Introduction
Mexico’s shrimp farming sector harvest a total of 177,000 tonnes in 2021 according to the National Commission of Aquaculture and Fisheries (CONAPESCA). Mexico has around 900 shrimp farms, 45 hatcheries and over 40 shrimp processing plants. Sinaloa is the main producer of farmed shrimp (40.3 %), followed by Sonora (39.7 %) and Nayarit (7.5 %) (TheFishSite, 2021).
Only 65% of the crustacean is used for human consumption, the rest corresponds to the exoskeleton and cephalothorax (Núñez-Gastélum et al., 2011). The waste is almost completely discarded, except that only a little is processed as an excellent animal feed supplement (Nwanna et al., 2004).
Seafood by-products are valuable natural resources that show range of functionalities and hence potential materials for biomedical and nutraceutical industries (Senevirathne and Kim, 2012; Hernández-Zazueta et al., 2021a; Hernández-Zazueta et al., 2021b).
Shrimp industry wastes have attracted attention due to the presence of valuable bioactive compounds as proteins, lipids, pigments (carotenoids), chitin/chitosan, oligosaccharides, vitamins, etc. (Sachindra et al., 2006; Nirmal et al., 2020). Bioactive evaluations reported include antimicrobial activity (Stenotrophomonas maltophilia, Enterobacter cloacae and Bacillus subtilis; Lactobacillus helveticus, L. innocua, S. aureus, Citrobacter freundii, E. coli, and P. fluorescens) (Vilar et al., 2016; Djellouli et al., 2020), antioxidant, ACE (Angiotensin I converting enzyme) inhibitory activity, and antiinflammatory (Nirmal et al., 2020).
Natural products take the leading place in drug discovery of antimicrobial agents highlighting the fact that approximately 70% of antibiotics clinically used for treatment of infectious diseases are derived from nature (Brown et al., 2014).
To the best of our knowledge, this is the first report of of these L. vannamei by-products extracts activity against this set of clinical relevance bacteria (E. faecalis, S. aureus, S. epidermidis, E. coli, K. pneumoniae, P. aeruginosa, S. typhimurium; and M. bovis BCG).
Materials and methods
Shrimp material
A sample of white shrimp (L. vannamei) was obtained in the central-western region of the state of Sonora, in the town of Bahía de Kino (28 ° 49′22 ″ N 111 ° 56′27 ″ W). The raw material was separated into exoskeleton and cephalothorax, ground (Osterizer, Oster, USA) and stored in polyethylene bags at -18 °C until use.
Preparation of shrimp extracts
Twenty g of shrimp material were homogenized with 60 mL of solvent (proportion 1:3 weight/volume) (n-hexane, acetone, metanol or water, Químicos Fermont, Mexico) in a blender (Osterizer, Oster, USA) at high speed for 1 min and the resulting mixture was keppt in an Erlenmeyer flask at room temperature for 24 h in darkness. Solids were filtered out (filter paper Whatman no. 1), the extracts concentrated by evaporation under reduced pressure at 30 °C in a rotary evaporator (HS-2005S-N, Hahnvapor Hahnshin Scientific Co., Republic of Korea), and dried under N2 stream (López-Saiz et al., 2014; Osuna-Ruiz et al., 2016). The following extracts were obtained: exoskeleton hexanic, methanolic and acqueous extracts (ExHex, ExMe, ExAc); cephalothorax hexanic extract, acetonic and methanolic extracts (CeHex, CeAce, CeMe).
Antibacterial Activity
Bacterial strains
Bacterial strains used in this study: Gram-positive bacteria (Enterococcus faecalis American Type Culture Collection (ATCC) 51299, Staphylococcus aureus ATCC 25293, and Staphylococcus epidermidis) and Gram-negative bacteria (Escherichia coli ATCC 25922, Klebsiella pneumoniae, Pseudomonas aeruginosa ATCC 10145, and Salmonella typhimurium), obtained from the ceparium of the Department of Chemical Biological Sciences of the University of Sonora. Before testing, all bacterial strains were maintained frozen at - 70 °C in 10 % glycerol broth.
