Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Journal of the Mexican Chemical Society
versión impresa ISSN 1870-249X
J. Mex. Chem. Soc vol.56 no.1 Ciudad de México ene./mar. 2012
Article
Determination of Antioxidants in Fruit Tissues from Three Accessions of Habanero Pepper (Capsicum chinense Jacq.)
Lizbeth A. Castro–Concha, Ismael Canche–Chuc, and María de Lourdes Miranda–Ham*
Unidad de Bioquímica y Biología Molecular de Plantas, Centro de Investigación Científica de Yucatán, Calle 43 # 130, Chuburná de Hidalgo, Mérida, Yucatán, México. Tel.: (999) 9428330 Ext. 228. Fax: (999) 9813900. mirham@cicy.mx
Received January 31, 2011.
Accepted April 15, 2011.
Abstract
Three accessions of habanero pepper (Capsicum chínense Jacq.), differing in the color of the fruits throughout the maturation process, were analyzed for antioxidants, namely carotenoids, ascorbate and glutathione. The mature fruits from the three accessions contained high levels of the above mentioned antioxidants. Accession MR8H presented the highest content of red carotenoids, while in SBN01, the ascorbate level was the maximal found regardless of the maturation stage.
Key words: Capsicum chinense, carotenoids, ascorbate, glutathione.
Resumen
Se determinaron los antioxidantes: carotenoides, ascorbato y glutatión, en tres accesiones de chile habanero (Capsicum chinense Jacq.), que contrastaban en sus patrones de maduración. Los frutos maduros de las tres accesiones mostraron niveles altos de los tres tipos de antioxidantes mencionados. La accesión MR8H presentó el nivel más alto de carotenoides rojos, mientras que en la SBN01 se observó el contenido máximo de ascorbato sin importar el grado de maduración de los frutos.
Palabras clave: Capsicum chinense, carotenoides, ascorbato, glutatión.
Introduction
Recently, antioxidants have become an active research topic, given their protective properties towards oxidative damage in living organisms. The accumulation of reactive oxygen species have been linked to a number of diseases, including cancer, diabetes, cardiovascular and neurological disorders, and other aging related processes [1]. Antioxidants are frequently found in plant tissues, and include a wide range of metabolites from phenol acids, flavonoids, saponins, tannins, alkaloids, polysac–charides, to the most common one, ascorbic acid or vitamin C. Carotenoids also display an important antioxidant potential, and are vital for the mammalian diet, since they represent the precursors to vitamin A, whose deficiency causes a number of disorders ranging from impaired iron mobilization, growth retardation and blindness, to depressed immune response and increased susceptibility to infectious disease [2]. Besides the health benefits mentioned above, carotenoid accumulation in vegetables represent a desirable trait, since it confers them color, an attribute that influence consumers' choice.
Color intensity in fresh habanero peppers (Capsicum chinense Jacq.) is a valuable comercial feature [3], resulting from the accumulation of chlorophylls and yellow, orange and red carotenoids [4]. Interestingly, peppers also represent an excellent dietary source of antioxidants, including vitamins C and E, among others [1]. Prior to ripening, habanero pods are green–colored, turning to orange or red as they ripen. Some other cultivars can turn from green to orange, red or purple, while others turn from white or light green to red directly [1].
Habanero peppers are mainly appreciated for their extreme hotness or pungency, and most plant inventories focus on variations of capsaicin content among different varieties [5]. However, in general, peppers are considered an alternative source for vitamin C and other antioxidants, and thus these compounds may constitute an added value for their cultivation and human consumption. Antioxidant and other nutrimental properties can widely vary depending on genotype, developmental stage and plant growing conditions. Hence in the present work, we have analyzed the content of carotenoids, chlorophylls, ascorbate and glutathione in the pericarp and placental tissues from three accessions of C. chinense differing in the color of the fruits throughout the maturation process. These accessions were taken from a collection cultivated in the state of Yucatan, which possess the largest genetic diversity in México. Our results provide useful information to choose the best cultivar for human consumption given its nutraceutical value, based on anti–oxidant levels.
Results and Discussion
Pods from accessions MR8H, SBR01 and SBN01 were evaluated at different stages of maturation. Accession MR8H showed the largest variation in color during ripening: immature fruits were light green (1–15 d after anthesis), while the mature ones were bright red (36–45 d after anthesis), with intermediate bright green (16–25 d after anthesis) and orange (26–35 d after anthesis) stages. In contrast, accession SBR01 only turned from light green (1–30 d after anthesis) to bright red (31–45 d after anthesis), whereas accession SBN01, from bright green (1–30 d after anthesis) to orange (31–45 d after anthesis). Color in pepper pods results mainly from the accumulation of chlorophylls and carotenoids, which can function both as antioxidants as well as ultraviolet and visible light protectants [1].
