navarro and Cuautitlán de García Barragán in the state of Jalisco, and, within its boundaries, it is located the Las Joyas Scientific Station (ECLJ) (Jardel et al., 2004). The ECLJ is a property owned by the Government of the State of Jalisco and is part of the Manantlán-Las Joyas core zone in the Sierra de Manantlán Biosphere Reserve, Jalisco (Jardel et al., 2004). The center of the LJCS is located 22 km south-southeast of the city of Autlán de la Grana, 17 km east of Casimiro Castillo and 52 km north of the port of Manzanillo.
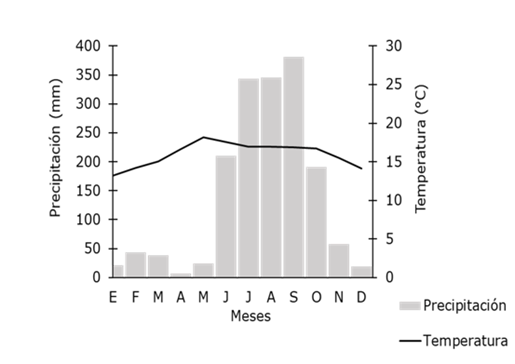
The vegetation corresponds to mesophilic mountain forest (MMF), Pinus forest (PF), Quercus forest (QF), gallery forest (GF), Pinus-Quercus forest (PQF), secondary vegetation (SV), pine shoot (PS), grassland and agricultural land (GA), and rock outcropping and erosion (RE) (Jardel et al., 2004). Since 1985, the ECLJ has been exempt from disturbances such as agriculture, livestock and forestry harvesting; however, some surface fires and the occurrence of cyclones such as Jova and Patricia in 2011 and 2015, respectively, eventually occur. The climate is markedly seasonal, with a cold season from October to January, a dry season from February to May and a rainy season from June to September. Based on the climatic records of the last 30 years, the average annual temperature and precipitation have been 15.5 °C and 1 663 mm, respectively; according to information from the local weather station (Figure 1).
Data collection
Specimens available in herbariums have been a source of information for phenological studies (Borcher, 1996; Solórzano et al., 2010; Tooke and Battey, 2010). In the case of the ECLJ and surrounding areas, there has been a continuous collection of botanical material from 1984 to date, material that is part of the collection of the ZEA Herbarium of the Centro Universitario de la Costa Sur, which represents a collection of tree species close to 3 000 specimens. The ECLJ also has a climatological station, located at an altitude of 1 850 m, which has been recording continuous information on the area since 1993. Both specimen collections and climatological information in the area allow, with a certain degree of confidence, to carry out an investigation such as the one presented in this document.
An exhaustive review of herbarium specimens of tree species collected in the ECLJ and surrounding areas was carried out. Specimens with well-developed flowers, with open petals and exposed anthers were considered as individuals in bloom (Tooke and Battey, 2010); while fruiting individuals were those with well-developed, ripe fruit or very close to it. With the information available on the labels of the herbarium specimens, a monthly database was created in Excel, which included: genus, species, family, date of collection, presence of flower or fruit, type of vegetation, (MMF, PF, PQF, QF, GF, SV), and altitude. The indicated vegetation types were considered because they are the ones referred to on the labels of the herbarium specimens. Specimens that were collected with flowers, fruits or both and that were collected during the first week or last week of the month were included in the previous or following month, in order to have a better representation in the phenological periods. The nomenclature of the species was based on the publication of Cuevas et al. (2021).
Data analysis
The Excel database was transferred to the InfoStat software, in which statistical analyses were performed (Di Rienzo et al., 2019). Descriptive statistics were performed using histograms. A data normality analysis was performed using the Shapiro-Wilks test, and homogeneity of variance tests were carried out utilizing the Kolmogorov Smirnoff procedure (Di Rienzo et al., 2019). A one-way analysis of variance was used to analyze the variation in the flowering and fruiting of tree species. A post-hoc Duncan significance test was performed comparing pairs of means in order to determine the differences between them; a p≤0.05 was considered as significant. Three seasons were considered for the analysis of flowering and fruiting patterns: the cold season, from October to January; the dry season, from February to May, and the rainy season, from June to September.
