Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Nova scientia
versión On-line ISSN 2007-0705
Nova scientia vol.6 no.11 León abr. 2014
Ciencias naturales e ingenierías
Estudio a nivel laboratorio de la Degradación atípica en un refractario tipo SiO2 utilizado en hornos de inducción
Laboratory scale study of uncommon degradation SiO2 refractories used on induction furnaces
J. Zuno-Silva1, A. Bedolla-Jacuinde2, J.M. Martínez-Vázquez3, A. Pérez-Perez3 y T. Quintero-Azuara4
1 Ingeniería Mecánica - ESCS - UAEH, Ciudad Sahagún, Hidalgo. E-mail: zunojorge@gmail.com.
2 Instituto de Investigaciones Metalúrgicas, UMSNH, Morelia Michoacán.
3 Ingeniería Metalúrgica, UPJR, Juventino Rosas Guanajuato.
4 Desarrollo de Nuevos Proyectos – CIFUNSA, Saltillo, Coahuila.
Recepción: 13-03-2013
Aceptación: 16-09-2013
Resumen
En este trabajo de investigación se realizó un estudio de la degradación atípica que se produce en las paredes de los hornos de inducción. Para esto, se utilizó un material refractario base - SiO2 (98.8%) con dos escorias de composición química diferente y se analizó su comportamiento mediante las pruebas experimentales del método de copa o crisol y el de inmersión, así como por un modelado asimétrico del metal líquido en el horno de inducción. La escoria, la estructura del material refractario y el mecanismo de penetración de la escoria fueron caracterizados por microscopia óptica, utilizando la técnica de luz reflejada. Los resultados demostraron que, con valores altos de frecuencia (240 Hz), para la operación del horno, se obtiene una fuerza de Lorentz muy alta (14.26 N) pero con una velocidad del metal liquido baja (6 m/s) la cual tiene un vector de dirección perpendicular a las paredes del horno, provocando presión sobre la escoria incrustada en los poros del refractario favoreciendo el proceso de degradación del material.
Palabras clave: Refractario base - SiO2, penetración de escoria, horno de inducción, calidad de chatarra.
Abstract
The refractory degradation on induction furnace walls at different points was studied. A SiO2 (98.8%) base refractory was analyzed with two different slag chemical composition through crucible and dipping laboratory degradation test and also applying an asymmetric modeling. The slag, refractory structure and slag penetration was analyzed by optical microscopy using reflecting light technique. The results demonstrated that slag penetration starts on the refractory pores produced by the sintering process and also by hollows generated by the came off grains. The modeling results evidenced that, with high frequency (240 Hz), a high Lorentz force (14.26 N) is originated but with a low liquid velocity (6 m/s) that have a perpendicular vector direction to the furnace walls, making pressure over the filtered slag.
Keywords: SiO2 - refractory, slag penetration, induction furnace, scrap quality.
Introduction
Refractories are inorganic, nonmetallic, porous and heterogeneous materials composed of thermally stable mineral aggregates, a binder phase and additives. The principal raw materials used on its manufacture are: the oxides of silicon, aluminum, magnesium, calcium and zirconium and some non-oxide refractories like carbides, nitrides, borides, silicates and graphite. Refractories are heat resistant materials used in all processes that work at high temperatures and corrosive conditions, as such metal factories. These are typically used to insulate and protect industrial furnaces and vessels due to their excellent resistance to heat, chemical attack and mechanical damage (Bhatia 2011, 3). At the commencement, materials refractories were used in bricks form to cover the interior of induction furnace, until in 1914, the so-called monoliths refractory was commercially released. At present, monoliths materials like SiO2 based are known as castables refractories, whereby a fluid mass of refractory aggregate is placed inside the furnace, forming an entire seamless wall which made the application of these materials far more efficient. These sort of refractories have a complex and granular microstructures consisting of a coarse particles called aggregates (millimeters size distinguished) and a fine fraction (which can be as fine as nanometric in size) called the matrix, which interlinks the coarser particles of the material. The fine fraction works as chemical binding at low temperature and ceramic bond formation at high temperature (sintering of the particles). Refractories are frequently subjected to thermal shocks and corrosion attacks (Ribeiro et al. 2010, 263). With increasing temperatures (≥ 1400°C) and specific chemical (SiO2, AlO2, MgO2 based), mechanical, and physical gradients, the propensity to degradation increases rapidly. Degradation, deterioration, decomposition, and wear are all words used to describe corrosion of these materials (Rigaud 2011, 387). Under this context, specialized lining materials to optimize induction furnace operation are required; thereby, materials refractories play a critical role to ensure long campaing and reliable operation of high temperature processing furnace, at the same time, refractories lining can be the controlling factor in the success or failure of a induction furnace service life, as well as the safe and profitable operation of the plant (Maity 2011, 29).