In order to compare the available accessions regarding to their antioxidants' contents, levels of carotenoid, ascorbate and glutathione were analyzed in the pericarp and the placental tissue separately. Both tissues constitute the edible parts of the fruit, being the placenta the one responsible for pepper hotness [6].
Chlorophyll and carotenoid contents in the pericarp and placenta of the fruits from the different accessions at different stages are shown in Table 1. At comparative stages (namely bright green), the MR8H accession accumulated highest contents of total chlorophylls, and even when both MR8H and SBR01 ripen to red, red carotenoid content in the former was twice of those in the second one (Table 1). The main carot–enoids in red pepper pods are capsanthin and capsorubin which display maximal absorbance in the region between 440 and 460 nm [3], coinciding with those observed in the chromatogram from pod extracts (data not shown). Interestingly, orange color in pericarps of SBN01 accession was not due to p–carotene, but to other yellow carotenoids, such as violaxanthin, anthe–raxanthin, lutein and zeaxanthin, that have been found in C. annuum [3].
Ascorbate and glutathione are amongst the major components of the antioxidant system in plants, and take part in the Halliwell–Asada cycle, that provides a regular supply of reduced molecules, which are involved in the detoxification of reactive oxygen species in most cells. Total ascorbate contents were in similar levels among the three accessions analyzed, along the fruit maturation process (Table 1). However, some variations were detected in glutathione levels (Table 1). In MR8H, glutathione increased as pods ripened (Table 1). An opposite trend was found for SBN01, which presented its highest glutathione levels prior to ripening. No variations were detected in pods from SBR01 (Table 1).
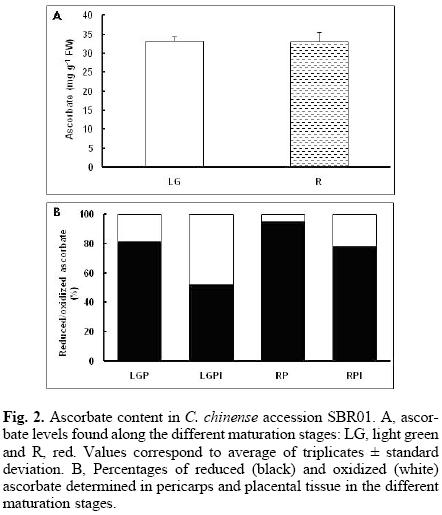
The correct functioning of the Halliwell–Asada cycle is cumbersome to cellular homeostasis since it can provide reducing power for growth and development. It is noteworthy that in pericarps from the four stages in MR8H, the balance is tipped to the reduced form of ascorbate, where it represents more than the 90% of the total (Fig. 1). However, in the immature stages in placental tissue, the oxidized form or dehydroascorbate represents approximately 50% of the total and, as the ripening process develops, this percentage gradually changes to present a maximum level of reduced ascorbate (Fig. 1). Similar patterns were detected in SBR01 and SBN01 accessions, i.e., pericarps presented the most ascorbate in the reduced form (above 80%), while in the placental tissue, this form increased its contents as ripening proceeded (Fig. 2).
