Introduction
Cell culture started at the beginning of the 20th century as a method to study the behavior of animal cells outside of the systemic variations that can occur in vivo. Therefore, cell culture can be defined as acquiring animal cells and their propagation in vitro1. To preserve most of their physiological, biochemical, and genetic properties in an artificial environment, freezing, thawing, seeding or trypsinization techniques are necessary to allow the maintenance, survival, and multiplication of cells of specific organs2,3.
Cell cultures are used in basic and applied research and can be classified into three types: monolayer, suspension, and three-dimensional4,5. Usually, cell cultures are worked in a completely sterile environment, avoiding contaminations6. In addition, the same sterile conditions must be maintained with all the reagents that are involved with the culture medium (e.g. FBS, antimycotics, among others)7,8.
FBS is the main culture media supplement since it provides more than 1,000 nutritional components for cells. These include amino acids, proteins, vitamins (particularly fat-soluble vitamins such as A, D, E, and K), carbohydrates, lipids, hormones, growth factors, minerals, and trace elements9. In addition, serum buffers inactivate the culture medium proteolytic enzymes, increase the average viscosity, and maintain the conditions for the growth surface of the culture container10,11.
The increased use of FBS for research, diagnostics, and pharmaceutical manufacturing have made it a global business that represents a significant economic impact (exp. 17,724.63 Mexican pesos for a unit of 500 mL SIGMA® brand). The global availability and demand may create opportunities for the production of the reagent12. Mexico has 35 million heads of livestock, of which approximately 13 million are raised on a free grazing basis, giving the opportunity that one out of every eight cows that are sent to the slaughterhouse arrives pregnant13-15. In this context, Mexican serum production can be suitable for producing this essential component for cell cultures16,17. Because sometimes there are problems with the acquisition of the serum due to border closure or sanitary regulation problem that prevents the importation of potentially contaminated reagents. The purpose of this project was, as a first stage, to characterize FBS obtained from the meat industry in Mexico and compare it with a commercial serum according to the tests requested by the International Serum Industry Association (ISIA) and evaluate its suitability for its use in cell culture.
Material and methods
This in vitro study consists of obtaining FBS for cell culture using the parameters of a commercial serum as a control. The FBS characterization included microfiltration, pH levels, osmolarity, total protein concentration, presence/absence of Mycoplasma sp., cell proliferation, and DNA concentration. A commercial FBS was used as a control (F2442, Fetal Bovine Serum, Mammalian and insect Cell Culture Tested, 17L436 from SIGMA®). Following the guidelines of the ethics regulation for the use of animals in teaching and research at the Autonomous University of Aguascalientes (CEADI-UAA) and the Official Mexican Standard NOM-024-ZOO-1995, "Specifications and zoosanitary characteristics for the transport of animals, their products and by-products, chemical, pharmaceutical, biological and food products for use in or consumption by animals”.
Obtaining bovine blood and separation of serum from fetal bovine blood
One lot of serum was collected from FREASA (Frigorífico y Empacadora de Aguascalientes), by trained personnel. Later, in freezing conditions -20 °C, it was transferred to the laboratory processing. Blood was obtained by cardiac puncture technique18, and it was collected in sterile 30 ml Falcon® tubes and a 500 ml blood containment bag with anticoagulant (500 ml ACD BLORECEP bag with 2.20 g trisodium citrate, 0.80 g citric acid, 2.45 g dextrose, and pyrogen-free H2O).
The blood was centrifuged at 3,000 rpm for 5 min19,20. Subsequently, the serum was extracted and placed in 30 ml Falcon® tubes. Finally, serum aliquots were made in tubes, and they were stored at -20 °C until use. When performing the blood component separation process, two samples were obtained: serum (centrifugation of blood sample without anticoagulant) and plasma (centrifugation of blood sample with anticoagulant). In short, the difference between both is the presence of the proteins responsible for coagulation processes21,22. Based on the samples collected, the experimental groups were as follows: Control group was commercial FBS (C-FBS), experimental groups were serum obtained from the meat industry (E-FBS), and plasma obtained from the meat industry (E-Plasma).
Filtration of the FBS obtained for the elimination of cellular components
The elimination of the cellular components was carried out with the microfiltration method. For this, 0.2 µm syringe filters were used. The same filtration process was applied to all the groups [C-FBS (F1), E-FBS (F1), and E-Plasma (F1)], and all the samples were placed in new sterile 2 ml tubes.