Consequently, SiO2 refractory is mostly indicated as material lining for induction furnace when cast iron is molten. In general, silica refractories contain typically >93% SiO2 with minor amounts of lime (CaO <3.5%), alumina (Al2O3 <2%), and iron oxide (Fe2O3 <1.5%) and it has three polymorphs (quartz, tridymite and cristobalite) and each polyphorms has an α – β type transformastion (quartz: 573°C, tridymite: 117°C, cristobalite: 200 to 275°C).
They have high corrosion resistance to acid slags but are sensitive to high temperatures and to thermal shock even at medium temperature levels. Its reduced porosity and lower alumina content may be used to prevent rapid degradation in the presence of alkalies conditions (Rigaud 2011, 387). That it is why is used on induction furnaces lining and also because quartz is stable up to 867°C, the tridymite is stable polymorph from 867 to 1470°C and the cristobalite is stable phase of silica between 1470°C and the melting point in silica refractories (Arahori, 1987, 2248).
According to the literature, for all the types of refractories the corrosion process involves a combination of different mechanisms, such as dissolution and invasive penetration characterized by diffusion, grain boundary and stress corrosion. On the other hand, oxidation–reduction reactions comes together with absorption, desorption, and mass transport phenomena and finally all variables mentioned above come into play (Rigaud 2011, 387). On his work, Narasimham (2007, 720) concluded that even though many factors affect the lining life, the major reasons are design and operational management. Some commom problems and failures are listed below:
-Hot spots (higher casing temperature)
-Excessive cracking
-Spalling of lining (thermal, mechanical, structural)
-Erosion and thinning of lining
-Chemical attack / corrosion from process gases (such as hydrogen, carbon monoxide, sulfur dioxide, alkalies)
-Partial melting and degradation of lining
-Excessive shrinkage
-Anchor failure and detachment of lining from wall
-Explosive spalling during dries out
-Mechanical damages
Of course, each problem, extent of damages and failure will vary from equipment to equipment. Sometimes the problem will appear within a short time of operation or during the smelting process which it would represents a major problem (Maity 2011, 29). In this manner, the silica refractory localized on the floor lining and the sidewall section of the induction furnace used on the grey and ductile iron foundries are degraded taking an unusual wear pattern of an elephant's foot and occurs within the active power coil, leading to accelerated erosion when the metal saturated - refractory is inductively superheated (Williams et al. 2000, 22). So far, this sort of degradation process could be considered within the common problems.
Currently, there are significant improvements on the refractories resistance which come together with common problems variations, as such slag penetration in the middle area of the induction furnace besides the elephant's foot erosion when ductile iron is molten. Thus, a well-planned and methodical maintenance routine for the lining is required in order to performance – enhancing process; nevertheless sometimes fail due to the quality of the metallic charge or scrap (Sinha, 1998, 152). All scrap grades shall be free of dirt, nonferrous metals, or foreign material of any kind, and excessive rust and corrosion in order to reduce slag chemical reactions, for instance, no initial C addition is needed when cast iron returns are used as an initial charge. Also, melting carbon steel generates more FeO and MnO, which are more aggressive to the silica refractory. (Scrap specifications Circular 2013, 17).