Preparation of working solution
Each organic extract was dissolved in 100 % dimethyl sulfoxide (DMSO, Sigma-Aldrich, USA) (20 mg mL-1) and maintained at room temperature for 1 h to assure their sterilization (Molina-Salinas et al., 2006). These extracts were diluted with fresh Mueller Hinton broth (BD DIFCO, Sweden) to their final concentrations of 50, 100, 200 and 400 µg mL-1.
Preparation of inoculum
Bacterial colonies grown on Mueller Hinton agar (MCD Lab, México) for 18 - 24 h (log phase of growth) were transferred to a sterile vial containing 15 mL of sterile 0.85 % saline solution. The bacterial suspension was disaggregated by agitation using a Genie II vortex, speed 3, for 1 minute, and left to stand for 10 min at room temperature. The super-natant was then adjusted to the optical density of OD630 nm= ~0.095, a turbidity matching the 0.5 McFarland standard (1.5 x 108 colony forming units CFU mL-1).
Antibacterial assay
In vitro antibacterial studies were carried out by the broth microdilution method as described previously (Velazquez et al., 2007; Navarro-Navarro et al., 2013). Briefly, 15 µL (2.25 x 106 CFU) of the inoculum (Velazquez et al., 2007) were inoculated into each well of a flat 96-well microplate (Costar, Corning, USA), containing 200 µL of different concentrations of the organic extracts (50-400 µg mL-1) in Mueller Hinton Broth (BD DIFCO, Sweden). Additionally, each antibacterial test included wells containing the culture media plus DMSO (2 %), in order to obtain a control of the solvent’s antibacterial effect. Gentamicin (12 µg mL-1) (AMSA, México) was used as positive control of bacterial growth inhibition. Bacterial cultures were incubated at 37 °C for 48 h. Plates were read at 630 nm in an enzyme -linked immunoassay (ELISA) microplate reader (Benchmark Microplate Reader, Bio-Rad, Hercules, USA) at 6, 12, 24, and 48 h. The optical density (OD630 nm) was corrected by subtracting the OD630 nm from wells with extracts alone in sterile broth. The minimal inhibitory concentration was defined as the lowest extracts concentrations that inhibited at least 50 % (MIC50) or 90 % (MIC90) of the bacterial growth after incubation at 37 °C for 24 h. MICs were determined using the following criteria (Baizman et al., 2000; Velazquez et al., 2007):
MIC50: (OD630 nm untreated bacteria - OD630 nm test con-centration)/(OD630 nm untreated bacteria) x 100 ≥ 50 %.
MIC90: (OD630 nm untreated bacteria - OD630 nm test con-centration)/(OD630 nm untreated bacteria) x 100 ≥ 90 %.
Antimycobacterial Activity of Shrimp Extracts Mycobacterial strain
Mycobacterium bovis bacillus Calmette -Guérin (M. bovis BCG) Danish strain was obtained from the ceparium of the BSL3 laboratory of the Biomedical Research Institute of the National Autonomous University of Mexico.
M. bovis BCG inoculum preparation
M. bovis BCG strain was cultivated in 50 mL of Middle-brook 7H9 broth (Becton Dickinson, USA) supplemented with 0.2 % (v/v) glycerol, and 10 % (v/v) ADC (albumin, dextrose, catalase enrichment) (Becton Dickinson, USA) (MDB 7H9) to which 0.02 % (v/v) tyloxapol (Sigma-Aldrich, USA) was added. Bacteria was incubated at 37 °C until an OD600 nm of 0.39 was reached (equivalent to 1 McFarland unit). Working bacteria solution was prepared by a 1:25 dilution in MDB 7H9 (Peñuelas-Urquidez et al., 2013; Guzmán-Gutiérrez et al., 2020).