Evaluation of sterility with microbiological tests
Soy broth (BD Bioxon®, Becton Dickinson de Mexico) was used for sterility assay. The instructions according to the manufacturer were followed. The sterility test consisted in preparing six tubes with soy broth, to which 200 µl of unfiltered C-FBS, E-FBS, and E-Plasma, an additional positive control (human saliva) was used. Once the tubes were prepared, they were placed in the bacteriological oven at 37 °C for 24 h.
Assessment of the presence/absence of Mycoplasma sp.
The Mycoplasma sp. presence/absence test was performed on selective growth with Mycoplasma sp. agar (MO660-500G from SIGMA). 100 ml of the medium was prepared for 15 small Petri dishes. Similarly, seven boxes with sterile swabs were seeded following the labeling, with the difference that the positive control was a scraping of the facial epidermis. Once the boxes were prepared, they were placed in the bacteriological oven at 37 °C for 24 h.
Evaluation of pH
The pH test was carried out with reagent strips (Hydrion® 9400, Plastic pH Indicators Strips, pH range 5.0-9.0). It is based on a colorimetric scale. The same groups previously described were evaluated; additionally, the FBS groups were evaluated with culture media dilution.
Preparation of dilution samples for total protein concentration and DNA concentration measurements
For the presence of DNA components in the FBS, the samples dilute at a final concentration of 1,030 µg/mL, which was a dilution factor (DF) of 32, which means that the FBS concentration was diluted 32 times. To obtain the DF, the following calculation was performed: DF= CI / CF; where: DF= dilution factor, CI= initial concentration, CF= final concentration. With the DF, it was proceeded to process the corresponding test samples.
Total protein concentration evaluation
Total protein measurement was based on Pierce's colorimetric method, and the PierceTM BCA Protein Assay Kit (ref. 23227) from Thermoscientific® was used. Following the protocol proposed by the manufacturer, the working reagent, the samples, and the calibration curve were prepared. Briefly, 25 µL of each standard or unknown was pipetted per replica (three replicates) into each well of the plate. Later, 200 µL of the working reagent was added and incubated at 37 °C for 2 h. Finally, readings were carried out at 620 nm, in the 96-well plate reader Multiskan FC (SN 357-914771) from Thermoscientific®.
DNA concentration evaluation
To quantify the DNA concentration with Nanodrop, the Phenol-Chloroform extraction method was carried out, which is divided into three phases:
1. Cell lysis: where the sample was centrifuged for 5 min at 270 xg, and then the supernatant was discarded. The pellet is resuspended in 100 µL of PBS. The sample was incubated at -80 °C for 30 min and transferred to the sonicator at 20 kilohertz and applied in two batches of 10 sec to break the cells. Once the lysis was done, the samples were centrifuged at 10,000 xg for 20 min. Finally, the supernatant was moved to a new sterile Eppendorf® tube to continue with the second phase.
2. Phase separation: 250 µL of Phenol-chloroform was added to the obtained supernatant and was passed through a continuous vortex process. They were centrifuged at 10,000 xg for 5 min to finally observe a separation of two phases, from which, the upper part was taken, and proceeded to next phase.
3. DNA purification: 200 µL of chloroform were added to the samples and were centrifuged at 10,000 xg for 10 min to obtain a supernatant and transferred to a new tube. Then, 1/10 volume of 3 molar sodium acetate and two volumes of 100% ethanol were added to the supernatants. Subsequently, they were left to precipitate overnight at -80 °C. After finishing, the samples were centrifugate at 10,000 xg for 30 min at 4 °C, the supernatant was extracted, and 100 µL of ethanol at 70% was added to the remaining content in the tube, and was centrifuged for 10 min at 10,000 xg (the 2 previous steps of 70 % ethanol and centrifugation were repeated 2 to 3 times to clean the genetic material). Finally, the largest possible supernatant was removed and the remaining ethanol was allowed to evaporate. Genetic material was resuspended in 100 µL of Milli-Q grade water and nucleic acid readings were performed on the Thermoscientific Nanodrop 2000 spectrophotometer.
Osmolarity assessment
Osmolarity was measured on a Model 5004 Automatic Osmometer. Briefly, the protocol consisted of connecting and calibrating the equipment, selecting the reading range to be measured, placing 100 µL of the sample in an Eppendorf® tube, and reading.
Evaluation of cell proliferation
For the cell proliferation test, a low glucose DMEM culture medium with L-Glutamine and Sodium Pyruvate (Biowest®) was used. In addition, the antibiotic/antifungal solution of Sigma® (A5955) was added, and finally, it was supplemented with the commercial FBS (the control serum) Sigma® (F2442). Three types of media were prepared for this evaluation, commercial serum (C-FBS), serum obtained from the meat industry (E-FBS), and the last with plasma (E-Plasma).
