Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.6 no.8 Texcoco nov./dic. 2015
Articles
Physiological quality of the seed and maize seedlings development at low temperatures
1Campo Experimental Bajío-INIFAP. Carretera Celaya-San Miguel de Allende, km 6.5, Guanajuato. C. P. 38110, México. (gamez.josue@inifap.gob.mx; avila.miguel@ inifap.gob.mx; gamez.francisco@inifap.gob.mx).
2Campo Experimental Valle de México-INIFAP. Carretera Los Reyes-Texcoco, km 13.5, Coatlinchán, Texcoco, Estado de México, C. P. 56250.A.P. 307 y 10. (virgen.juan@inifap.gob.mx, sangerman.dora@inifap.gob.mx).
3Universidad Autónoma Agraria Antonio Narro. Buenavista, Saltillo, Coahuila, C. P. 25315. (nruiz@uaaan.mx).
4Universidad Tecnológica de la Mixteca, carretera a Acatlima km 2.5, Huajuapan de León, Oaxaca. C. P. 69000. (aascencioal@ conacyt.mx).
The adaptation of maize to early plantings required improving their tolerance to low temperatures, in terms of the ability of the seeds to germinate and produce seedlings at such temperatures. The aim of this study was to identify the variables that seed and seedlings are involved in tolerance to low temperatures of lines and hybrids adapted to the high valleys of Mexico. Were evaluated, in the laboratory and greenhouse 17 genetic materials, including a hybrid of the tropics and a synthetic variety of temperate conditions. Genotypes were evaluated in the laboratory at temperatures of 4, 8, 12 and 25 °C. The genetic materials from the highest elevation area had a better tolerance, high germination rates, plumule dry weight and radicle; besides faster emergence. The dry weight of the radicle was proportionally less affected than the plumule by low temperatures. The response to low temperature tolerance was induced in susceptible genotype s to reduce from 8 to 4 °C temperature. The results suggest that, the cold test with 8 °C for 7 days before the induction germination can be used as a quick test of tolerance to low temperatures.
Keywords: Zea mays L.; germination; plumule dry weight; tolerance
La adaptación del cultivo de maíz a siembras tempranas requiere de mejorar su tolerancia a bajas temperaturas, en términos de capacidad de las semillas para germinar y generar plántulas que prosperen a tales temperaturas. El objetivo del presente trabajo fue identificar las variables que a nivel de semilla y plántula están involucrados en la tolerancia a bajas temperaturas de líneas e híbridos de maíz adaptados a los Valles Altos de México. Se evaluaron en condiciones de laboratorio e invernadero 17 materiales genéticos, incluidos un híbrido del trópico y una variedad sintética de condiciones templadas. Los genotipos se evaluaron en laboratorio en temperaturas de: 4, 8, 12 y 25 °C. Los materiales genéticos originarios de la zona de mayor altitud presentaron mejor tolerancia, altos porcentajes de germinación, peso seco de plúmula y radícula; además de mayor velocidad de emergencia. El peso seco de la radícula fue proporcionalmente menos afectada que la plúmula por bajas temperaturas. La respuesta de tolerancia a bajas temperaturas se indujo en los genotipos susceptibles al reducir de 8 a 4° C la temperatura. Los resultados sugieren que la prueba fría con 8° C durante 7 días previo a la inducción de la germinación puede considerarse como una prueba rápida para detectar tolerancia a bajas temperaturas.
Palabras clave: Zea mays L.; germinación; peso seco plúmula; tolerancia
Introduction
Maize (Zea mays L.) is a species of subtropical origin, sensitive to low temperatures, which can die with exposure for short periods of time at temperatures near 0 °C (Restrepo et al., 2013). In Mexico, for the past 25 years, nearly 260 000 hectares planted with maize are affected annually by low temperatures and States with the highest incidence of this type of disaster are: Chihuahua, Durango, Sonora, Baja California, Puebla, Oaxaca, Hidalgo, Tlaxcala, Zacatecas and the State of Mexico (CONAGUA, 2013), mainly above 2 200 meters of elevation. Early planting of maize in these places require low temperature tolerant genotypes during germination and seedling. Most indicators have been evaluated regarding tolerance between the phases of germination and third ligulate of leaf, so that the initial vigour is essential for the expression of important classification variables, such as germination, emergence, survival and dry matter accumulation in seedling, valued at suboptimal temperatures (Aguilera et al., 1999).