A monthly average of the values of the climatic variables registered at the ECLJ weather station, such as average insolation (hr day-1), average temperature (°C), relative humidity (%), evaporation (mm), and precipitation (mm) was calculated. The relationship between each of these variables and the number of flowering and fruiting species was obtained using Pearson correlations.
Results
Flowering and fruiting of tree species at the community level
Of the 3 000 specimens examined, only 1 724 met the criteria to be considered as flowering or fruiting according to the definition of the method. 124 species, 96 genera, and 49 families of tree species were registered. The families with the most specimens were Fagaceae, with 181 (10.50 %); Solanaceae, with 130 (7.54 %); Fabaceae, with 102 (5.92 %); Malvaceae,with 88 (5.10 %), and Betulaceae, with 74 (4.29 %). The flowering of tree taxa in the study area exhibited a bimodal distribution pattern, with one flowering peak in March-May and another in November-January, and a noticeable decrease in the number of species from August to October, although taxa were flowering all year round (Figure 2). As for fruiting, it was richest from February to July, with a decrease from August to November, but fruiting taxa were present throughout the year (Figure 2).

Riqueza de especies = Species richness; Meses = Months; Floración = Flowering; Fructificación =Fruiting.
Figure 2 Richness of flowering and fruiting species during the year.
The richness of flowering species was higher in the dry season; there were no significant statistical differences regarding the cold season, (F= 13.02, gl=2, p≤0.0022) (Figure 3a). The highest richness of fruiting species occurred in the dry season and was statistically different from the cold and rainy seasons (F= 5.46, gl= 2, p≤0.0280) (Figure 3b).

Especies en floración = Species in flowering; Especies en fructificación = Species in fruiting; Estaciones = Seasons; Fría = Cold; Seca = Dry; Lluviosa = Rainy. Different letters indicate significant differences at a p≤0.05.
Figure 3 a. Richness of flowering species by season; b. Richness of fruiting species by season.
Flowering and fruiting patterns by plant community
The MMF was the community with the highest number of flowering and fruiting specimens and where the greatest richness of families, genera, and species was identified; while the lowest richness of taxa and specimens was observed in the GF (Table 1).
Table 1 Register of specimens by plant communities and their richness of taxa.
| Vegetation | Sflowers | Sfruits | Species | Genera | Families |
|---|---|---|---|---|---|
| GF | 94 | 77 | 41 | 38 | 28 |
| SV | 101 | 92 | 46 | 39 | 26 |
| PF | 127 | 148 | 63 | 48 | 31 |
| PQF | 159 | 118 | 64 | 49 | 26 |
| QF | 161 | 168 | 76 | 54 | 32 |
| MMF | 397 | 383 | 115 | 91 | 47 |
GF = Gallery forest; SV = Secondary vegetation; PF = Pinus forest; PQF = Pinus-Quercus forest; QF = Quercus forest; MMF = Mesophilic mountain forest; Sflowers = registered specimens with flowers; Sfruits = registered specimens with fruits.
The flowering pattern by plant community was consistent with the one registered for tree species as a whole. There is a peak in the concentration of richness of flowering species from February to May, and another, from November to December, with some small variations in the PF, where the number of flowering species exhibited a notorious increase from December to March; while in the QF, the first peak of flowering was observed in March and April, and the second, from November to January (Figure 4).
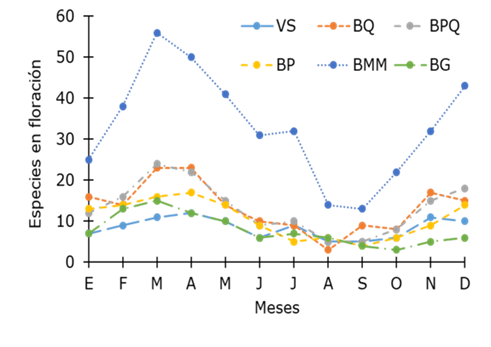
VS = Secondary vegetation; BQ = Quercus forest; BPQ = Pinus-Quercus forest; BP = Pinus forest; BMM = Mesophilic mountain forest; BG = Gallery forest. Especies en floración = Species in flowering; Meses = Months.
Figure 4 Flowering patterns by plant communities.