The cell viability test was carried out with the Abcam® brand MTT Assay Kit (Cell Proliferation) method. The experiment was performed on 96-well plates and read on the Thermoscientific® Multiskan FC 96-well plate reader (SN 357-914771) at 620 nm. The hFOB 1.19 ATCC line of osteoblasts was used to measure cell viability, osteoblasts are a type of cell without specific requirements for growth and proliferation, although standard conditions for cell culture were performed in this case: 37 °C in an atmosphere of 5% CO2, 95 % air9. Measurements at 3, 7, 14, and 21 d were performed to observe how the cells were growing in the plate from an initial seeding of 1,000 cells.
Statistical analysis
For the statistical analysis, means, medians, and standard deviation were calculated, the normality distribution of data was evaluated with Q-Q plot, and homogeneity of variance with Levene’s test, if the statistical assumption were made a two-tailed or one-way ANOVA test was performed with the statistical program R version 4.0.3. To evaluate the proliferation cell kinetic, a Likelihood ratio (LR) test was used to compare the kinetic curves, a confidence level of 95 % was considered.
Results
A general characterization was performed on E-FBS to verify the serum status. Table 1 shows the results obtained. The sample was taken from a fetus; then some parameters may be outside the reference limits established for a fully grown organism.
Table 1 Characteristics of the E-FBS obtained from the meat industry
| Analyte | Value | Reference value |
|---|---|---|
| Color | Ambar | Ambar |
| Glucose, mg. dL | 37 | 80-120 mg. dL |
| Creatinine, mg. dL | 2.73 | 1.2-1.9 mg. dL |
| Uric acid, mg. dL | 2.0 | 1.21-3.47 mg. dL |
| Phosphorus, mg. dL | 10.5 | 2.5-5.0 mg. dL |
| Calcium, mg. dL | 16 | 12.0-14.0 mg. dL |
| Bilirubin, mg. dL | 0.8 | 0.2-0.5 mg. dL |
| TGP, U/L | 8 | 11-40 U/L |
| ALP, U/L | 280 | 86-285 U/L |
| Total proteins, g. dL | 3.81 | 6.0-8.0 g. dL |
| Albumin, g. dL | 2.63 | 2.5-3.5 g. dL |
| Serum iron, Ug. dL | 169 | 37-170 Ug. dL |
| Serum amylase, U/L | 49 | 30-110 U/L |
| Globulin, g. dL | 1.18 | 2.5-4.5 g. dL |
| Atherogenic index | 3.6 | 0-5 |
Summary of the FBS properties obtained from the meat industry. The test was carried out by an external company and served as support for the results obtained. TGP= alanin-aminotrasnferase; ALP= alkaline phosphatase.
Evaluation of sterility with microbiological tests
For microbiological tests, two measurements were made. One at the time the serum was obtained and 1 mo after stored at -20 °C. Figure 1A shows the absence of microbiological growth (translucent) in the experimental groups after 24 h at 37 °C incubation period. The same negative result was obtained after one month of storage, in the saliva group turbid appearance characteristic of microbiological growth can be observed. The Mycoplasma sp. evaluation is shown in Figure 1B (E-FBS), Figure 1C (E-Plasma), and Figure 1D (C-FBS), since from the previous evaluation the presence of Mycoplasma sp. Is not found in the agar, except for the skin group (Figure 1E).
DNA concentration
According to the data obtained (Table 2), in the DNA concentration, there were significant differences between groups and the filtered or non-filtered factor (P<0.05). In the filtering section, there are statistical differences between groups. Nevertheless, in the non-filtered area, there are no differences between the E-Plasma and C-FBS groups, but statistically significant differences were observed between the E-Plasma/E-FBS and E-FBS/C-FBS groups.