Temperature is the main factor that determines the adaptation of species for different locations, since it alters several vital functions. The activities affected are the rate of chemical reactions; changing the state of water (ice-liquid-vapour), changes in the structure and activity of macro-molecules, the functions associated with the membrane and enzyme activity.
Plants under low temperatures grow slowly and alterations in fatty acid composition, fluidity of cell membranes, rate of metabolic activity (Nishida and Murata, 1996), loss of cell solutes, reduced transport arise through the plasmalemma, dysfunction breathing, inducing high levels of activated oxygen species (EAO), indoleacetic acid, abscisic acid and oxidases also change in the composition of proteins in cell membranes (Anderson et al., 1994), especially at growing points. When raising the breathing process in the presence of light and inducing stomatal closure, EAO production can cause significant fotoxidative damage, accentuated in the maize' seedling epicotyl, increasing root/shoot relationship (Richner et al., 1996).
The process of germination and seedling development, as all physiological processes are affected by temperature. This primarily affects the enzymatic activity required for the degradation of substances reservation. The most harmful effect of low temperatures with humidity occurs during the enzymatic swelling- activation of the seed (entry of water into the seed). This is known as "cold imbibition damage", the cold water inlet into the seed causes damage to the cell membranes, the latter plus the exudates of the cellular contents, facilitates the entry of fungi (Olivares et al., 1990).
The main aspects that influence the sensitivity of plants to cold are the species, age, previous history and environmental conditions. In general, very young seedlings germinating seeds and flowers are the most affected by low temperatures, while the dormant seeds are the most resistant. Typically, the roots are more sensitive than the aerial parts and the stems more than the buds (Sung and Amasino, 2004) stems. Low temperatures are a major factor determining the geographical distribution of species and crops. Damages in the crops are quite substantial, it is estimated that a 1 °C drop in the annual average temperature would cause a 40% decrease in world rice harvest. The expectation of using sensitive crops at low temperatures in cold climate regions is based on the possibilities of manipulating the natural plant responses to these temperatures. In recent years there have been made efforts for learning how plants "sense" the environment and respond to environmental changes by the potential application of this knowledge (Thomashow, 2001). In the course of evolution, plants acquired numerous coping mechanisms related to the cold. To survive this stress, plants use avoidance mechanisms and tolerance. Evasion is to minimize the occurrence of stress; however, tolerance is the ability to resist changes caused by the cold through extremely internal complex mechanisms that are controlled by genes "triggered" by low temperatures (Olivares et al., 1990). One of the strategies of tolerance is cold acclimation, the process by which the plants increase their tolerance to freezing after being exposed to low temperatures for a period of time. Environmental cues that trigger it are short days and a gradual decrease in temperature; plants are conditioned as the temperature drops in autumn, this requires energy and involves changes in gene expression that result in qualitative changes in the pattern of synthesized proteins (Amasino and Sung, 2004).
Generally, acclimated plants survive with more water frozen in their tissues; resistance to freezing depends on both, the ability of the extracellular spaces to control the volume of the crystal and the ability to resist protoplast dehydration. It has been observed that cold acclimation correlates with decreased osmotic potential and active photosynthesis. The degree of acclimatization achieved depends on the temperature at which the plant has been exposed (Örvard et al., 2000).
The adaptation of plants to low temperatures is a genetic trait that does not show consistently throughout the growing season and can be induced by preconditioning with temperatures below -10 °C, a phenomenon known as "acclimatization or pre response-conditioning to low temperature" (Thomashow, 2001). The response at low temperatures has been studied by comparing acclimated and non-acclimated seedlings (Prasad, 1997; Madakadze et al., 2003) or by crosslinking genotypes with different origins and tolerance in several stages of development. However, this tolerance in inbred lines of maize has not been defined just yet, whether if it is determinant or not for the behaviour which can form hybrids (Revilla et al., 2000).
In the Valley of Toluca-Atlacomulco, the average annual temperature of 12 °C, for early plantings of maize genotypes (15 March- 20 April) tolerant to low temperatures are required during the initial phase of development; where germination and early seedling development permit a higher crop cycle and therefore the risks due to the presence of early frosts at the end of the cycle (September), better use of the period of rainfall and higher yields are reduced. The aim of this study was to classify parents and single crosses H-52, H-68, for its tolerance to low temperatures and identify the variables involved with the initial seed vigour and seedling lines and maize hybrids generated by the Experimental Field Valley of Toluca, part of the National Research Institute of Forestry, Agriculture and Livestock (INIFAP).