Fruiting by plant community is a little more heterogeneous in its behavior while tending to the fruiting pattern exhibited by all taxa. Greater richness of fruiting species was observed from February to July, with a decrease from August to October, and an increase in November and December (Figure 5).
Flowering and fruiting patterns in relation to environmental variables
Flowering patterns had a statistically significantly negative correlation with relative humidity (r = -0.91 p≤0.0001) and precipitation (r = -0.79 p≤0.0003). No significant relationship with temperature, evaporation, or insolation was observed (Table 2). Fruiting, without being significant, exhibited a strong negative correlation with relative humidity (r =-0.58 p≤0.0561) and also a decrease in the richness of fruiting species with increasing precipitation (Table 2).
Table 2 Correlations between the patterns of flowering and fruiting species richness; species richness by plant communities with environmental variables.
| Temperature | Evaporation | Precipitation | Relative humidity | Insolation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | |
| Flower | -0.40 | 0.194 | 0.16 | 0.628 | -0.79 | 0.0003 | -0.91 | ‹0.0001 | -0.06 | 0.8523 |
| Fruit | 0.15 | 0.636 | 0.37 | 0.242 | -0.41 | 0.187 | -0.58 | 0.0561 | 0.33 | 0.2889 |
| Flowering by plant community | ||||||||||
| SV | -0.34 | 0.277 | -0.01 | 0.984 | -0.72 | 0.0078 | -0.75 | 0.0046 | -0.16 | 0.6118 |
| QF | -0.52 | 0.084 | -0.03 | 0.933 | -0.81 | 0.0015 | -0.88 | 0.0002 | -0.31 | 0.3293 |
| PQF | -0.52 | 0.086 | -0.02 | 0.945 | -0.82 | 0.0010 | -0.90 | 0.0001 | -0.26 | 0.4189 |
| PF | -0.60 | 0.038 | -0.01 | 0.987 | -0.91 | ‹0.0001 | -0.97 | ‹0.0001 | -0.25 | 0.4357 |
| MMF | -0.36 | 0.247 | 0.14 | 0.655 | -0.74 | 0.0063 | -0.84 | 0.0006 | -0.04 | 0.9079 |
| GF | -0.40 | 0.204 | 0.30 | 0.346 | -0.55 | 0.0664 | -0.86 | 0.0004 | 0.10 | 0.7674 |
| Fruiting by plant community | ||||||||||
| SV | -0.17 | 0.598 | 0.05 | 0.868 | -0.05 | 0.8688 | -0.12 | 0.7100 | 0.26 | 0.4233 |
| QF | 0.01 | 0.970 | 0.23 | 0.474 | -0.19 | 0.5602 | -0.29 | 0.3566 | 0.36 | 0.2544 |
| PQF | -0.35 | 0.262 | -0.53 | 0.075 | -0.29 | 0.3545 | 0.06 | 0.8531 | -0.33 | 0.2973 |
| PF | -0.17 | 0.605 | -0.08 | 0.808 | -0.19 | 0.5630 | -0.07 | 0.8238 | 0.12 | 0.7052 |
| MMF | -0.12 | 0.713 | 0.36 | 0.256 | -0.47 | 0.1261 | -0.58 | 0.0469 | 0.31 | 0.3284 |
| GF | -0.37 | 0.231 | 0.20 | 0.527 | -0.69 | 0.0127 | -0.82 | 0.0012 | 0.08 | 0.8028 |
Flower = Richness of flowering species; Fruit = Richness of fruiting species; SV = Secondary vegetation; QF = Quercus forest; PQF = Pinus-Quercus forest; PF = Pinus forest; MMF = Mountain mesophilic forest; GF = Gallery forest; r = Correlation; p = p value.
Flowering patterns showed a significant negative correlation with relative humidity and precipitation in the plant communities, except the GF, where the richness of flowering species diminished, although the correlation was not significant (Table 2). Fruiting showed a significant negative correlation only in the GF with precipitation, and in the MMF and GF, with relative humidity (Table 2).
Flowering and fruiting patterns across the altitudinal gradients
The richness of flowering and fruiting species was greatest at the altitude range of 1 700 to 2 000 masl, with a very noticeable drop above 2 100 masl (Figure 6).