Table 2 Summary of statistical data
| Variable evaluation | Exper. Groups | Mean ± SD | ANOVA P-value | 1 vs 1 comparison | Cohen d | Tukey P-value |
|---|---|---|---|---|---|---|
| DNA Concentration (ng/µL) |
C-FBS | 9.46 ± 0.358 | <0.00001 | C-FBS vs E-FBS | 1.3400 | 0.21777 |
| E-FBS | 2.88 ± 0.303 | |||||
| E-Plasma | 9.60 ± 0.860 | E-FBS vs E-Plasma | 3.0400 | 0.00434 | ||
| C-FBS(F1) | 6.16 ± 0.167 | |||||
| E-FBS(F1) | 7.50 ± 2.04 | E-Plasma vs C-FBS | -1.7000 | 0.10154 | ||
| E-Plasma (F1) | 4.46 ± 0.288 | |||||
| Total protein concentration (g/dL) |
C-FBS | 0.744 ± 0.0322 | <0.001 | C-FBS vs E-FBS | -0.06688 | 0.00172 |
| E-FBS | 0.677 ± 0.0238 | E-FBS vs E-Plasma | -0.00390 | 0.96217 | ||
| E-Plasma | 0.674 ± 0.00494 | E-Plasma vs C-FBS | -0.07078 | 0.00108 | ||
| C-FBS(F1) | 0.666 ± 0.0324 | <0.001 | C-FBS(F1) vs E-FBS(F1) | -0.06418 | 0.00414 | |
| E-FBS(F1) | 0.602 ± 0.0278 | E-FBS(F1) vs E-Plasma (F1) | 0.06956 | 0.00244 | ||
| E-Plasma (F1) | 0.671 ± 0.00729 | E-Plasma (F1) vs C-FBS(F1) | 0.00538 | 0.93867 | ||
| Osmolarity (mOsm/Kg H2O) |
C-FBS | 253 ± 14.7 | 0.48 | C-FBS vs E-FBS | N/A | N/A |
| E-FBS | 242 ± 6.88 | |||||
| E-Plasma | 252 ± 9.92 | E-FBS vs E-Plasma | N/A | N/A | ||
| C-FBS(F1) | 245 ± 2.07 | |||||
| E-FBS(F1) | 246 ± 3.91 | E-Plasma vs C-FBS | N/A | N/A | ||
| E-Plasma (F1) | 257 ± 26.5 | |||||
| Cell viability (%) |
C-FBS | 0.131 ± 0.00829 | 0.08 | C-FBS vs E-FBS | N/A | N/A |
| E-FBS | 0.124 ± 0.00593 | E-FBS vs E-Plasma | N/A | N/A | ||
| E-Plasma | 0.121 ± 0.00439 | E-Plasma vs C-FBS | N/A | N/A |
All the comparisons were made with independent methods; One-way or two-way ANOVA analysis was applied independently for each variable according to the relationship of the factors: filtered or unfiltered and the experimental groups. The 1vs1 comparisons were made with the posthoc Tukey method. C-FBS: Control-FBS; E-FBS: Experimental-FBS; E-Plasma: Experimental-Plasma sd; Standard deviation. Significance: P-value<0.05.
Total protein concentration
Table 2 shows how the total protein concentration presents significant differences between the groups (P<0.05). In the filtered section, differences between C-FBS/E-FBS and E-Plasma/C-FBS can be seen. However, in the non-filtered area, no differences can be observed between the E-Plasma/E-FBS groups, but they are observed between E-Plasma/C-FBS and E-FBS/C-FBS groups.
pH evaluation
The pH obtained from the groups before filtering was 7.0 (C-FBS and E-FBS) except E-Plasma, which presented a pH of 6.0. The pH of the 3 groups after filtering was 7.0.
Osmolarity
The obtained osmolarity values were similar between the three experimental groups regardless of the filtration factor (Table 2). Therefore, no significant differences were seen at P>0.05.
Cell viability
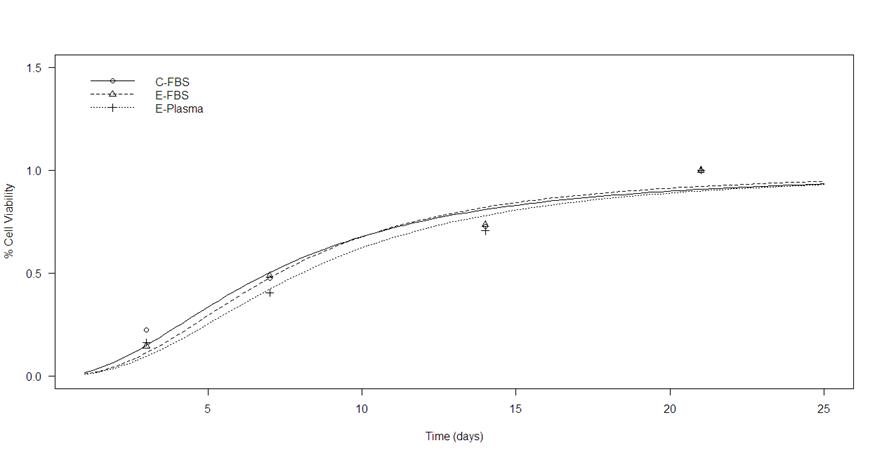
Cell growth among the experimental groups was evaluated from the percentage of cell viability through time (Figure 2). The comparison was made using a Likelihood Ratio Test with a value of LR=0.031124 and P=0.999. No significant differences were observed between groups.