Materials and methods
Seeds of the M-43, M-44, M-60 and M-61 lines were used, the first two lines form the first H-52 hybrid of intermediate cycle and the last two the H-68 of early cycle. With each pair of parent lines, and in each case with the populations F1, F2, RC1 and RC2 (backcross to the female and male line, respectively) we formed a family. In addition to both families, 12 genotypes were included, three controls adapted to the Valley of Toluca-Atlacomulco (H-52, VS-46 and landrace), H-516 formed for dry tropical conditions and Synthetic-1 adapted to conditions of Bajio type; having a total of 17 genotypes. The seed is produced in the spring-summer agricultural cycle, 2005 in Metepec, State of Mexico. The genotypes developed for the Valley of Toluca-Atlacomulco were described and evaluated by María et al., 2003).
Laboratory and greenhouse tests were made in the facilities of the Antonio Narro Agrarian Autonomous University (UAAAN). In the laboratory was evaluated the exhibition of the seed at temperatures of 4, 8 and 12 °C as well as performing the germination test at 25 °C. In order to achieve this, we used 100 seeds of each genotype, placing them between wet anchor papers towels (four replicates of 25) folding them in rolls. Then they were placed in chambers calibrated at the temperatures indicated for a period of seven days. Subsequently, the seeds (the rolls) were placed in a germinating machine at 25 °C in darkness for six days. As a control, seeds were evaluated in standard germination test (ISTA, 2004), four repetitions of 25 seeds. The evaluation was performed according to the criteria of (ISTA, 2004), except that the counts were the fourth and sixth day. In the latter also the dry weight of plumule and radicle were measured. The experimental design was completely randomized, with factorial arrangement 17 x 4. The linear model used was: γij= μ + αi + τj + ατ ij + εijk.
In the greenhouse, an experiment was established to evaluate the speed of emergence under irrigated conditions, using as substrate a mixture of peat moss, perlite and vermiculite in a 4: 1: 1 (v/v). The sowing was done in a seedbed at a depth of 7 cm. The experimental design was completely randomized with four replications, where the experimental unit consisted of 25 seeds. Data were taken from the fourth day after planting, at 11:00 h in each case, and was completed in 13 days. The emergence speed index was estimated according to Maguire (1962).
The seed used in both experiments passed through the sieve of oblong holes of 5 x 19 mm and 7 mm round, but it was retained on the 6.35 mm sieve.
Statistical analysis. The analysis of variance was made for the factorial arrangement; principal component to explore the variation of genotypes and classification of the tolerance of genotypes (Table 1) and; the regression analysis between the genotypes and temperatures. The statistical analyses were performed using the procedures PROC GLM, PROC PRINCOMP and PROC REG (SAS, 1999), considering a fixed effects model.
Results and discussion
In the principal component analysis (Figure 1) it was determined that, the first two components justify 75.2% of the variance, and allow classifying the genotypes for tolerance at low temperatures. In the distribution of genotypes (Figure 1A) it shows that H-516, M-44 and M-61 (genotypes p, b and h, respectively) form the group of susceptible materials at low temperatures. The susceptibility of the H-516 is mainly because it was not selected to tolerate suboptimal temperatures, which coincides with that reported by Madakadze et al. (2003), about the pressure of ecophysiological selection is crucial for tolerance to low temperatures. Groups of tolerant and susceptible materials can even be separated into two distinct subgroups, one of them located in quadrants III and IV (Figure 1B), grouping the temperatures of 4 and 25 °C.
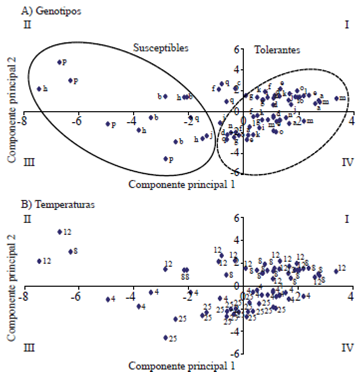
Figure 1 Dispersion of the genotypes (A) and temperatures (B) based on two major components (75.2% variance). CP1= [germination 4th and 6th day (+), dry weight of radicle (-), dead seeds (-) and abnormal seedling to the 6th day (-)]; CP2= [dead seeds at day 4th day (+), dry weight of plumule (+), plumule length (+) and radicle (+)]. Genotypes: a= M-43; b= M-44; c= a x b, d= cF2; e= c x a; f= c x b; g= M-60; h= M-61; i= g x h; j= iF2; k= i x g; l= I x h; m= H-52; n= VS-46; o= landrace; p= H-516 and; q= synthetic-1.