Proper care is necessary to avoid this, but the question is: how the erosion process starts eventought Silica refractories has a low porosity percent and good bulk sintered structure? . On they work; Lee and Zhang (1999, 180), explain that, other physiochemical effects which acts on a refractory – slag erosion together with the simple penetration due to wetting effect, are: (a) microstructural effects where a smooth surface and dense material may resist ingress whereas a porous one may not, (b) slag line attack effect and (c) the velocity of slag flow which is often a result of the Marangoni effect (faster movement will present fresh slag to the slag/refractory interface). A vigorous agitation of the liquid metal is undesirable as it tends to pull out the fine grains in the refractory by erosion (Lee and Zhang 1999, 183). These effects contribute to produce annormal degradation on SiO2 based refractories. The metal liquid agitation is directly relationed with electromagnetic fields – EMF - (current and voltage as main parameter) which besides melt the scrap produce high velocity movement of the liquid metal with parallel and vertical directions to the hot face of wall refractories. In addition, as was said before, the purity of the alloy elements involved in the casting have a corrosive effect on the lining, since the scrap has different type of metallic materials leading to change the chemical composition of the liquid metal.
On the current work, the degradation of the SiO2 refractory used in an induction furnace to melt ductile iron is analyzed. An unspected slag penetration and eroded process was found in the middle of the lining and close to the furnace crown in spite of the refractory was added with B2O3 (dry ramming mix) to help the consolidation at lower temperatures (reducing porosity). The campaign operation was planned to four weeks expecting a wear process on the floor of the furnace similar to the elephant's foot, however, after two or five days, the refractory fails.
Thus, the main objective is to determine how the slag infiltration started on this kind of refractory when is supposed that it has a good bulk density. In addition, determine the scrap quality effect on the refractory degradation. Also, it will try to propose a practical routine at laboratory scale to evaluate refractory sintering process. For these, an optical microscopy study of the degradation mechanism of a SiO2 based refractory was carried to determine how the erosion begins, most of the research works explains this process in a schematically form but, an optical microscopy analysis is limited. Also, the influence of the electromagnetic field (EMF) effect was analyzed together with the slag composition.
Method
In an iron foundry process, several variables and parameters are involved, (i.e. temperature, pouring time, current, voltage, chemical composition of the casting and more), but as was argued before, the quality of the metallic charge plays a fundamental roll due to consist of different class of scrap, which in turn, it will assist the refractory degradation mechanism. On the other hand, electric current forms a magnetic field (MF) in the coil, producing thermal energy, melting the metallic charge and causing an intense stirring action of the liquid metal. At the same time, slag is generated from oxidation, dirt, sand and other impurities that come from the scrap. Normally, the slag is deposited along the upper region of the lining or crucible walls and above the heating coils, but also, it can be deposited in areas midway down the crucible lining where sufficient enough metal turbulence from magnetic stirring occurs, which is our case. Thus, the investigation was carried out as follow:
Chemical composition of the materials involved
The kind of refractory material used is SiO2 based; its chemical composition is showed in Table 1. Of course, slag is the main variable to take account, so it is vital to have information about its chemical composition that is presented on Table 2.
Static and Dynamic degradation test
Two methods were used to analyze the slag attack and degration mechanism. The first one is the crucible, cavity, cup or brick test (static), which consist in a hollow drilled piece of refractory or a brick that is filled with slag and exposed to high temperature to promote slag – refractory interaction, Figure 1(a). The advantages of this method are that it is simple and many samples can be tested quickly. For this test, molten slag (1300°C) was poured into the drilled bricks and cooled down at room temperature. Crucible method allows evaluating the slag-penetration into refractories or reduction of wall thickness (by spalling or dissolution), also to evaluate by visual inspection as: unaffected, lightly attacked, attacked or corroded refractory and together with optical microscopy analysis it will provide the enough knowledge of the refractory texture and raw materials binding (Freud, 2010, 3). The second ones, it is known as the dipping, immersion or finger test (dynamic) where one or more cylindrical or square pillar shaped refractory samples are held in the corrosive slag for a certain period of time in an induction furnace.
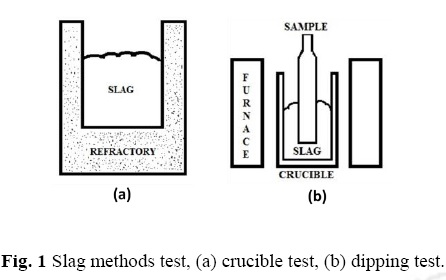
In this method, the atmosphere is easily controlled and slag with different chemical composition can be added to get a rapid saturation with reaction products which are controlled by using variable volume of slag relative to the size of samples, Figure 1(b). In this case, two ductile iron pieces (used as scrap) were melted (1380°C) and adding Slag 1. Once the slag was dissolved, a refractory sample was submerged into the liquid/slag mix. The same procedure was applied using Slag 2.
