Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the coronavirus that causes the infectious disease known as coronavirus disease 2019 (COVID-19). Its global spread led the WHO to declare it a public health emergency of international concern. SARS-CoV-2 is the seventh zoonotic coronavirus to infect humans. Its RNA genome is 80% similar to that of SARS-CoV1. It encodes several structural proteins, including spike (S), which regulates viral entry by binding to angiotensin-converting enzyme 2 (ACE2). This cellular receptor is expressed in respiratory epithelium, vascular endothelium, alveolar monocytes, and macrophages, among others2.
The binding of ACE2 and S proteins triggers a membrane fusion reaction initiated by transmembrane serine protease 2 by cleaving S protein. Subsequently, the virus enters the cell by clathrin-mediated endocytosis3 and its genetic material is released into the cytosol where it is translated into non-structural and structural proteins. The former assemble in membranes derived from the endoplasmic reticulum and replicate viral RNA. The latter assemble into viral particles and exit the cell through the lysosomal pathway4.
During infection, the SARS-CoV-2 virus primarily enters type II pneumocytes, where an innate immune response is initiated. Macrophages initiate this response in the pulmonary alveoli, which secrete mediators such as tumor necrosis factor (TNF) and recruit lymphocytes and neutrophils, which release proinflammatory cytokines (interleukin [IL]-1, IL-6, and IL-8) and reactive oxygen species on entry into the alveoli2.
SARS-CoV-2 can also infect monocytes, macrophages, dendritic cells, and lymphocytes. These cell lines in severe COVID-19 undergo dysregulated cytokine release, or "cytokine storm" syndrome, which dramatically increases leukocyte recruitment and causes endothelial cell and pneumocyte injury. This leads to pulmonary capillary leakage and surfactant abnormalities that compromise alveolar gas exchange, resulting in acute respiratory distress syndrome, multi-organ failure, and, in the worst case, death2.
As of December 2020, COVID-19 disease had a global case fatality rate of 2.3% and a case fatality rate of 8.8% in Mexico5. COVID-19 is a systemic disease that presents with asymptomatic or presymptomatic infection. In symptomatic patients, mild, moderate, severe, very severe and critical disease occurs, as described in table 1, with the most common symptoms being fever, cough, dyspnea, and loss of smell and taste6,7.
Table 1 Clinical phases of COVID-19
| Clinical phases | Symptoms | Symptom management |
|---|---|---|
| Mild | Fever, cough, altered sense of smell or taste, fatigue, myalgia or arthralgia, expectoration, chest pain, without evidence of pneumonia | Antipyretic/analgesic Outpatient |
| Moderate | Lower respiratory tract disease. Radiological evidence of pneumonia | Antibiotics. Hospitalized, no oxygen requirement |
| Severe | Pneumonia, RR > 30, SpO2 < 90% PI > 50% (PaO2/ FiO2) < 300 mmHg | Venous thromboembolism prophylaxis, antibiotics, and corticosteroids. Without oxygen therapy or with low flow |
| Very severe | PaO2/FiO2 < 200 mmHg Hyperinflammation, acute respiratory distress syndrome | Antimicrobial treatment, venous thromboembolism prophylaxis, and pulmonary vasodilator. Non-invasive or high flow oxygenation. Intensive care unit |
| Critical | Acute respiratory distress syndrome, sepsis, hypoxemic respiratory failure and hemodynamic instability, hypercoagulability, and multiorgan failure. PaO2/FiO2 < 150 mmHg | Antimicrobial treatment, venous thromboembolism prophylaxis, pulmonary vasodilator, corticosteroids, endotracheal intubation, and mechanical ventilation. Intensive care unit |
COVID-19: coronavirus disease 2019; PaO2/FiO2: ratio of arterial partial pressure of oxygen to fraction of inspired oxygen; PI: pulmonary infiltrates; RR: respiratory rate measured in breaths per minute; SpO2: blood oxygen saturation levels7,8.