Resazurin microtiter assay (REMA) to evaluate inhibitory activity against M. bovis BCG
This assay was adapted from Collins and Franzblau (1997). In 96-well polystyrene flat bottom plates, 200 µL of sterile distilled water were added to the perimetral wells, and 100 µL of MDB 7H9 broth were added to the remaining wells. Working solutions of extracts (2000 µg mL-1) were distributed into the first well of each row, and 2-fold dilution series were made using the following four wells. 100 µL of inoculum was added to each test well. The final concentrations in-test ranged from 31.25 to 500 µg mL-1. The controls of this experiment were: rifampicin (Sigma-Aldrich, USA) (concentrations of 16-9.7x10-4 µg mL-1), MDB 7H9, MDB 7H9 with bacteria, extracts (without bacteria), 2.5 % DMSO, and 2.5 % DMSO with bacteria. The microplate was sealed with parafilm and incubated for 6 days at 37 °C. Further, 30 µL of 0.01 % resazurin sodium salt (weight/volume) (Sigma-Aldrich, USA) (Palomino et al., 2002) were added to each well and plates were reincubated for 48 h. The minimum inhibitory concentration (MIC) was defined as the minimum concentration of crude extract that prevented the color shift from resazurin (blue) to resorufin (pink). Experiments were performed in triplicate.
Determination of the fatty acid profile
The fatty acid profile was determined as fatty acid methyl esters (FAMEs), which were prepared adding to 10 mg of the CeHex, 200 µL of benzene and 200 µL of the derivatizing reagent Meth-Prep II (GraceTM AlltechTM). This reagent is a 0.2 N methanolic solution of (m-trifluoromethylphenyl) trimethylammonium hydroxide. The transesterification reaction was carried out at room temperature for 30 minutes to obtain the FAMEs mixture. 1 µL of the FAMEs mixture was injected to the gas chromatograph (Agilent 6890) equipped with a flame ionization detector (FID), and the AT-FAME column (30 m x 0.25 mm). The analytical conditions were: injection 1 mL, injector temperature 250 °C, detector temperature 250 °C. The temperature gradient in the column oven starts at 180 °C for 15 min, followed by 10 °C / min increments up to 230 °C. The FAME standards retention times were used to identify the chromatographic peaks of the samples. Fatty acid content was calculated, based on the normalized peak area of detected FAMEs.
Results and discussion
Antibacterial activity
CeHex was the most potent extract, active against all Gram-positive and Gram-negative bacteria tested with a MIC50= 400 µg mL-1; subsequently, CeAce was active against E. faecalis, E. coli and K. pneumoniae (MIC50= 100, 400 and 400 µg mL-1, respectively). While CeMe resulted inactive. It is clear that the shrimp cephalothorax extracts were the most potent antibacterial samples tested. Non-polar or low polarity compounds may be the main responsible of the antibacterial activity described in CeHex. Despite antibacterials are collectively classified as large and polar compounds with relatively low lipophilicity (Mugumbate et al., 2015), in this research increasing polarity of solvents diminished the antibacterial activity. Solvent polarity is usually the most influential factor on the yield of the extraction (Bayona et al., 2018), has a great impact on selectivity, and influence directly on the solutes extracted, related to the chemical structure of the compounds (Lefebvre et al., 2020). Recent research described that the shrimp oil extracted from cephalotorax (with a 1:1 hexane/isopropanol mixture) possesses an important amount of cholesterol (89.1 ± 0.6 mg/g) (Raju et al., 2021), then it is possible that cholesterol is also present and abundant in the acetonic and methanolic extracts, affecting the antibacterial activity.
All exoskeleton extracts were inactive except for ExMe (MIC50= 50 ug/mL-1), active against E. faecalis, which was the most susceptible bacteria to shrimp extracts, followed by E. coli and K. pneumoniae (Figure 1, Table 1).