It is important to make clare that, because the SiO2 lining is hard and fragile, it was quite difficult to cut and drills a hole on it, so a small furnace and some bricks of vibrable material were made it following the standard sintering process, see Figure 2. The SiO2 material was vibrable compacted on a metallic mould and heated for 4 hours at 600°C, after this, the brick was pull out and heated again during 4 hours more at 1200°C until got it hard.
Visual inspection and optical microscopy
Firstly, a visual inspection was carried out over refractory samples extracted from industrial induction furnace. The main point is to know the depth of the slag attack on the refractory lining. The principal characteristics of these specimens are that were extracted from a furnace area where supposedly the erosion must be not aggressive; eventought the sintered process satisfied the standard requirements.
In order to be microscopy analyzed, the material refractory was mechanically grinding by traditional routine, starting with abrasive paper of 260 grade and finishing with 1200 grade, in this case, no diamond past was used. The samples were alcohol cleaned to avoid any slag oxidation. Light microscopy was used to analyses the slag penetration on the SiO2 base refractory using an Olympus BX-41RF- LED microscope. A combination of transmitted light, polarized light and Differential Interference Contrast (DIC) technics was used for distinguishing between refractory material and slag features.
Electromagnetic field modeling
Lorentz law is the universal expression of the force exerted by electromagnetic fields (EMF) on a volume containing a distribution of electrical charges and currents. If electric and magnetic dipoles are present in a material medium, they are traditionally treated by expressing the corresponding polarization and magnetization distributions in terms of bound-charge and bound-current densities, which are subsequently added to free-charge and free-current densities, respectively (Mansuripur 2012, 5). For the electromagnetic field (EMF) modeling, an axisymmetric model was performed using the Ansys® software, for this, it was necessary to take into account the coils characteristics, cast iron properties and the SiO2 base refractory features. In this case, for the furnace analysis, real commercial kiln dimensions were used (for an industrial capacity of 12 Ton of metallic charge). A designed grid with the Plane 53 element tool was used for the Lorentz force (fL), as shown on Figure 4, with this grid, a total of 7854 elements and 24507 nods was obtained and for the velocity fields (Vf), a square grid of four nods, it was made it with Fluid 141 tool. Basically, the operative variables were the same that are used in a foundry process, as such frequency, current, voltage and potency. As first target of the simulation work, it was to determine the modeling conditions to known whether or not there is any preferential velocity vector of the metal liquid against the furnace walls. The properties of the cast iron are shown in Table 3, and the SiO2 base refractory and the coil properties are presented on Table 4.
The potency (P) and voltage (V) values used to operate the furnace were also employed to calculate the current (I) throughout the relationship I = P/V, the data results are displayed on Table 5, and taking into account the area and properties of the coil material, the current density (Js) was the next (see Table 5).
Results and discussion
Slag chemical composition:
Generally speaking, nearly all melting coreless induction furnaces have acid – silica- linings due to is best suited for scrap melting. Thus, according to the chemical composition of the refractory showed on Table 1, this requirement is well accomplished since the SiO2 content is 98.8 %.
However, despite amorphous silica has an excellent volume and chemical stability at temperatures up to 1200°C, metal penetration and chemical corrosion by slag still persist (Hemrick et al, 2005, 30), as presented on Table 2 where it can be observed that SiO2 amount is higher on Slag-2 (55.823% - 2nd class scrap) than Slag-1 (33.627% - 1st class scrap) as consequence of the degradation process.
Visual inspection:
According to the visual inspection, the Slag-2 penetrates up to two centimeter ahead of the hot face (slag area), Figure 5a, and also propagate along the slag/refractory line, Figure 5b, and spalling the SiO2 material (arrowed), it is clear that the grain boundary works as path corrosion but mainly across the aggregate grains but, the slag infiltration had must started in any porous. In refer to this, on they work, Kaupuzs et. Al (2003, 40) argue that silica has the advantage of strong expansion effect for getting high material density after heating and for closing cracks after reheating but, its chemical resistance is still, relatively, lower than other refractory materials. Very often coarse refractory grains break off when the matrix is rate determining for the corrosion rate (Pötschke 2005, 111).