Discussion
Certain authors have documented (Williams and Meave, 2002; Solórzano et al., 2010) the limitations of the use of herbarium specimens in studies of reproductive phenology, including the absence of a record of the intensity of the phenophases and of the abundance of individuals in flowering or fruiting, or the fact that this is very limited and differentiated among species. In addition, abundant and continuous collections in an area over several years are required in order to obtain a good representation of the phenology of the species. Except for the first two limitations, the continuous collection of botanical material during more than 30 years at the ECLJ and the surrounding areas allows a good degree of confidence in the patterns of reproductive phenology determined at the community level and by vegetation type.
The 124 tree species recorded for the ECLJ represent 27 % of this biological form in the Sierra of Manantlán (Vázquez et al., 1995). The families with the most flowering or fruiting specimens corresponded to those with the greatest richness of tree species at the ECLJ (Cuevas et al., 2004; Cuevas et al., 2021).
The flowering pattern registered at the ECLJ, exhibiting a greater richness of flowering tree species during the dry season (Figures 2, 3a), is in line with what has been reported in seasonal humid tropical forests and in temperate forests (Janzen, 1967; Reich and Borchert, 1984; Wright and van Schaik, 1994; Moore, 2008; Ochoa et al., 2008; Cortés-Flores et al., 2015), as well as in mesophilic forests of Mexico (Hernández and Carreón, 1987; Solórzano et al., 2010). This pattern may be attributed to the fact that during this period the species are less photosynthetically active, after the rainy season, which allowed the accumulation of reserves (Singh and Kushwaha, 2006); furthermore, there is a lower incidence of insect pests during the dry season (Wright and van Schaik, 1994).
There is another set of factors that may exert an influence, some of which have been pointed out by other authors (Janzen, 1967; Moore, 2008); they include the abundance of certain groups of pollinators, mainly honeybees and bees; the absence or decrease in the number of leaves renders the flowers more visible to pollinators; the flowers do not fall, nor does their nectar concentration diminish, because of the rains. The importance of the insect group as pollinators appears to be supported, since, based on the available information (Cuevas et al., 2021), and according to the types of flowers, it is suggested that 74 % of the tree species in the area exhibit entomophilous pollination syndromes, while pollination is anemophilous in 21 %, and ornithophilous in 5 %.
Fruiting is at its richest from February to July, coinciding with most of the dry season and the beginning of the rainy season, with a noticeable decrease in the months with more precipitation (Figures 2, 3b) ―a previously registered pattern (Hamann, 2004; Cornejo and Ibarra, 2007; Solórzano et al., 2010). This has been explained as logical, in the sense that fruit production in the dry season and the onset of rainfall minimizes seed exposure to predators and provides time for diaspores to germinate and seedling establishment to occur more successfully, since it profits from the moisture available in the environment during the short rainy season, especially in the soil (Hamann, 2004; Singh and Kushwaha, 2006).
Species that exhibit their fruiting peaks at the end of the rainy season possibly have diaspores with dormancy periods, which should allow them to be viable until the next rainy season.
Given that there are 20 or more flowering and fruiting species in the months with the least richness (Figure 2) although the area has clear seasonal rainy and dry seasons, as well as a cold period, it is considered that the mountain area of western Mexico has a higher humidity in the environment than dry tropical regions, which generates conditions for the presence of species with reproductive structures throughout the year. This coincides with what has been reported in other studies for Mexico, where the predominant vegetation is mountain mesophilic forest (Hernández and Carreón, 1987; Solórzano et al., 2010).
It is important to consider that, in the plant communities of the area, the presence of organic matter in the soil should contribute to the maintenance of soil moisture, which generates conditions for the tree species to be evergreen or deciduous for short periods, with the existence of a certain degree of photosynthetic activity, and, therefore, flowering and fruiting species are observed throughout the year.
The rebound in the richness of flowering and fruiting species observed from October to December (Figure 2) could correspond to a decrease in precipitation starting in October (Figure 1). The decrease in the richness of flowering and fruiting species in January would be the result of a drop in temperature or a lower incidence of specimen collection in that month.
The richness of flowering and fruiting species was higher in the MMF (Figures 4, 5; Table 1), which was expected, given that this plant community has the largest wealth of tree species in the world (Cuevas et al., 2004; Cuevas et al., 2021). The pattern of flowering and fruiting is in agreement with the expectations and reflects the behavior of the reproductive phenology of the complete tree flora of the ECLJ. The reasons for this pattern would be the same as those previously indicated for all the tree species in the area.