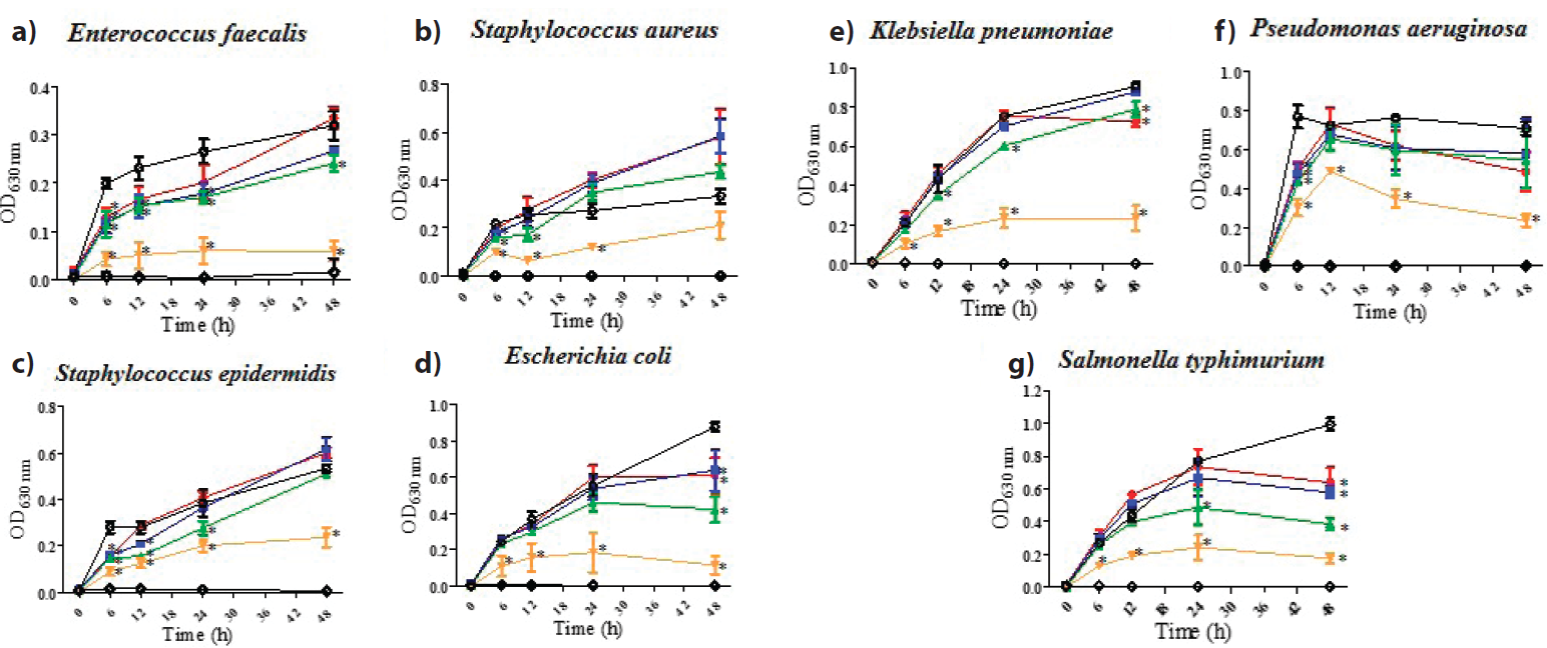
Figure 1 Antibacterial activity of shrimp cephalothorax hexanic extract, CeHex, evaluated at 50-400 µg mL-1 (●, 50 µg mL-1; ■, 100 µg mL-1, ▲, 200 µg mL-1; ▼, 400 µg mL-1; ◊, gentamicin 12 µg mL-1; ○, bacteria). All values represent mean of triplicate determinations ± SD. Significant differences (p < 0.05) from bacterial growth control are marked with an asterisk.
Figura 1 Actividad antibacteriana del extracto hexánico del cefalotórax de camarón, CeHex, evaluada a 50-400 µg mL-1 (●, 50 µg mL-1; ■, 100 µg mL-1, ▲, 200 µg mL-1; ▼, 400 µg mL-1; ◊, gentamicina 12 µg mL-1; ○, bacteria). Todos los valores representan el promedio de un triplicado ± Desviación Estándar. Dife-rencias significativas (p < 0.05) respecto al control de crecimiento bacteriano son marcados con un asterisco.
Table 1 Growth-inhibitory activity of shrimp exoskeleton, muscle, and head extracts against different Gram-positive and Gram-negative bacteria.