Meanwhile, the corresponding to the treatment of 8 and 12 °C temperatures are located within the quadrants I and II. That is, the temperatures of 12 and 8 °C have similar stress conditions, so if we want to perform a quick test, we would have to choose between the possibilities. The difference between the two groups is the high frequency of dead seeds, as well as the highest level of dry plumule, which were obtained in the treatment of 8 and 12 °C.
The materials are classified by their tolerance to low temperatures (first principal component), placing materials susceptible to tolerance, from left to right on the x-axis (Figure 1A), leaving the tropical susceptible control (H -516, p), male parental lines (b and h), the susceptible Bajio (Synthetic-1, q), female parental lines (a and g), backcrosses (e, f, k and l), the populations F2 (d and j) and finally the hybrids (c, i, n, o and m) and native control; the latter classified as more tolerant to low temperatures.
The factor analysis of developed laboratory experiments, detected statistical differences (p≤ 0.01) between the environments evaluated the genetic materials and the genotype x environment interaction; i.e., temperatures of evaluation (25, 12, 8 and 4 °C) were contrasting enough to allow the genotypes to show a differential behaviour in each of the evaluated variables.
In germination, a contrasting response between selected genotypes in Metepec and susceptible checks and tropical shallows (Figure 2A) was obtained; M-43 female line had a similar response in the four temperatures, surpassing statistically (Tukey, p≤ 0.05) for all lines, and even the simplest crosses M-43 x M-44. The latter single cross shows recovery in germination at 4 °C, which could be attributed to the higher ability to induce tolerance response, probably associated with the behaviour of the female line (M-43), which showed a constitutive tolerance to low temperatures. In contrast, H-516 initially showed reduced germination; recovering later, which can be associated with induction of tolerance promoted by the treatment of 4 °C; however, it did not exceed the M-44 line, also classified as susceptible.
In the case of the hybrid (H-516 and M43 x M44) there is a temperature between 8 and 4 °C, which triggers a survival response to low temperatures, which coincides with that reported by Castro et al. (2008) who pointed out the acclimation to low temperatures is reached with temperatures below 10 °C. This same phenomenon is not so evident with the lines in question, which have a more uniform response, probably due to the genetic selection process developed in the Valley of Toluca, where low temperatures are common.
In contrast in the second family, formed with the parental lines of crosses M60 x M61 (Figure 2B), the female line M-60 was overcome by the simple crosses and the control H -52, in the test of 8 °C, so that the hybrid vigour allowed a higher response than even the best parent. As in the previous case also it shows that the male line (M61) was the progenitor more susceptible to low temperatures, as it presented the lowest percentages of germination in all tests at low temperatures.
In the analysis of the response to suboptimal treatment, the temperature of the families, formed by parental lines and their hybrids (Figure 2) stands out in both cases, the expression of the response associated with the female parent, who gives the tolerance to low temperatures in maize, which coincides with Revilla et al. (2000) and Cano et al. (2000) who associate this response with the double maternal response, determined during the process of fertilization and its implications for the quality and quantity endosperm of the seed.
This figure shows again that, the cold test at 8 °C allowed a better classification of genotypes for tolerance to low temperatures. Besides considering the differences in suboptimal temperatures tolerance among females M-43 and M-60 lines, they could be associated with the level of precocity of both, as M-43 is an intermediate cycle and was primarily selected on early plantings, since M-60 is of early cycle, which was selected after the planting dates.
Low temperatures caused the reduction of germination (95 to 85%), as was reduced from 25 to 8 °C; which coincides with what was reported by Revilla et al., (2000), who associated with low temperatures oxidative damage to membranes and cell death. Germination recovered when genetic materials were subjected to 4 °C, which Pastori et al. (2000), explained through the accumulation of antioxidant compounds. Furthermore, when comparing the assessment of days to germination (4th and 6th day), there were no statistical differences with cold test at 12 and 8 °C; so the cold test with 8 °C could be a quick way to detect low temperature tolerance.