Relative humidity and precipitation were the two environmental factors with the greatest influence on flowering, which appears to decrease in response to them (Table 2). The explanation for this is that these environmental factors exert an influence on both the flowers and the activity of pollinators (Janzen, 1967). Higher humidity and water entail a greater difficulty for the activity of pollinators, whose mobility they hinder; the flowers also become less rewarding, as the nectar is diluted and its nutritional quality decreases; furthermore, the number of flowers is reduced due to the impact of the drops of water, which increase their weight; there is also more cloudiness and, consequently, the daylight hours are fewer, which reduces the pollinators’ activities (Janzen, 1967).
The variables temperature, evaporation, and insolation do not seem to fluctuate much in the area, nor did not show a significant relationship with the richness of flowering, contrary to the evidence registered in other regions (Siegmund et al., 2016) (Table 2).
The richness of fruiting species exhibited a clear tendency to decrease with increasing relative humidity (p≤0.0561) and with the increase in precipitation, as indicated by the literature (Cornejo and Ibarra, 2007; Cortés-Flores et al., 2012); this tendency is attributed to the fact that the species maximize the time of moisture availability for the diaspores to germinate and for the seedling establishment to take place (Janzen, 1967).
The richness of flowering and fruiting species was higher at an altitude range of 1 700 to 2 000 masl, with a very noticeable decrease above 2 100 m, due to an area effect, as 67 % of the surface area of the ECLJ is located within the altitudinal range of 1 700-2 000 masl, and <1 %, above 2 200 masl (Figure 6).
Conclusions
The flowering and fruiting patterns of the tree species in the study area coincide with those registered for dry tropical forests, humid forests with a certain degree of seasonality in precipitation, and humid temperate forests in Mexico, as well as in other regions of Central America.
The most important seasonal condition to which the reproductive phenology of the trees of the LJCS seems to respond is determined by relative humidity and precipitation; however, there are always species with flowers or fruit all year round.
In the study area, environmental factors such as temperature, insolation, and evaporation do not vary much throughout the year, and their effects on the reproductive phenology patterns of tree species do not seem to have much influence.
For a better understanding of the phenological patterns of plant species in the ECLJ, it is necessary to analyze the reproductive phenology of shrubs and herbaceous plants, including in the analysis the pollination and dispersal syndromes of the species.
Acknowledgments
The authors are grateful to the staff of the ECLJ for their help in the fieldwork and for providing the climatological information for the area. The project received financial resources from the University of Guadalajara, through the PRO-SNI and Strengthening of Research Institutes, Centers and Laboratories programs; from the National Council for Science and Technology (Conacyt), through the National System of Researchers, and from the Ministry of Public Education, through the PIFI and PROFEXCE programs and support for the consolidation of Academic Bodies.
REFERENCES
Acevedo, J. A. A. y G. M. V. Hernández. 2013. Fenología de ambientes tropicales en el marco de la teledetección. GeoFocus 13 (2): 195-211. http://www.geofocus.org/index.php/geofocus/article/view/294 (14 de abril de 2021). [ Links ]
Borcher, R. 1996. Phenology and flowering periodicity of Neotropical dry forest species: evidence from herbarium collections. Journal of Tropical Ecology 12 (1): 65-80. Doi: https://doi.org/10.1017/S0266467400009317. [ Links ]