During the 1st year of the pandemic, management of COVID-19 included infection control measures, symptom management, and intensive care support for severe or critical illness1. However, clinical trials were also initiated, starting with drug repositioning. As a result of the tremendous effort and guidance on clinical management strategies to deal with COVID-19, numerous clinical trials were completed; however, they encountered various problems, including several that were never completed due to difficulties in patient recruitment or follow-up.
At present, despite the availability of various types of RNA- and DNA-based vaccines, it is still necessary to develop effective therapies to prevent severe cases of COVID-19 and death. For example, in 2022, the mortality rate was 4.7% in Mexico and 1% worldwide6. This review identified drugs that showed efficacy in clinical trials for treating SARS-Cov-2 and COVID-19 disease during the 1st year of the pandemic.
Many natural and synthetic compounds have been described and suggested in databases as potential inhibitors of COVID-19 development and progression. However, many of the compound repositioning studies have been performed by in silico computational studies without being supported by in vitro, in vivo, or clinical studies to demonstrate their activity. Therefore, such results were excluded from the present review. Similarly, papers on the use of hyperimmune immunoglobulin from patients who have developed COVID-19 disease were excluded due to the complexity of evaluating each patient from whom the anti-SARS-CoV-2 sera were obtained. Studies related to vaccine development were also excluded because this type of biologic is classified as a prophylactic treatment rather than a drug for treating patients with COVID-19.
Methods
A search was performed in specialized databases available at UNAM and open access databases, using the following search strategy in the Title, Abstract, and Keywords fields: (sars AND cov 2 AND inhibitor AND NOT [docking OR "in silico" OR "virtual screening" OR computational OR antibody OR antibodies OR plasma OR "network model" OR immunoinformatics OR epitope]).
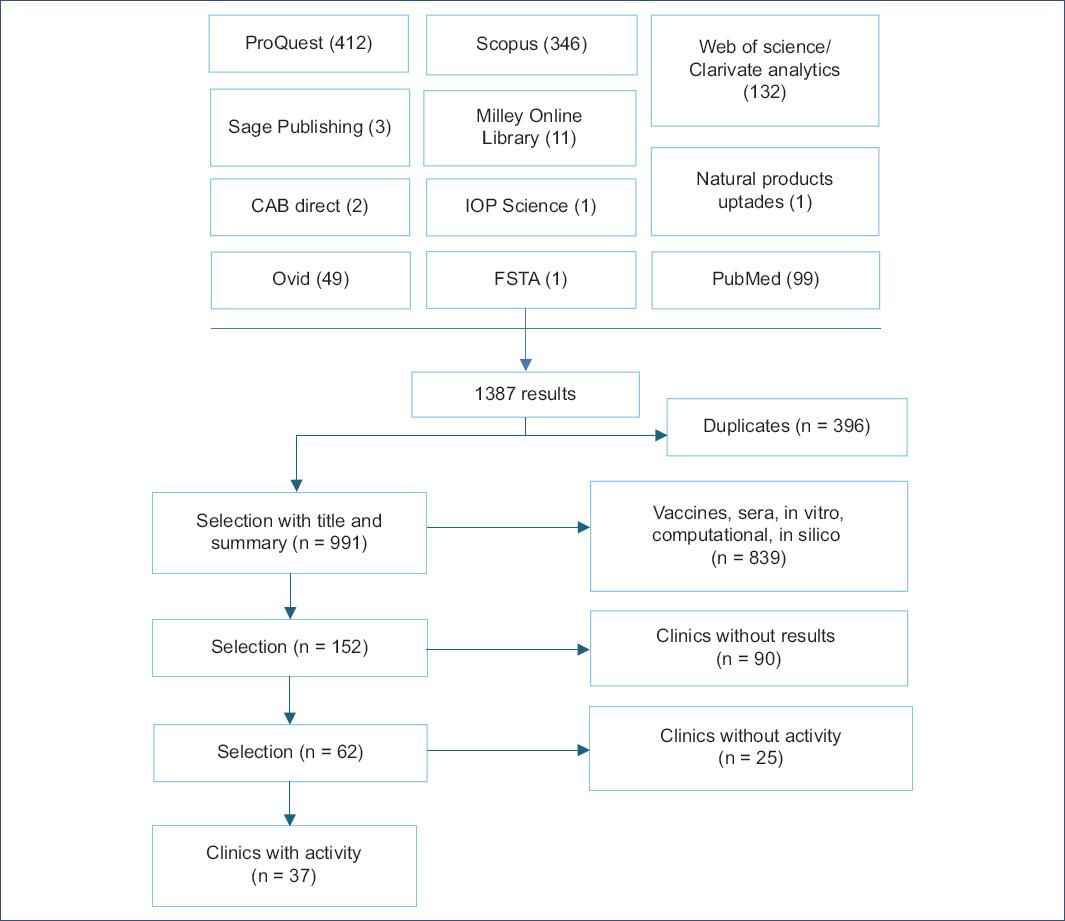
The following types of studies were included in this work: Adaptive clinical, randomized, non-randomized, cross-assignment, placebo, blinded, double-blind, triple-blind, quadruple-blind, exploratory, interventional, longitudinal, observational, parallel, prospective, retrospective, single-center, multicenter, single-group, and triple-group studies describing the activity of drugs against SARS-CoV-2 and COVID-19 and their effect on reducing mortality, respiratory failure, viral load, disease progression, hospitalization, or recovery time. Only full-length articles in English and Spanish in which the study started between December 31, 2019, and December 31, 2020, were considered, and the results of these studies were reviewed until November 01, 2021. The search procedure is shown in Fig. 1.