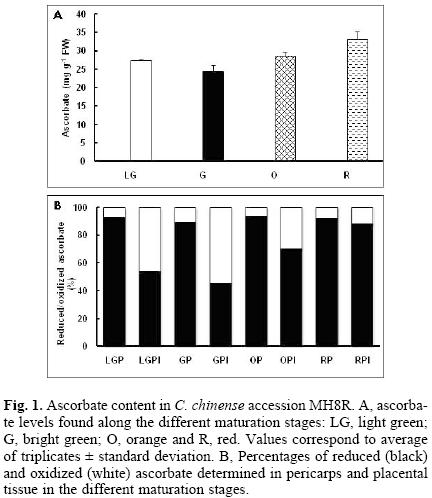
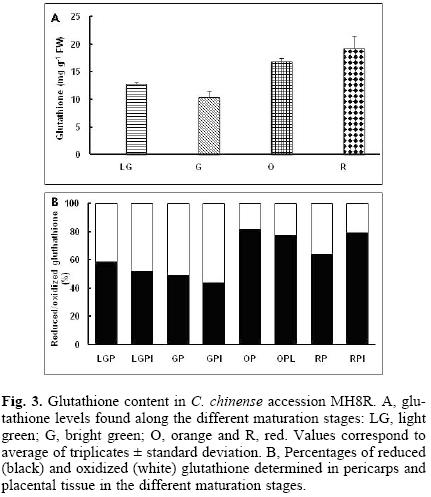
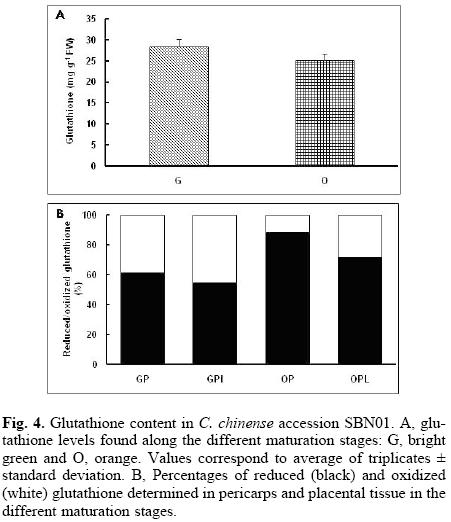
Total glutathione content in accession MR8H increased along ripening (Fig. 3), but the balance between the oxidized and the reduced forms was different to that observed for ascorbate. Ratio between reduced and oxidized glutathione was 1, both at light and bright green stages (pericarps and placentas), whereas at more mature stages (orange and red), the balance tended towards the reduced form, representing between 65 to 80% of the total content (Fig. 3). In the other two accessions, though total glutathione contents were similar for the different maturation stages, when the ratio between the reduced and oxidized forms was analyzed, a trend that has been previously observed in the MR8H accession, appeared, i.e. both the pericarp and placental tissue from the green fruits showed a ratio of 1, while in the mature (orange) stage, the reduced form was the major one (Fig. 4).
Yellow and red carotenoids contents are comparable to those found in Capsicum annuum (0.09–0.36 and 4.19 mg/100 g fresh weight (FW), respectively) [3], tomato puree (β–carotene, 0.63 mg/100 g FW) [7] and gazpacho (β–carotene, 0.345 mg/100 g FW) [8]. In contrast, the ascorbic acid content in C. chínense pepper pods is several–fold higher in the studied accessions than the levels found in other Capsicum species: seven–fold higher than in C. annuum c.v. Signal (392 mg/100 g FW) [3], ten to twenty–fold in several C. annuum and C. fru–tescens varieties (280– 137.5 mg/100 g FW) [9], and fifty–fold in freshly squeezed orange juice (47.96 mg/100 ml) [10].
Conclusions
The present study demonstrated that the largest concentrations of carotenoids, ascorbate and glutathione were found in the pericarps from ripe fruits from the three C. chínense accessions, thus they may provide more potential benefits for the maintenance of health compared to immature habanero peppers and even to other classical sources of antioxidants, such as tomato or orange juice. Therefore, habanero peppers should not only be considered a valuable spice, but also an important alternate source of antioxidants.
Experimental Section
Plant material. Chili peppers from accession MR8H were collected in Cuzamá Yucatan (20° 40' N; 89° 18' W), while accessions SBR1 and SBN1 were obtained from Kanasín Yucatán (20°53' N; 89°28' W) . The three varieties were collected in October–December 2009. Fruits were obtained at different stages, ranging from immature (light or bright green) to fully mature (orange or red). Fruits from each accession were collected from at least ten individual plants. They were transported to the laboratory and they were washed in soapy water and rinsed thoroughly before pericarps and placental tissues were delicately separated. Tissues were weighed, then frozen with liquid nitrogen and stored at –80°C until analysis.
Pigment extraction. Pigments (chlorophylls and carotenoids) were extracted as described by Hernández et al. [11], with some modifications. Fresh tissue (1 g fresh weight, FW) was ground with liquid nitrogen, extracted with 4 ml acetone (HPLC grade, J.T. Baker) and stored in the dark at 4°C for 24 h. The homogenate was centrifuged at 2561 x g for 20 min at 4°C to remove cell debris. The clear supernatant was collected and stored in the dark and 4°C until analysis.
HPLC separation and quantitation of carotenoids. It was performed using an HPLC HP Agilent 1200 system, with a PDA detector and a C–8 ZORBAX reverse–phase column (4.6 × 150 mm, 5 urn). The composition of mobile phase was a binary gradient elution system of: A) methanol/1 M ammonium acetate (70:30) and B) 100% methanol at a flow rate of 1 mL/min. Detection of pigment separation was performed at two wavelengths, 440 and 667 nm. Standards and samples were filtered and mixed with 1 M ammonium acetate in a ratio of 2:1. Twenty μL of the mixture were injected into the HPLC system, where the temperature was set to 25°C. Concentrations were calculated using their corresponding standard curves.