The number of dead seeds was the main variable increased by reducing the temperature at 8 °C (Figure 3), but at 4 °C was significantly reduced in susceptible materials. Important differences in this variable when evaluated were detected at 4 or 6 days of development in the germination chamber at temperatures no statistical assessment. Allowing assessing either term.
As for the variables, dry weight of plumule and radicle, the highest effect on low-temperatures was presented in reducing the dry weight of plumule, especially when the temperature was lower than 8 °C (Figure 4). In this regard, Fryer et al. (1998) and Leipner et al. (1999) reported a reduced rate of metabolic activity at the plant for temperatures below the threshold. In both treatment, the variables 12 and 8 °C were statistically similar, (Tukey, p≤ 0.01) even better than the treatment at 4 °C. The dry weight of the radicle was less affected than plumule in this sense, Martin and Dodd (2011), reported that this is the main cause of the root- aerial part ratio increases in plants grown at low temperatures.
The emergence speed index (Figure 5) of the genotypes classified as tolerant to low temperatures M60 x M61 and M43 x M44, overcame their female parental lines, probably due to hybrid's vigour, with rates between 2.5 and 3. The susceptible materials H-516, M44 and M61 had the lowest rates (values between 1.1 and 2.3). The response to low temperatures of the adapted controls was similar to the H-52 as the control of higher vigour, followed by the VS-46 and the landrace from the high valleys (above 3.5 index), which are commercial materials that exceeded in vigour and adaptation to the single crosses and their parents. This behaviour could be associated with the expression of the response under field conditions, especially in the early stages of establishment and development of the crop, as indicated by Tiryaki and Andrews (2001).
Conclusions
The genotypes with the best tolerance to low temperatures were simple crosses (M-43 x M44 and M-60 x M-61) and female lines M-43 and M-60, in addition to the controls from the high valleys: H-52 landrace and VS-22. The male lines M-44 and M-61 exhibited a low and intermediate tolerance, in a third group were the controls: Synthetic-1 and H-516, which are for Bajio and Tropic standard conditions.
The low temperature tolerant genotypes presented higher germination, dry weight of plumule, dry weight of radicle and rate of seedling emergence speed.
The best behaviour of the tolerant materials was similar through different temperatures, probably due to a constitutive response of the genes tolerant to low temperatures.
The response to low temperature tolerance was induced in susceptible genotypes to reduce the temperature of the cold test from 8 at 4 °C.
The cold test in 8 °C allows classifying tolerant materials in a rapid test for laboratory evaluation that requires little time.
Literatura citada
Aguilera, C.; Stirling, C. M. and Long, S. P. 1999. Genotypic variation within Zea mays for susceptibility to and rate of recovery from chill-induced photoinhibition of photosynthesis. Physiology Plantarum. 106: 429-436. [ Links ]
Anderson, M. D.; Prasad, T. K.; Martin, B. A. and Stewart, C. R. 1994. Differential gene expression in chilling-acclimated maize seedlings and evidence for the involvement of abscisic acid in chilling tolerance. Plant Physiology. 105: 331-339. [ Links ]
Cano, R. P.; Ramírez, R. G.; Ortegón, P. J.; Esparza, M. J. H. y Rodríguez, H. S. 2000. Análisis dialélico para vigor de semilla en melón. Agrociencia. 34(3): 337-342. [ Links ]
Castro, M. A.; Reyes, M. D.; Alberdi, M.; Jara, V. R.; Sanhueza, C.; Corcuera, L. J. and Bravo, A. L. 2008. Effects of low temperature acclimation on photosynthesis in three Chilean Proteaceae. Rev. Chilena de Historia Natural 81. 321-333 pp. [ Links ]
Coordinación General del Servicio Meteorológico Nacional. 2013. Reporte anual. CONAGUA, México D. F. [ Links ]
