Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.6 spe 12 Texcoco nov./dic. 2015
Articles
Selenium and its effect on antioxidant status and mineral composition of lettuce
1Departamento de Horticultura, Universidad Autónoma Agraria Antonio Narro, Buenavista, Saltillo, Coahuila. C. P. 25315 México.
2Departamento de Materiales Avanzados, Centro de Investigación en Química Aplicada, Blvd. Enrique Reyna 140, Saltillo, Coahuila. C. P. 25294.
Selenium is an element that does not appear on the lists of essential elements for plants and it is not considered in soil analysis, water and plant tissues. Different reports indicate that it seems to be associated with changes in cellular redox state. The aim of this study was to apply selenium in lettuce (Lactuca sativa) to assess the effect on growth, composition and antioxidant metabolism. The experiment consisted on the application of sodium selenite as foliar in concentrations of 0, 5 and 10 mg L-1 at 15, 30 and 45 days after transplantation. In plants the redox potential, biomass, mineral concentration and leaf catalase activity was determined. The application of selenium was effective in changing the redox potential and increase catalase activity without causing changes in mineral content and biomass.
Keywords: catalase; lettuce; minerals; sodium selenite
El selenio es un elemento que no aparece en los listados de elementos esenciales para las plantas y no se considera en los análisis de suelos, aguas y tejidos vegetales. Diferentes reportes indican que el Se parece asociarse con cambios en el estado redox celular. El objetivo de este estudio fue realizar aplicaciones de selenio en plantas de lechuga (Lactuca sativa) para verificar el efecto sobre el crecimiento, composición y metabolismo antioxidante. El experimento consistió en la aplicación de selenio de sodio en forma foliar en concentraciones de 0, 5 y 10 mg L-1 a los 15, 30 y 45 días después del trasplante. En las plantas se determinó el potencial de óxido-reducción, la biomasa, la concentración de minerales y la actividad catalasa foliar. La aplicación de selenio fue efectiva para modificar el potencial de óxido-reducción y elevar la actividad catalasa sin causar modificaciones en el contenido de minerales y la biomasa.
Palabras clave: catalasa; lechuga; minerales; selenito de sodio
Introduction
Selenium is an essential element for humans but qualified as non-essential for plants. However, the presence of selenium in the substrate or applied by foliar spraying, plants accumulate it in their tissues, working as primary source of this element in the diet (Broadley et al., 2006). In most soils worldwide selenium concentration is low, from 0.01 to 2 mg kg-1 with an average of 0.4 mg kg-1, but can be found in soils called seleníferous with concentrations of up to 1200 mg kg-1 of It (Fordyce, 2005). It is considered that selenium is essential in humans for its role as cofactor of enzymes related with antioxidant metabolism (Rayman, 2008). In plants selenium has a positive effect on the antioxidant capacity, acting more effectively as selenite than selenate (Cartes et al., 2005). In Mexico studies on the relationship between selenium and nutritional quality of food in terms of its antioxidant capacity are few and therefore the aim of this study was to document the effect of selenium applications on lettuce, checking plant growth, mineral composition of leaves and antioxidant capacity of foliar cell extracts. This under the assumption that the application of this element will modify the cellular redox balance enhancing the antioxidant capacity of plant tissues.
Materials and methods
This research was carried out in the Universidad Autonoma Agraria Antonio Narro (UAAAN) in Saltillo, Coahuila, Mexico, under greenhouse conditions, with an average temperature and relative humidity of 17.8 °C and 68% respectively. Lettuce seeds (Lactuca sativa) variety Great Lakes were used, these were placed in germinating trays 200 cavities, using as substrate peat moss and perlite for germination. After 40 days after sowing, seedlings were transplanted in 20 L polyethylene pots using as substrate a mixture of peat moss and perlite with 70:30 V:V. Treatments consisted of foliar application of selenium in concentrations of 0, 5 and 10 mg L-1 at 15, 30 and 45 days after transplantation. Sodium selenite (Na2SeO3, Sigma Aldrich, USA) was used as selenium source without mixing with adjutants.
Leaves from three plants at physiological maturity per treatment at 20 and 35 days after transplanting (DAT) were collected, these were ground, obtaining a fresh leaf extract where the redox potential (ORP) was determined ( mV) using a potentiometer HI98185-01 (HANNA, Inc., USA) using the technique described by Benavides-Mendoza et al. (2002). Catalase activity (EC 1.11.1.6) was also quantified in leaves with physiological maturity at 35 days after transplanting (DAT) following Ramos et al. (2010) technique.