Ascorbate and glutathione determinations. Two–gram samples of pericarp or placental tissue were first ground with liquid nitrogen, and then homogenized with 2.6 mL 2.5 M H3PO4. Samples were centrifuged at 15,710 × g for 15 min at 4°C. Then, enough 1.25 M K2CO3 was added to reach pH 5.6. Extracts were centrifuged again at 15,710 × g for 5 min to remove any precipitate, and the clear supernatants were used to assay ascorbate and glutathione contents. Ascorbate and dehy–droascorbate were determined as described by Foyer et al. [ 12]. The assay is based on the oxidation of ascorbate by ascorbate oxidase in an acidic solution at 265 nm.
Reduced (GSH) and oxidized (GSSG) glutathione were determined according to Griffith [13]. Total glutathione content was quantified by reducing GSSG to GSH using yeast glutathione reductase, 5, 5'–dithio–bis–nitrobenzoic acid and NADPH and measuring its absorbance at 412 nm. GSSG was determined by the same method in the presence of 2–vinylpyridine. GSH content was calculated by subtracting the GSSG content from total glutathione level.
Acknowledgements
We gratefully acknowledge support for this project from Consejo Nacional de Ciencia y Tecnología, México (CONACyT), application 0104051. We are indebted to Dr. Felipe A. Vázquez–Flota for his critical review, and Dr. Nancy Santana for supplying us with C. chínense accessions SBN01 and SBR01.
References
1. Sun–Hwa, H.; Jung–Bong, K.; Jong–Sug, P.; Shin–Woo, L.; Kang–Jim Ch. J. Exp. Bot. 2007, 58, 3135–3144. [ Links ]
2. Cuttriss, A. J.; Mimica, J. L.; Howitt, C. A.; Pogson, B. J., in: The Structure and Function of Plastids, Cap. 16, Wise, R. R.; Hoober, J. K., Eds., Springer, New York, 2007, pp. 315–334. [ Links ]
3. Matsufuji, H.; Ishikawa, K.; Nunomura, O.; Chino, M.; Takeda, M. Int. J. Food Sci. Technol. 2007, 42, 1482–1488. [ Links ]
4. Hornero–Méndez, D.; Ladrón–Gómez, R. J. Agric. Food Chem. 2000, 48, 3857–3864. [ Links ]
5. Canto–Flick, A.; Balam–Uc, E, Bello–Bello, J.; Lecona–Guzmán, C.; Solís–Marroquín, D.; Áviles–Viñas, S.; Gómez–Uc, E.; López–Puc, G.; Iglesias–Andreu, L.; Santana–Buzzy, N. HortScience 2008, 43, 1344–1349. [ Links ]
6. Vázquez–Flota, F.; Miranda–Ham, M.L.; Monforte–González, M.; Gutiérrez–Carbajal, G.; Velázquez–García, C.; Nieto–Pelayo, Y. Revista Fitotecnia Mexicana 2007, 30, 353–360. [ Links ]
7. Sánchez–Moreno, C.; Plaza, L.; De Ancos, B.; Cano, M. P. Eur. Food Res. Technol. 2004, 219, 151–160. [ Links ]
8. Plaza, L.; Sánchez–Moreno, C.; De Ancos, B.; Cano, M. P. Eur. Food Res. Technol. 2006, 223, 210–215. [ Links ]
9. Kumar, O. A.; Tata, S. S. Not. Sci. Biol. 2009, 1, 50–52. [ Links ]
10. Plaza, L.; Sánchez–Moreno, C.; Elez–Martínez, P.; De Ancos, B.; Martín–Belloso, O.; Cano, M. P. Eur. Food Res. Technol. 2006, 223, 487–493. [ Links ]
11. Hernández Sandoval, F. E.; Ibarra Martínez, L. I., in: Métodos y Herramientas Analíticas en la Evaluación de la Biomasa Microalgal, Cap. 8, Arredondo–Vega, B. O.; Voltolina, D., Eds., CIBNOR, La Paz, 2007, 69–79. [ Links ]
12. Foyer, C. H.; Halliwell, B. Planta 1983, 157, 239–244. [ Links ]
13. Griffith, O. W. Anal. Biochem. 1980, 106, 207–212. [ Links ]
Note
Dedicated to Dra. Estela Sánchez de Jiménez in celebration of a life full of achievements, and to the memory of a great teacher, Q. Alicia Benítez de Altamirano.