After obtaining a total of 1387 articles, we eliminated duplicates, leaving a total of 991 unique articles. A selection was then made based on the title and abstract, excluding studies related to hyperimmune sera from recovered patients and those focused on vaccine development. Computational, in silico, and in vitro studies were also excluded because they used different terms to define the type of research, making it difficult to identify them using Boolean operators. In the final screening stage, 152 relevant articles were identified. However, 90 studies that were not completed by November 2021 and 25 drug-related studies that showed no activity were excluded. As a result, a total of 37 articles were included in this review.
Results
Effective treatments
Drugs, an herbal remedy, and a vitamin were documented; 24 were used alone, and 12 in combination with others. These successfully reduced mortality, hospitalization, respiratory failure, viral load, disease progression, or recovery time in patients with COVID-19. Most are available internationally and have been identified in repositioning studies. Table 2 lists the drugs, vitamins, and remedies that were administered individually, while table 3 lists the combined therapies. For each treatment, the identifier and type of trial, the severity of the disease, the sponsor and country in which the trial was conducted, and the trial results are presented.
Table 2 Clinical studies of individually administered drugs against COVID-19 that showed effectiveness
| Drug | Study identifier | Type of study | Sponsor/Country of study | Phase, (Patients), Severity | Results | References |
|---|---|---|---|---|---|---|
| Acalabrutinib | NCT00001467; NCT0 1200953 | IN | Astra Zeneca/US | (19) Severe | Improved lung function and decreased inflammation | 47 |
| Anakinra* (Kineret) | NCT04357366 | OG, NoR, OL | II (1000) Severe | Reduced respiratory failure and mortality by 50% | 11 | |
| Aviptadil (Zyesami) | NCT04311697 | R, PL, MC | NeuroRx, Inc/US | II (196) Critical | Reduced respiratory failure and mortality | 10 |
| Baricitinib | NCT04393051 | IN/NRC | Azienda Ospedaliero U. Pisana/ IT | II (126) Moderate- Severe | Reduced inflammation, viral load, clinical worsening, and mortality | 38 |
| Bevacizumab* | NCT04275414 | IN, OG, OL | Qilu Hospital, Renmin Hospital of Wuhan/CN, IT | II (27) Severe, very severe | Reduced fever, duration of oxygen support, and mortality | 70 |
| Pyridostigmine bromide | NCT04343963 | IN, R, QB, PL | Instituto Nacional Salvador Zubirán/ MX | II (436) Very severe | Reduced the need for mechanical ventilation and mortality | 15 |
| Chloroquine | NCT04323527 | IN, R, DB | Fundação Medicina Tropical Dr. H. V. /BR | IIb (81) Critical | At low doses, CQ decreased mortality | 19 |
| Colchicine† | NCT04322682 | IN, R, DB, PL | Montreal Heart Institute/CA | III (4488) Mild | Reduced mortality and hospital admissions | 31 |
| Eculizumab* | NCT04346797 | R, PR, OL, OG | Publique Hopitaux de Paris/ FR | II (8) Severe | Six patients improved significantly | 32 |