Tabla 1 Actividad inhibidora del crecimiento de bacterias Gram-positivas y Gram-negativas por extractos de exoesqueleto y cefalotórax de camarón.
| Strains | Crude shrimp extracta | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ExHex | ExMe | ExAc | CeHex | CeAce | CeMe | ||||||||
| MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | ||
| Enterococcus faecalis | >400 | >400 | 50 | >400 | >400 | >400 | 400 | >400 | 100 | >400 | >400 | >400 | |
| Gram-positive bacteria | Staphylococcus aureus | >400 | >400 | >400 | >400 | >400 | >400 | 400 | >400 | >400 | >400 | >400 | >400 |
| Staphylococcus epidermidis | >400 | >400 | >400 | >400 | >400 | >400 | 400 | >400 | >400 | >400 | >400 | >400 | |
| Escherichia coli | >400 | >400 | >400 | >400 | >400 | >400 | 400 | >400 | 400 | >400 | >400 | >400 | |
| Gram-negative bacteria | Klebsiella pneumoniae | >400 | >400 | >400 | >400 | >400 | >400 | 400 | >400 | 400 | >400 | >400 | >400 |
| Pseudomonas aeruginosa | >400 | >400 | >400 | >400 | >400 | >400 | 400 | >400 | >400 | >400 | >400 | >400 | |
| Salmonella typhimurium | >400 | >400 | >400 | >400 | >400 | >400 | 400 | >400 | >400 | >400 | >400 | >400 | |
aConcentration in µg mL-1
ExHex: Exoskeleton hexanic extract; ExMe: Exoskeleton methanolic extract; ExAc: Exoskeleton acqueous extract; CeHex: cephalothorax hexanic extract;
CeAce: cephalothorax acetonic extract; CeMe: cephalothorax methanolic extract.
Regarding L. vannamei waste, various studies have reported the inhibitory and bactericidal activity of chitosan prepared from shell waste against Gram-negative bacteria and Gram-positive bacteria (Vilar et al., 2016); moreover, its antibacterial activity against Xanthomonas sp. isolated from leaves affected with citrus canker was proven (Mohanasrini-vasan et al., 2014).
A very interesting review about the utilization of seafood processing by-products was published describing peptides, oligosaccharides, fatty acids, enzymes, oils, and biopolymers isolated from fishes, crustacean shells, and shellfish, with many biological activities as antibacterial, antiviral, and anticancer (Senevirathne and Kim, 2012).
Moreover, marine sponges, produces interesting antibacterial compounds, in example: Arenosclera brasiliensis produces alkaloids active against resistant S. aureus and P. aeruginosa (Torres et al., 2002); Cribrochalina sp. produces alkaloids (cribrostatin 3) active against Neisseria gonorrheae (Pettit et al., 2000), just to mention a few (Laport et al., 2009).
Antimycobacterial activity
M. bovis BCG was used as an alternative to Mycobacterium tuberculosis H37Rv as it owns similar profiles of anti-biotic susceptibility and offers a safer option for screening anti-tubercular compounds in a high-throughput format (Taneja and Tyagi, 2007; Altaf et al., 2010).
CeHex was the most active extract with a MIC100 of 250 µg mL-1, followed by CeAce with a MIC100 of 500 µg mL-1. The rest of the extracts (CeMe; ExHex, ExMe, and ExAc) were inactive against M. bovis BCG (Figure 2).
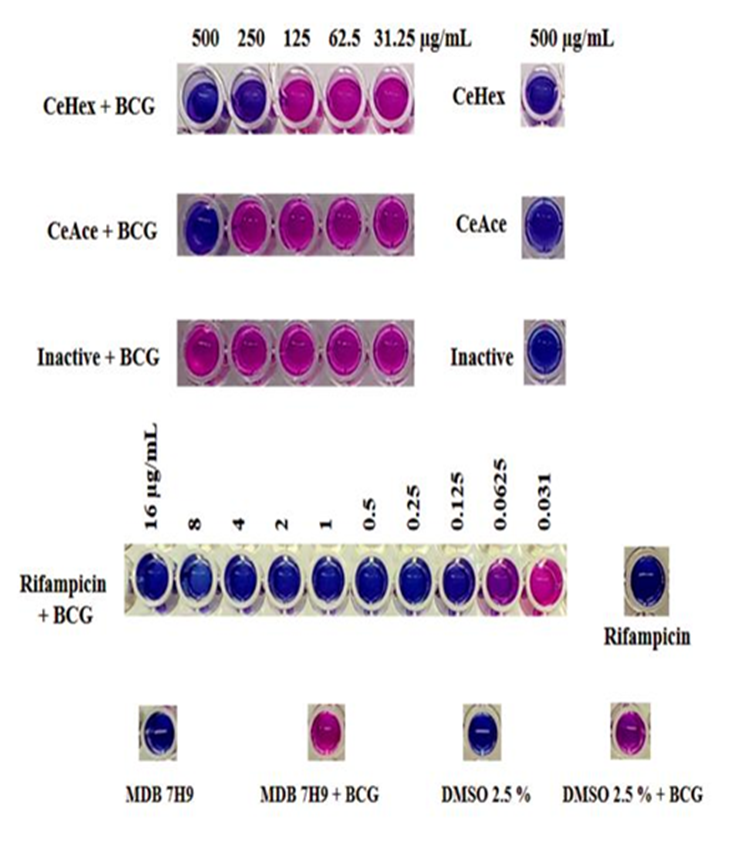
These results are in accordance to previous reports where non-polar extracts are recommended to find anti-mycobacterial compounds (Coronado-Aceves et al., 2016).