Laboratory test
Despite the fact that working life of SiO2 base refractory linings used in foundry process has been extended greatly in the last decade, common problems still persist but with some variations on its failure behavior, thus, it is important to understand the degradation mechanism of these improved refractories under different slag chemical concentration. On Figure 6, the crucible test result is showed.
It can be observed that the cavity was well filled with Slag-2 and the same result was produced using Slag-1. At first sight, the fabricated SiO2 brick seems look very well compacted, so the industrial sinterised conditions were applied correctly. Similarly, the dipping test is presented on Figure 7, where a laboratory inductions furnace was used to add Slag-1 and Slag-2. Optical microscopy samples were carefully cut to be analyzed by reflected light technique.
Optical microscopy analysis
SiO2 brick refractory as sintered conditions
As was said before, laboratory SiO2 brick was well sintered with the help of B2O3 addition, not excessive spalling grain was observed before and after being cut which allows evaluating the slag effect on it. The matrix looks mostly uniform and some of the aggregate grains appear to be well bound having a particle size ranged from ~0.5 to 1.5 mm. Figure 8.
However, a noticeable aggregate grains sized from ~1.5 to 2.5 mm (or more) was found forming some kind of clusters as pointed on Figure 8(b), and not signicant porosity was observed at these magnifications. In reference to this, Kalpakli (2008, 85) improved the properties of a material refractory reducing the aggregate grains size from ~3 mm to ~1.5 mm, and the apparent porosity was decreased from 18.5 % to 16.5% respectively, but in our case, the aggregate grains are grouping quite similar to the grains showed on Figure 5 where the crack is propagated along their boundaries.
Not surprising, analyzing the refractory at 200X magnifications, some particles of irregular form were found close to the aggregate grains, some of them remain partially there but other just gone leaving hollows, Figure 9. So far, we can consider as normal these materials characteristics since certain porosity is allowed, about 10%.
The same kind of pores was observed on the SiO2 refractory matrix, most of them produced by a particle came off, sometimes there is a small pieces on the bottom of the hole or attached on the pore walls, Figure 10. On his work, Jansson (2005, 283-284) explain that slag resistance is also affected by the refractory porosity. Refractory corrosion is favored by slag penetration which soaks into the pores, dissolving the material refractory and causing stresses and cracks in the lining due to differential expansion, of course, all these factors are affected by the melting temperature which is controlled by the current and voltage variables.
In addition, difference of thermal expansion between the aggregates and the matrix of SiO2 refractory can lead, during heating and cooling stages, to the development of a large microcracks network within the microstructure, having a strongly effect on the thermo-mechanical properties: thermal expansion coefficient, Young's modulus and stress–strain law (Huger 2009, 578).
SiO2 brick refractory as tested conditions
The results obtained from the crucible and dipping test developed similar slag penetration behavior to the specimen extracted from the industrial induction furnace. The favorable points to initiate the slag filtering were the pores as was explained before, Figure 11(a). The Slag-2 is filtered in the pinholes and kept inside, starting a refractory degradation both in walls and bottom of the hollow, as can be seen on Figure 11(b) and (c). It can be observed that the pore is shaped very similar to the one showed on Figure 10. Similar behavior is reported by Wang et al. (2008, 111 ) who explains that the corrosion reactions occurs around pores and along crack toward the interior due to the grain boundary acts as path of corrosion reaction.
Lee and Zhang (1999, 186) explain that, capillaries, such as pores and microcracks are the main way to initiate slag penetration into a refractory material. Thus, the penetration rate dl/dt of slag into a capillary can be expressed by Poiseullie's law which is reported by Jansson (2005, 285) as follow:
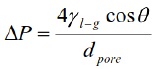
Where ∆P is the capillary pressure difference (Pa), γl-g is the surface tension liquid – gas (N/m), θ is the wetting angle slag – refractory (°) and dpore is the diameter of pore/capillary (m). From this relationship, there is no doubt that a pressure is required to fill the pores of the refractory material - in this case a refractory SiO2 base - where the pressure force is dependent of the wettability of the refractory by the slag. This capillary effect is well illustrated on Figure 12 for Slag-2. It can be observed how the slag is embedded into a pore which is partially occupied by a refractory grain, Figure 12(a), and it seems to be the beginning of the refractory spalling as can be seen on Figure 12(c), also some bubbles (probably of gas) are located in the slag/refractory limit. Similarly, Lee and Zhang (1999, 86) explains that the slag penetration give arise to refractory degradation, where dissolution at refractory/slag interfaces is governed by (a) chemical reaction (or solution) at the interface or (b) transport (or diffusion) of reacting species through the liquid. Phases of dissimilar chemical composition tend to react at high temperature, so that to limit the extent of dissolution, the refractory material and metal liquid should be of similar nature which is not happening here due to the scrap quality, 2nd class slag. The corrosion rate of refractories is increased with the increasing of the FeO content in the molten, which come from the steel scrap, some reported values indicate that with 12% of FeO a corrosion rate of 2.5 g/h could be produced (Liu 2008, 51).