Brennan, K. 1996. Flowering and fruiting phenology of native plants in the Alligator Rivers Region with particular reference to the Ranger uranium lease area. Supervising Scientist Report 107. Barton, Canberra, Australia. 40 p. [ Links ]
Cornejo T., G. and G. Ibarra M. 2007. Plant reproduction phenology in a temperate forest of the monarch butterfly biosphere reserve, Mexico. Interciencia 32 (7): 445-452. http://www.redalyc.org/pdf/339/33932704.pdf (10 de mayo de 2021). [ Links ]
Cortés-Flores, J., E. Andresen, G. Cornejo-Tenorio and G. Ibarra-Manríquez. 2012. Fruiting phenology of seed dispersal syndromes in a Mexican Neotropical temperate forest. Forest Ecology Management 289: 445-454. Doi:https://doi.org/10.1016/j.foreco. 2012.10.038. [ Links ]
Cortés-Flores, J. , G. Cornejo-Tenorio and G. Ibarra-Manríquez. 2015. Flowering phenology and pollination syndromes in species with different growth forms in a Neotropical temperate forest of Mexico. Botany 93: 1-7. Doi: https://doi.org/10.1139/cjb-2014-0218. [ Links ]
Cortés-Flores, J. , G. Cornejo-Tenorio, L. A. Urrea-Galeano, E. Andresen, A. González-Rodríguez and G. Ibarra-Manríquez. 2019. Phylogeny, fruit traits, and ecological correlates of fruiting phenology in a Neotropical dry forest. Oecologia 189 (1): 159-169. Doi:https://doi.org/10.1007/s00442-018-4295-z. [ Links ]
Cuevas G., R., S. Koch, E. García M., N. M. Núñez L. y E. J. Jardel P. 2004. Flora vascular de la Estación Científica las Joyas. In: Cuevas G., R. y E. J. Jardel P. (eds.). Flora y vegetación de la Estación Científica Las Joyas. Universidad de Guadalajara. Autlán de Navarro, Jal., México. pp. 119-176. [ Links ]
Cuevas, G., N. M. Nuñez L. y E. V. Sánchez R. 2021. Flora arbórea de la Estación Científica Las Joyas y áreas adyacentes en la Sierra de Manantlán, México. Universidad de Guadalajara. Autlán de Navarro, Jal., México. 456 p. [ Links ]
Di Rienzo, J. A., F. Casanoves, M. G. Balzarini, L. Gonzalez, M. Tablada y C. W. Robledo. 2019. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. http://www.infostat.com.ar (18 de abril de 2021). [ Links ]
Diez, J. M., I. Ibáñez, A. J. Miller R., S. J. Mazer, T. M. Crimmins, M. A. Crimmins, C. D. Bertelsen and D. W. Inouye. 2012. Forecasting phenology: from species variability to community patterns. Ecology Letters 15 (6): 545-553. Doi:https://doi.org/10.1111/j.1461-0248.2012.01765.x. [ Links ]
Elzinga, J. A., A. Atlan, A. Biere, L. Gigord, A. E. Weis and G. Bernasconi 2007. Time after time: flowering phenology and biotic interactions. Trends in Ecology and Evolution 22 (8): 432-439. Doi:https://doi.org/10.1016/j.tree.2007.05.006. [ Links ]
González E., A. R., I. De la Cruz C., M. Castro M. y C. A. Riley S. 2016. Estrategias fenológicas de especies de Annona en una selva baja caducifolia de Chiapas, México. Botanical Sciences 94 (3): 531-541. Doi: https://doi.org/10.17129/botsci.645. [ Links ]
Hamann, A. 2004. Flowering and fruiting phenology of a Philippine submontane rain forest:climatic factors as proximate and ultimate causes. Journal of Ecology 92 (1): 24-31. Doi: https://doi.org/10.1111/j.1365-2745.2004.00845.x. [ Links ]
Hernández, H. M. y Y. Carreón A. 1987. Notas sobre la ecología reproductiva de árboles en un bosque mesófilo de montaña en Michoacán, México. Botanical Sciences (47): 25-35. Doi:https://doi.org/10.17129/botsci.1329. [ Links ]
Janzen, D. H. 1967. Synchronization of sexual reproduction of trees within the dry season in Central America. Evolution 21: 620-637. Doi:https://doi.org/10.1111/j.1558-5646.1967.tb03416.x. [ Links ]