A UV-Vis Biomate 5 (Thermo Electron, USA) spectrophotometer at 590 nm was used and the results were expressed in mM consumption of H2O2 min-1 total-1 protein (mg/g). Fresh weight of aerial parts and root was determined at 50 DAT taking three full plants per treatment. These were carefully washed and root was separated from stem and leaves at crown height. Weights were measured with an Adventurer Pro (OHAUS, Inc., USA) scale. Then the same samples were placed in a Robertshawa dehydrator oven at 60 °C for 72 h to weight them on an analytical scale Pioneer (OHAUS, Inc., USA). The dried samples were determined mineral concentration in leaves.
For total nitrogen the micro Kjelhdal (AOAC, 1980a) method was used, while for phosphorus used a spectrophotometric (AOAC, 1980b) method, K, Ca, Mg, Na, Fe, Mn, Cu and Zn were measured through an atomic absorption spectrophotometer Varian AA-1275 following Fick et al. (1976) technique. To determine selenium first digesting of 0.5 g of dry sample in concentrated nitric acid until all the organic part using a spectrophotometer of plasma atomic emission IRIS ADVANTAGE (Thermo Jarrell, USA). The statistical design was randomized complete block with three replications. Data analysis consisted of ANOVA and Tukey tests and was carried out with SAS version 6.0 (SAS Institute, 2001).
Results and discussion
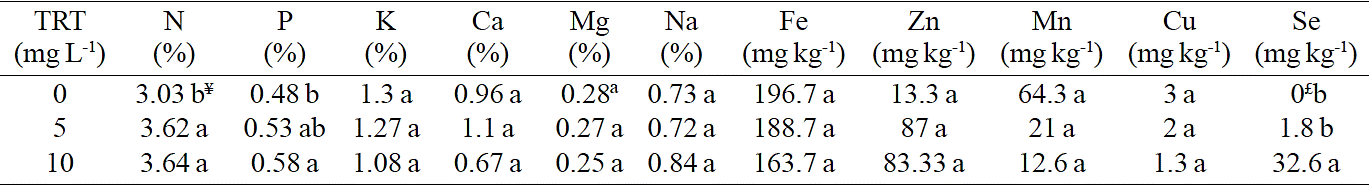
Foliar application of selenium has a substantial effect on redox potential (ORP) (Figure 1). ORP values indicate the antioxidant capacity, i.e. the ability of the system under analysis to donate electrons compared to hydrogen electrode (Benavides et al., 2002); as lower the value of ORP greater the capability to donate electrons and work as an antioxidant.
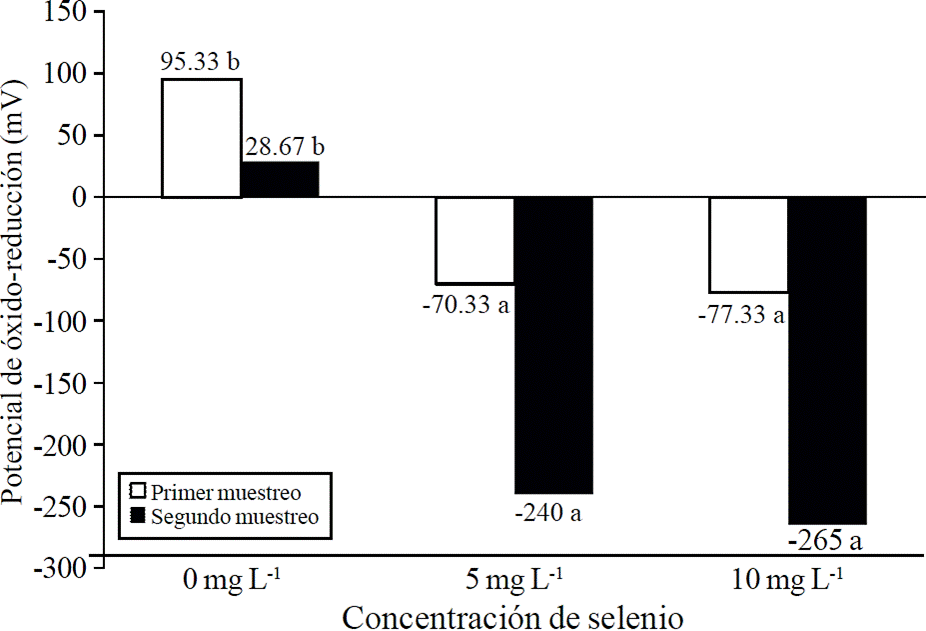
Figure 1 Average and standard deviation of oxide reduction potential (ORP mV) in fresh lettuce seedlings extract to which was applied selenium as sodium selenite. Bars with different literal difference indicated by Tukey (p≤ 0.05).
This result is possibly related to an increased antioxidant enzyme activity, which is known to increase in presence of certain selenium concentrations (Freeman et al., 2010). This seems to be confirmed by the results obtained with leaf catalase activity (Figure 2) showing positive and significant trend by applying selenium to plants. These results are similar to those obtained by Lingan et al. (2005) who reported a positive effect of selenium on catalase activity.