In refer to the slag produced by the 1 st class – scrap; the degradation effect was less aggressive than produced by the 2nd class, as can be seen on Figure 13. The Slag-1 wet the material refractory and appears to form a thin slag film covering the material (Figure 13a) and delaying the refractory degradation (Figure 13b). Some research works explain that one of the reaction products of the slag is a solid phase that forms as a continuos, adherent layer on the refractory surface, then this layer may act to delay the overall rate of corrosion of the refractory material (Lee and Zhang-1999, 87, Den Hoed-2000, 57).
Liquid metal modeling
On the other hand, the molten modeling results showed that there is a preferential vector direction of the liquid metal against to the induction furnace walls. Table 5 presents the modeling results. It can be seen that at low frequency (50 Hz) the Lorentz force is low (7.375 N) but the velocity is high (29.09 m/s) and its vector direction is parallel to the furnace walls, so is not a direct pressure that eroded the SiO2 refractory as can be observed on Figure 14. However, at high frequency (240 Hz) the Lorentz force is incremented (14.269 N) but the liquid metal velocity is lower (6.08 m/s), but its vector direction is perpendicular (against) to the furnace wall favoring the refractory erosion, see Figure 14 (circled area). Thus, this explain that capillary effect observed on the Figure 12, since the Lorentz force is higher at high frequency and providing the pressure needed to fill the pores. In refer to this, Lee and Zhang (1999, 88) explain that other physiochemical variables that effects a refractory – slag system as well the simple penetration due to wetting considered before are: (a) microstructural effects where a smooth surface and dense material may resist ingress whereas a porous one may not, (b) slag line attack and (c) the velocity of slag flow which is often a result of the Marangoni effect. More rapid movement will present fresh slag to the slag/refractory interface.
A vigorous agitation of the liquid metal is undesirable as it tends to pull out the fine grains in the brick by erosion (physical wear). An electromagnetic stirring velocity up to 50 cm/s is enough to provide a bulk material (slag and iron) with changing composition that favor the SiO2 degradation (Pötschke 2005, 112).
Conclusions
1.- A SiO2 base vibrable refractory was well sinterised obtaining a compacted matrix with not excess spalling, however the aggregates grains was not fully uniform distributed and forming cluster that favor the crack propagation across the grain boundary once the corrosion process start.
2. - Addition of B2O3 reduced the porosity percent of the SiO2 refractory but the remains pores favor the slag corrosion and contribute to the crack propagation due to the thermal changes.
3. - Scrap quality modified the molten composition producing a sort of slag more aggressive due to contain chemical elements (FeO and MgO) that will react with the temperature and liquid flow.
4. - The uncommon refractory degradation began on the material pores produced by the sintering process which became to be a variation of a typical corrosion failure, since an elephant's foot wear was expected instead.
5. – SiO2 refractory degradation was promoted as well by field electromagnetic forces (EMF), which was concentrated on furnace walls leading to an increasing temperature with a high stiring liquid flow that have a vector direction perpendicular to the lining face and increasing the pressure over the porosity of the refractory material.
Acknowledgements
The authors are very grateful with the Consejo Nacional de Ciencia y Tecnologia (CONACYT) Mexico, for supporting this work, to the PROMEP program, IIM/UMSNH and the UPJR for the facilities to do this work.