Previous studies have considered that an extract is active against mycobacteria if MIC is ≤250 µg mL-1 (as exerted by CeHex); however, it is also relevant to consider toxicological studies (Coronado-Aceves et al., 2016; Jurno et al., 2019).
Chitosan obtained by deacetylation of chitin extracted from shrimp shell wastes has been used for the encapsulation of panchovillin, isolated from Erythrina schliebenii, and its antimycobacterial activity was demonstrated over Mycobacterium indicus pranii using Galleria mellonella larvae as an in vivo infection model (Rwegasila et al., 2016).
Another study investigated two edible marine algae, Ulva lactuca and Ulva intestinalis, finding that both extracts inhibit the mycobacterial biofilm development (Mukherjee et al., 2021).
Finally, remarkable reviews have been published describing more than 250 antimycobacterial metabolites from marine natural products (Daletos et al., 2016; Wang et al., 2018).
Determination of the fatty acid profile
Some investigations have been carried out to elucidate the content of primary and secondary metabolites present in shrimp, as well as its biological activity of extracts of different polarity and/or fractions of the muscle, exoskeleton, head, and tail (Núñez-Gastélum et al., 2011; López-Saiz et al., 2014).
Shrimp muscle is reported to have a high content of high-quality protein and a low proportion of fatty acids (López-Saiz et al., 2016; AlFaris et al., 2021) and consequently, it is the part of the shrimp with the highest commercial value. The exoskeleton, head, and tail are often discarded or transformed into feed for aquaculture or supplemented as feed for animals (Nwanna et al., 2004). Various bioactive compounds have been described from all parts of the shrimp (Mandeville et al., 1992; Heu et al., 2003; Sachindra et al., 2006; López-Saiz et al., 2014; Bharathi et al., 2019), which is why the waste generated from this crustacean has attracted attention in recent years. It has been possible to separate chitin, proteins, and lipids from shrimp waste (Núñez-Gastélum et al., 2011) and it has been reported that pigments such as astaxanthins and fatty acids are found within the lipid fraction (Armenta et al., 2002; Kandra et al., 2012; López-Cervantes et al., 2010).
The presence of polyunsaturated fatty acids has been described in shrimp oil obtained from cephalothorax, mainly EPA (eicosapentaenoic acid) and DHA (docosahexaenoic acid) (Núñez-Gastélum et al., 2011; Takeungwongtrakul et al., 2012; Gulzar and Benjakul, 2018). These fatty acids belong to the group of ω-3 and therefore, they are widely valued for their nutraceutical and medicinal applications.
Oleic acid has been detected within the most abundant fatty acids extracted from shrimp cephalothorax (Take-ungwongtrakul et al., 2012).
In order to know a more complete profile of fatty acids present in CeHex, derivatization was carried out to form the methyl esters of the fatty acids and they were analyzed by gas chromatography (GC) (Figure 3). Fatty acid methyl esters were identified with the use of standards.