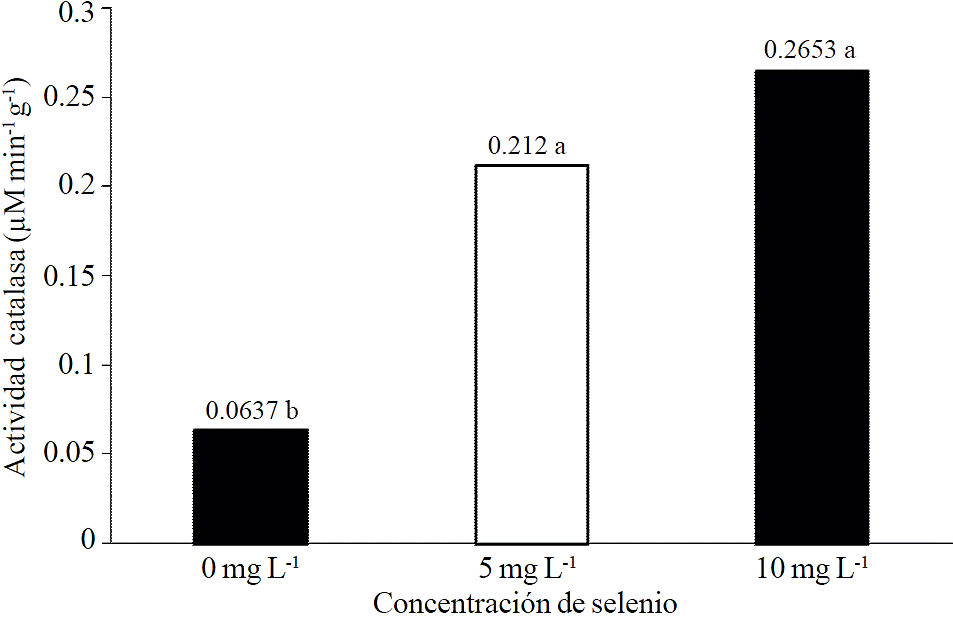
Figure 2 Mean and standard deviation of catalase activity in lettuce seedlings to which was applied selenium as sodium selenite. Bars with different literal difference indicated by Tukey (p≤ 0.05).
It is likely that the effect of selenium on the antioxidant potential is not associated with a higher photosynthetic activity and perhaps this explains the lack of differences in fresh and dry weight of root and leaves on lettuce plants in this study (Table 1). Becvort et al. (2012) obtained similar results applying selenium to tomato plants, obtaining a significant increase in antioxidant status but without differences in fresh and dried weight of fruits and root between treatments.
Table 1 Effect of foliar application of selenium on the fresh and dry weights of lettuce plants.
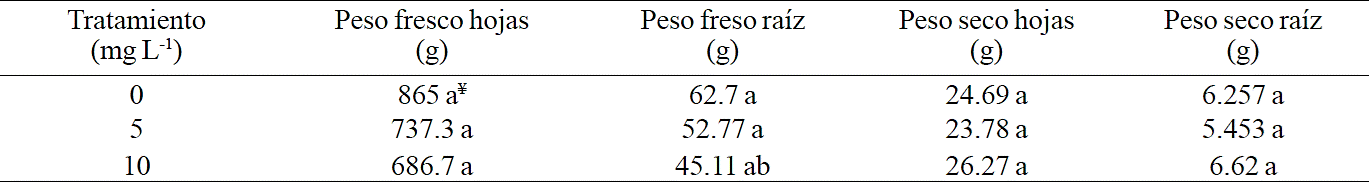
¥Promedios seguidos de distintas literales son estadísticamente diferentes según Tukey (P≤0.05).
As for mineral content in lettuce plants (Table 2) found differences only in nitrogen and phosphorus content by applying selenium. Nowak et al. (2002) found an increase in nitrogen content with the application of selenite in wheat plants. The data obtained regarding P match with Wu and Huang (2004), who found an increase of this element in clover plants when low amounts of selenite was applied to the substrate with 0, 10, 20 and 30 mg kg-1. Regarding the concentration of selenium it was found greater amount in treatment of 10 mg kg-1, Kápolna et al. (2009) increasing radical selenium concentration from 0.045 to 2.0 μg g-1 dry weight by applying 100 mg L-1 of selenium by foliar spraying. These data is consistent with Smrkolj et al. (2006) who indicate the need to perform repeated spraying if the objective is accumulation of selenium.
Conclusions
The application of selenium as foliar induced a higher antioxidant status in lettuce plants, increasing the concentration of N and P in leaves without changing the concentration of other elements or biomass.