Jardel P., E. J., E. Ezcurra, R. Cuevas G., A. L. Santiago P. y P. Cruz C. 2004. Vegetación y patrones de paisaje. In: Cuevas G., R. y E. J. Jardel P. (eds.). Flora y vegetación de la Estación Científica Las Joyas. Universidad de Guadalajara. Autlán de Navarro, Jal., México. pp. 119-176. [ Links ]
Moore, P. D. 2008. Tropical forest. Fact on File, Inc. Nueva York, NY, USA. 245 p. [ Links ]
Newstom, L. E., G. W. Frankie and H. G. Baker. 1994. A new classification for plant phenology base on flowering patterns in lowland tropical rain forest trees at La Selva, Costa Rica. Biotropica 26 (2): 141-159. Doi: https://doi.org/10.2307/2388804. [ Links ]
Ochoa G., S., I. Pérez H. y B. H. J. de Jong. 2008. Fenología reproductiva de las especies arbóreas del bosque tropical de Tenosique, Tabasco, México. Revista de Biología Tropical 56 (2): 657-673. Doi: https://doi.org/10.15517/rbt.v56i2.5615. [ Links ]
Reich, P. B. and R. Borchert. 1984. Water stress and tree phenology in a tropical dry forest in the lowlands of Costa Rica. Journal of Ecology 72 (1): 61-74. Doi:https://doi.org/10.2307/2260006. [ Links ]
Siegmund, J., M. Wiedermann, J. Donges and R. Donner. 2016. Impact of temperature and precipitation extremes on the flowering date of four German wildlife shrub species. Biogeosciences 13: 5541-5555. Doi: https://doi.org/10.51914/bg-5541-2016. [ Links ]
Singh, K. P. and C. P. Kushwaha. 2006. Diversity of flowering and fruiting phenology of trees in a tropical deciduous forest in India. Annals of Botany 97: 265-276. Doi:https://doi.org/10.1093/aob/mcj028. [ Links ]
Smythe, N. 1970. Relationships between fruiting seasons and seed dispersal methods in a neotropical forest. The American Naturalist 104 (935): 25-35. Doi:https://doi.org/10.1086/282638. [ Links ]
Solórzano, S., L. Ávila, S. Castillo, J. A. Meave y G. Ibarra M. 2010. Fenología de los árboles del bosque mesófilo de la Reserva de la Biosfera El Triunfo, Chiapas. In: Pérez, F., M. A., C. Tejeda C. y E Silva R. (eds.). Los bosques mesófilos de montaña en Chiapas: situación actual, diversidad y conservación. Universidad de Ciencias y Artes de Chiapas. Tuxtla Gutiérrez, Chis., México. pp. 121-160. [ Links ]
Tooke, F. and N. H. Battey. 2010. Temperate flowering phenology. Journal of Experimental Botany 61 (11): 2853-2862. Doi:https://doi.org/10.1093/jxb/erg165. [ Links ]
Topete C., C., R. Cuevas G., E. V. Sánchez R., A. Moreno H., J. G. Morales A. y N. M. Núñez L. 2020. Estructura poblacional y hábitat de un árbol tropical con frutos comestibles, Annona purpurea (Annonaceae), en el occidente de México. Revista de Biología Tropical 68 (4): 1171-1184. Doi:https://doi.org/10.15517/rbt.v68i4.42195. [ Links ]
van Schaik, C. P., J. W. Terborgh and S. J. Wright. 1993. The phenology of tropical forests: adaptive significance and consequences for primary consumers. Annual Review of Ecology and Systematics 24: 353-377. Doi:https://doi.org/10.1146/annurev.es.24.110193.002033. [ Links ]
Vázquez G., J. A., R. Cuevas G., T. S. Cochrane, H. H. Iltis, F. J. Santana M. y L. Guzmán H. 1995. Flora de Manantlán. Botanical Research Institute of Texas. Forth Worth, TX, USA. 312 p. [ Links ]
Williams L., G. y J. Meave. 2002. Patrones fenológicos. In: Guariguata, M. y G. Kattan (eds.). Ecología y conservación de bosques neotropicales. Editorial Libro Universitario Regional. San José, Costa Rica. pp. 407-431. [ Links ]
Wright, S. J. and C. P. van Schaik. 1994. Light and the phenology of tropical trees. American Naturalist 143: 192-199. Doi:https://doi.org/10.1086/285600. [ Links ]
Zimmerman, J. K., S. J. Wright, O. Calderón y M. A. Pagan. 2007. Flowering and fruiting phenologist of seasonal and aseasonal neotropical forest: the role of annual changes in irradiance. Journal of Tropical Ecology 23 (2): 231-251. Doi:https://doi.org/10.1017/s0266467406003890. [ Links ]
Received: June 10, 2021; Accepted: November 12, 2021











 texto en
texto en