Fryer, M. J.; James, R. A.; Oxborough, K.; Blowers, D. A. and Baker, N. R. 1998. Relationship between CO2 assimilation, photosynthetic electron transport, and active O2 metabolism in leaves of maize in the field during periods of low temperature. Plant Physiol. 116: 571-580. [ Links ]
International Seed Testing Association. 2004. International rules for seed testing. Bassersdorf Switzerland. 72 p. [ Links ]
Leipner, J.; Fracheboud, Y. and Stamp, P. 1999. Effect of growing season on the photosynthetic apparatus and leaf antioxidative defenses in two maize genotypes of different chilling tolerance. Environ. Exp. Bot. 42: 129-139. [ Links ]
Maguire, J. D. 1962. Speed of germination: AID in selection and evaluation for seedling emergence and vigor. Crop Sci. 2: 176-177. [ Links ]
Madakadze, I. C.; Stewart, K. A.; Madakadze, R. M. and Smith, D. L. 2003. Base temperatures for seedling growth and their correlation with chilling sensitivity for warm season. Crop Sci. 43: 874-878. [ Links ]
Maria, R. A.; Rojas, I.; Avila, P. M. A. y Gámez, V. A. J. 2003. Producción de maíz de temporal en el Estado de Tlaxcala. Folleto para productores Núm. 3. SAGARPA. INIFAP. México, Tlaxcala, Tlaxcala. 16 p. [ Links ]
Martin, A. I. and Dodd, I. C. 2011. Root-to-shoot signalling when soil moisture is heterogeneous: increasing the proportion of root biomass in drying soil inhibits leaf growth and increases leaf ABA concentration. The Lancaster Environment Centre Lancaster University LA1 4YQ. United Kingdom. [ Links ]
Nishida, I. and Murata, N. 1996. Chilling sensitivity in plants and cyanbacteria: the crucial contribution of membrane lipids. Ann. Rev. Plant Physiol. Plant Mol. Biol. 47: 541-568. [ Links ]
Olivares, A.; Johnston, M. y Fernandez, G. 1990. Efecto de la temperatura en la germinación de siete especies de la pradera anual mediterránea y caracterización de su emergencia. Simiente 60: 123-131. [ Links ]
Örvar, B. L.; Sangwan, V.; Omann, F. and Dhindsa, R. S. 2000. Early steps in cold sensing by plant cells: the role of actin cytoskeleton and membrane fluidity. The Plant J. 23: 785-794. [ Links ]
Pastori, G.; Foyer, C. H. and Mulineaux, P. 2000. Low temperature-induced changes in the distribution of H2O2 and antioxidants between the bundle sheath and mesophyll cells of maize leaves. J. Exp. Bot. 51(342):107-113. [ Links ]
Prasad, T. K. 1997. Role of catalase in inducing chilling tolerance in pre-emergent maize seedlings. Plant Physiol. 114: 1369-1376. [ Links ]
Restrepo, H.; Gómez, M. I.; Garzón, A.; Manrique, L.; Alzate, F.; López, J. y Rodríguez, A. 2013. Respuesta bioquímica de plántulas de maíz (Zea mays L.) a diferentes condiciones de temperaturas nocturnas. Rev. Colombiana de Ciencias Hortícolas. 7(2):252-262. [ Links ]
Revilla, P; Malvar R, A; Cartea M, E; Butrón, A. and Ordás, A. 2000. Inheritance of cold tolerance at emergence and during early Season growth in maize. Crop Sci. 40: 1579-1585. [ Links ]
Revilla, P.; Malvar, R. A.; Cartea, M. E. and Ordás, A. 1998. Identifying open-pollinated populations of field corn as sources of cold tolerance for improving sweet corn. Euphytica 101:239-247. [ Links ]
Richter, B. D.; Baumgartner, J. V.; Powell, J. and Braun, D. P. 1996. A method for assessing hydrologic alteration within ecosystems. Conservation Biol. 10: 1-12. [ Links ]
Statistical Analysis System. 2010. SAS. Version 9.3. SAS Institute, Inc. Cary, NC, USA. [ Links ]
Sung, S. and Amasino, R. M. 2004. Vernalization and epigenetics: how plants remember winter. Current Opinion in Plant Biology. 7: 4-10. [ Links ]
Thomashow, M. F. 2001. So what's new in the field of plant cold acclimation? Lots. Plant Physiol. 125: 89-93. [ Links ]
Tiryaki, I. and Andrews, D. J. 2001. Germination and seedling cold tolerance in sorghum: I. Evaluation of rapid screening methods. Agron. J. 93: 1386-1391. [ Links ]
Received: March 2015; Accepted: August 2015











 texto en
texto en 