Foliar application of selenium may be used as a tool to improve the nutritional quality of lettuce in terms of antioxidants.
Literatura citada
AOAC (Association of official analytical chemiste). 1980 a. Official Methods of Analysis 13th edition. Association of Official Analytical Chemists. Washington, DC., USA. 547-562 pp. [ Links ]
AOAC (Association of official analytical chemist). 1980 b. Official Methods of Analysis of the Association of Official Analytical Chemists. 30th edition. Association of Official Analytical Chemist. Washington, D.C. USA. 39 p. [ Links ]
Becvort-Azcurra, A.; Fuentes-Lara, L. O.; Benavides-Mendoza, A.; Ramírez, H.; Robledo-Torres, V. y Rodríguez-Mendoza, M. N. 2012. Aplicación de selenio en tomate: crecimiento, productividad y estado antioxidante del fruto. Terra Latinoamericana. 30:291-301. [ Links ]
Benavides-Mendoza, A.; Ramírez, H.; Robledo-Torres, V.; CornejoOviedo, E. and Maiti, R. K. 2002. Productivity, CO2 assimilation, and mineral tissue concentrations in onion plants under colored plastic films. Crop Research. 24:26-39. [ Links ]
Broadley, M. R.; White, P. J. M.; Bryson, R. J.; Meacham, M. C.; Bowen, H. C.; Johnson, S. E.; Hawkesford, M. J.; McGrath, S. P.; Zhao, F. J.; Breward, N.; Harriman, M. and Tucker, M. 2006. Biofortification of UK food crops with selenium. Proc. Nutr. Soc. 65:169-181. [ Links ]
Cartes, P.; Gianfrera, L. and Mora, M. L. 2005. Uptake of selenium and its antioxidative activity in ryegrass when applied a selenate and selenite forms. Plant Soil. 276:359-367. [ Links ]
Fick, K. R.; Miller, S. M.; Funk, J. D.; McDowell, L. R. and Houser, R. H. 1976. Methods of mineral analysis for plant and animal tissues. University of Florida institute of food and agriculture. Sciences, Departament of Animal Sciences, Gainesville, F L. USA. 81 p. [ Links ]
Fordyce, F. 2005. Selenium deficiency and toxicity in the environment. pp. 373-415. In: O. Selinus, B. Alloway, J. Centeno, R. Finkelman, R. Fuge, U. Lindh, and P Smedley (Eds.). Essentials of Medical Geology. Elsevier. Amsterdam, The Netherlands. [ Links ]
Freeman J. L.; Tamaoki, M.; Stushnoff, C.; Colin, F.; Cappa, J. D.; Sirine, F.; Matthew, M.; McGrath, S.; Doug-Van, H. and Pilon-Smits, E. A. H. 2010. Molecular mechanisms of selenium tolerance and hyperaccumulation in Stanleyapinnata. Plant Physiol. 153:1630-1652. [ Links ]
Kápolna, E.; Hillestrom, P. R.; Laursen, K. H.; Husted, S. and Larsen, E. H. 2009. Effect of foliar application of selenium on its uptake and speciation in carrot. Food Chem. 115:1357-1363. [ Links ]
Lingan, M. W. and Dongling, B. 2005. Selenium modulates the activities of antioxidant enzymes, osmotic homeostasis and promotes the grown of sorrel seedling under salt stress. Plant Growth Regul. 45:155-163. [ Links ]
Ramos S. J.; Faquin, V. L.; Guilherme, R. G.; Castro, E. M; Ávila, F. W.; Carvalho, G. S.; Bastos, C. E. A.; Oliveira, C. 2010. Selenium biofortification and antioxidant activity in lettuce plants fed with selenate and selenite. Plant Soil Environ. 12:584-588. [ Links ]
Rayman, M. P. 2008. Food-chain selenium and human health: emphasis on intake. Brit. J. Nutr. 100:254-268. [ Links ]
SAS Institute. 2001. PROC user’s manual. 6th ed. SAS Institute. Cary, NC, USA. 252 p. [ Links ]
Smrkolj, P.; Germ, M.; Kreft, I. and Stibilj, V. 2006. Respiratory potential and Se compounds in pea (Pisum sativum L.) plants grown from Se-enriched seeds. J. Exp. Bot. 57:3595-3600. [ Links ]
Wu, L. and Huang, Z. 2004. Chloride and sulfate salinity effects on selenium accumulation by tall fescue. Crop Sci. 31:114-118. [ Links ]
Nowak, J.; Kaklewski, K. and Ligocki, M. 2004. Influence of selenium on oxidoreductive enzymes activity in soil and in plants. Soil Biol. Biochem. 36:1553-1558. [ Links ]
Received: June 2015; Accepted: November 2015











 texto en
texto en