Discussion
The overall aim of the article was to the FBS characterization obtained from the meat industry for use in cell culture and to compare it with a commercialized FBS. As is already known, Mexican livestock is suitable for obtaining and producing this reagent because Mexico has been free of the main quarantine diseases since its appointment in 2016 to date17,23.
To obtain the FBS, the blood to produce this reagent must be from fetuses, and it is obtained by cardiac puncture to avoid risks of contamination and must be taken by highly trained personnel18. The main reason for obtaining the sample from that gestational stage is because the fetus is protected by the “placental barrier”, which is a natural protection that defends the developing organism from any infection24. Another aspect that helps the defense of the fetus is that at the time of fertilization and the ovum reaches the stage of implantation in the womb, most of the signaling processes to be carried out are inflammation, which means that immune cells are always present at all times, increasing defenses against contaminants, confirming that obtaining serum from fetuses for cell culture is indeed the best option25.
One of the limitations of the present study was the tests chosen for this initial stage of serum characterization. There were based on the Certificate of Analysis Guidance (CoA) described for ISIA26, however, the virus testing (Cytopathic, hemadsorbing, and bovine viral diarrhea virus) IgG and GGT were not performed at this stage because the main objective was the performance testing.
The osmolarity test measures the concentration of nutrient solutes in the reagent. Maintaining adequate levels (within standards) is of vital importance since presenting adequate levels allows the cells of the cell culture to grow quickly and avoid morphological malformations due to a lack of nutrients. Ryan JM.27 used different concentrations of FBS to verify the useful life of cultured chicken cells (5%, 10%, 20%, and 30%), and Kwon, et al28, verified the effect of the concentration of FBS on the efficiency of cell reprogramming for the generation of pluripotent stem cells (5%, 10%, 20%, and 30%), with the result that a good concentration of FBS allows for good proliferation and maintenance performance of the cells of interest. For this reason, it is pertinent to have a serum with its established concentration (260 - 340 mOsm / Kg H2O) since a low or no FBS supplementation would jeopardize the cells.
Focusing on the final cell viability test, the three groups at 21 d of growth maintain the same viability percentage. In this context, it can be assumed that plasma could be used to supplement culture media. Although, it may not be correct in all cases because of the presence of fibrinogen. Fibrinogen is a zymogen, which is an inactive enzyme precursor that participates mainly in the coagulation cascade. Its primary function is the formation of fibrin for the creation of the clot that "covers" the injured blood vessel. It is also known as a proenzyme that does not require a protein activator, but simply with a biochemical change in the environment, can be activated29,30. Fibrinogen not only has this function, it is also involved in processes such as platelet distribution, adhesion and signaling, the proliferation of fibroblasts and endothelial cells, healing, and inflammatory response; In addition, it is capable of binding to proteins such as fibronectin (facilitating its incorporation into the extracellular matrix), growth factors for fibroblasts (FDF-2, β-FGF) and vascular endothelium (VEDF) that stimulate angiogenesis, and interleukin-1β that intervenes in inflammation, therefore, it is a proenzyme with a wide field of action24,25. That is why serum is still the best option to supplement culture media compared to plasma.
Finally, significant differences were found in some of the characterization tests carried out but not in the viability test. However, none of these data has a significant effect when supplementing the culture medium with serum obtained from the meat industry (E-FBS). Therefore, although the project is only focused on the first stage of characterization, it can be suggested the serum obtained can be used to supplement cell cultures. It is intended to carry out a second stage of the project in which the missing characterization tests are carried out, among which are the measurement of endotoxins, hemoglobin, hormones, and vitamins, additionally, the evaluation in different cell lines it is necessary to verify the performance of the E-FBS.
Conclusion and implications
According to the results, it can be concluded that the serum obtained from the meat industry did not show significant differences in the aspects of cell maintenance and proliferation compared to the commercial serum. Although plasma is not the commonly used supplement for cell cultures, if FBS is not available, plasma could be used in emergency cases as a substitute to maintain certain cell cultures. Although the FBS obtained from the meat industry has not undergone all the requested standard tests, since it is in the first stage of characterization, it can be suggested that the serum produced with Mexican livestock can be used to supplement cell cultures.











 texto en
texto en