Table 2 shows the retention times of the detected peaks, the area percentage, and the methyl ester to which it corresponds according to its retention time. The fatty acid methyl esters that were identified in the highest proportion are oleate, linoleate, and palmitate (Figure 4). Other fatty acid methyl esters that were identified in lower proportions are stearate, palmitoleate, linolenate, and behenate (Figure 4). In another investigation where shrimp head oil was analyzed by gas chromatography, 14 fatty acid methyl esters were identified, of which it is confirmed that the main fatty acids are oleic, in a similar proportion to linoleic, followed by palmitoleic.
Tabla 2 Identificación de ésteres metílicos de ácidos grasos.
| # peak | Methyl ester | Fatty acid | Retention time (min) | % Area |
| 3 | Mehyl palmitate | C16:0 | 4.691 | 25.764 |
| 4 | Methyl palmitoleate | C16:1n7 | 5.098 | 2.866 |
| 6 | Methyl stearate | C18:0 | 8.772 | 8.338 |
| 7 | Methyl oleate | C18:1n9 | 9.357 | 29.294 |
| 9 | Methyl linoleate | C18:2n6 | 10.875 | 27.896 |
| 11 | Methyl linolenate | C18:3n3 | 13.427 | 1.806 |
| 23 | Methyl behenate | C22:0 | 20.311 | 4.036 |
Similarly, the methyl esters of fatty acids C16: 1n7, C18: 0, and C18: 3n3 were identified in lower proportions (Núñez-Gastélum et al., 2011).
DHA, EPA, among other fatty acids could not be identified by gas chromatography due to the lack of standards. Additionally, other studies of shrimp by-products show that the highest proportion of fatty acids are unsaturated (Heu et al., 2003; Núñez-Gastélum et al., 2011; Takeungwongtrakul et al., 2012; Gulzar and Benjakul, 2018).
Regarding the antibacterial activity of the FFA found in CeHex, their main target is the cell membrane, producing disruption of the electron transport chain (ETC) and oxidative phosphorylation, interfering with cellular energy production, inhibition of fatty acid biosynthesis enzyme activity, impair active nutrient uptake, and induces autolysis and leakage of cell metabolites by pore formation (Desbois and Smith, 2010; Yoon et al., 2018).
Oleic acid treatment in S. aureus increases membrane permeability and fluidity, leading to cell death (Chamberlain et al., 1991); while, a linolenic acid treatment induces the release of intracelular content. Morerover, linoleic acid induces the disruption of the ETC in S. aureus (Greenway and Dyke, 1979). Also, oleic acid or linoleic acid, produce lysis of Strepto-coccus faecalis (Carson and Daneo-Moore, 1980).
Finally, regarding to the anti-M. bovis BCG activity of CeHex, MIC values of linolenic acid (α- and γ- form) and conjugated linoleic acid (CLA) against the viability of M. tu-berculosis were determined as 75 µg mL-1 and 100 µg mL-1, respectively (Choi, 2016). Palmitic, linoleic and oleic acid have MICs of 25-50, 50-100, and 100 µg mL-1 against M. tuberculosis H37Rv (Sandoval-Montemayor et al., 2012). Increased levels of myristic, palmitic, oleic, and linoleic fatty acids in sera of guinea pigs induced tuberculocidal effect toward M. bovis BCG (Kochan and Berendt, 1974); long-chain fatty acids (oleic, linoleic, myristic, lauric, and palmitic) were reported to be mycobactericidal; while unsaturated fatty acids showed strong bactericidal activity in low concentrations (Kondo and Kanai, 1972; Kanetsuna, 1985).
Conclusions
Shrimp cephalothorax was the most promising by-product tested with antibacterial and antimycobacterial potential. Hexanic (non-polar) extract of shrimp cephalothorax resulted the most active against Gram-positive, Gram-negative bacteria, and M. bovis BCG. GC analysis of CeHex demonstrated the presence of three main, and four minoritarian, fatty acids. The strong antibacterial activity of CeHex and the identification of its main chemical constituents justify further studies on its biomedical and nutraceutical applications of this marine by-product. To the best of our knowledge, this is the first report of the antibacterial properties and chemical characterizaction of CeHex.











 nueva página del texto (beta)
nueva página del texto (beta)