References
-Arahori T . y Suzuki T . (1987). Transformation of tridymite to cristobalite below 1470° C in silica refractories, Journal of Materials Science, (22-6): 2248 - 2252. [ Links ]
-Bhatia A. B.E. (2011), Overview of refractory Materials. http://www.pdhonline.org/cgi-bin/quiz/courses/courselist.cgi?class_name=m158 (01 de Julio de 2013). [ Links ]
-Den Hoed P, (2000), An Anatomy of Furnace Refractory Erosion: Evidence from a Pilot-Scale Facility, Ponencia presentada en 58th Electric Furnace Conference, USA. [ Links ]
-Freund Mareike, (2010). Corrosion behaviour of refractory materials in the systems Cr2O3 and Cr2O3 – Al2O3 against glass melts in subject to their composition and the thermal conditions, Refractories World Forum, (2): 1-4. [ Links ]
-Hemrick James G and Peters KM, (2005), Energy saving strategies for the use of refractory materials in molten material contact, Energy Technology Perspectives, Ponencia presentada en Symposia held during TMS Annual Meeting and Exhibition. USA. [ Links ]
- Huger M, Ghassemi Kakroudi M, Gault C, y Chotard T. (2009). Anisotropic behaviour of andalusite particles used as aggregates on refractory castables. Journal of the European Ceramic Society, (29): 571–579. [ Links ]
-Institute of Scrap Recycling Industries, (2013). Scrap specifications circular 2013. http://www.isri.org/ (01 de Julio de 2013). [ Links ]
-Jansson S. Brabie V. and Jonsson P. (2005). Corrosion mechanism and kinetic behavior of MgO-C refractory material in contact with CaO-Al2O3-SiO2-MgO slag. Scandinavian Journal of Metallurgy, (34-5): 283-292. [ Links ]
-Kalpakli Y. Kutmen, (2008). Effects of particles size distribution on the refractory properties and corrosion mechanism of ultra-low cement castables. Archives of Materials Science and Engineering, (2): 81-88. [ Links ]
-Kaupuzs J, Frishfelds V, Jakovics A and Nacke B, (2003), Influence of Melt Flow and Temperature on Erosion of Refractory and Deposit Formation in Induction Furnaces , Ponencia presentada en International Scientific Colloquium Modelling for Electromagnetic Processing, Hannover. [ Links ]
-Lee W. E. and Zhang S, (1999), Melt corrosion of oxide and oxide-carbon refractories, Internationals Metarilas Reviews, (44): 180-190. [ Links ]
- Liu Q, Zheng H, Lu C y Gao W. (2008). Corrosion resistance of high-alumina graphite based refractories to the smelting reduction melts, Journal of Materials Science and Engineering, (2): 49-53. [ Links ]
-Maity M. (2011). Updates on improving refractory lining service life. Hydrocarbon Processing, (1): 29-37. [ Links ]
-Mansuripur Masud. (2012). Trouble with the Lorentz Law of Force: Incompatibility with Special Relativity and Momentum Conservation. Physical Review Letters, (19): 1-5. [ Links ]
- Narasimham, A.V.L. (2007). Refractory lining failures in FECR furnaces an over view. Ponencia presentada en en Foro Alloy Industry, India. [ Links ]
-Pötschke J. y Deinet T. (2005). Premature corrosion of refractories by steel and slag. Ponencia presentada en Millennium Steel – Steel Making, Alemania. [ Links ]
-Ribeiro S. y Rodríguez J. A. (2010). The influence of microstructure on the maximum load and fracture energy of refractory castables. Ceramics International,( 36): 263–274 [ Links ]
-Rigaud M. (2011). Corrosion Handbook. Corrosion of refractories and ceramics. Edited by R. Winston Revie, 387 – 398. Canada. [ Links ]
-Sinha P, and Chandra S, (1998), An Optimum Design of the Lining of a Medium Frequency Induction Melting Furnace, Int. Trans. Opl Res. (5): 255-259. [ Links ]
- Wang M.C, Hon M.H. y Hsu C.C. (2008). Reaction between Magnesia–Chrome Brick/Slag Interface by Electric Furnace Static Slag Corrosion Test. Materials Transactions, (49): 107-113. [ Links ]
-Williams, D.C. y Ko, Y.H. (2000). Reducing elephant's foot erosion in coreless induction furnaces. Modern Casting, (1): 22 -26. [ Links ]