| Favipiravir | CTRI/2020/05/025114 | OL, R, OG, PR, MC, TE | IN | III (150) Mild- moderate | The duration of clinical signs and symptoms decreased | 61 |
| Favipiravir versus umifenovir | ChiCTR2000030254 | PS, OL, MC, R | CN | (240) Moderate | Higher patient recovery rate at day 7 with favipiravir | 62 |
| Fluvoxamine | NCT04342663 | DB, R, PL | Washington University/ US | II (152) Mild | Reduced disease progression | 21 |
| Nitric oxide gas | NCT04305457 | IN, R, OL, TE. | Massachusetts General Hospital/ US | II (29) Severe | Reduced respiratory distress and prevented readmissions | 72 |
| LMWH | NCT04323761 | OB, PS | University Hospital of Pisa/IT | (244) Moderate- severe | Reduced risk of disease progression and mortality | 24 |
| Imatinib mesylate | EudraCT 2020-001236-10 | IN | Amsterdam Medical Center Foundation/ NL | (385) Very severe | Reduced mortality and duration of mechanical ventilation | 37 |
| Drug | Study identifier | Type of study | Sponsor/Country of study | Phase, (Patients), Severity | Results | References |
| Nigella sativa oil‡ | NCT04401202 | IN, R, PR, PS | King Abdulaziz University/SA | II (183) Mild | Significantly reduced recovery time | 27 |
| Nigella sativa and honey‡ | NCT04347382 | IN, R, PR, PL | Sohaib Ashraf/ PK | III (313) Moderate- severe | Reduced symptoms, viral load, and mortality rate | 26 |
| Opaganib | NCT04414618 | IN, R, PR, PL. | RedHill Biopharma Limited/US, IL | II (42) Severe | Reduced supplemental oxygen requirement | 51 |
| Ravulizumab* | NCT04369469 | IN, R, PR, OL | Alexion Pharma /US | III (22) Severe | Reduced terminal complement C5 convertase | 34 |
| Remdesivir | NCT04292730 | IN, R, PR, OL, ST | Gilead Sciences/US, CN, FR, DE, HK, IT, JP, KR, NL, SG, ES, SE, CH, TW, GB | III (584) Moderate | The 5-day treatment improved the clinical condition | 57 |
| Remdesivir | NCT04280705 | IN, R, DB, PL. | Europe, Asia, and America | III (1062) Moderate-Severe | Reduced recovery time | 23 |
| Talidomide | NCT04273529 | R, PR, QB | H. Wenzhou Medical University/CN. | II (12) Critical | Recovery in half the time of the control group | 17 |
| Tocilizumab* | NCT04331795 | NoR, OG, OL | University of Chicago/ US | II (32) Severe | It was associated with clinical and laboratory improvement in patients | 22 |
| Tofacitinib | NCT04469114 | R, PL, PR | Albert Einstein Israelite Hospital /BR | III (289) Moderate | Reduced mortality | 44 |
| Umifenovir | PS, NoR | CN | (62) Mild | Reduced symptoms of fever, cough | 63 | |
| Vitamin D3† | NCT04560608 | Quasi-experimental | Angers University Hospital/FR | (77) Moderate | Reduced disease progression and mortality | 75 |
*Biologics.
†Natural products or their semi-synthetic derivatives.
‡Herbal remedy. COVID-19: coronavirus disease 2019; DB: double-blind; IN: interventional; MC: multicenter; NoR: non-randomized; NRC: non-recruiting; OB: observational; OG: one group; OL: open-label; PL: placebo; PR: parallel; PS: prospective; QB: quadruple-blind; R: randomized; ST: standard therapy; TB: triple-blind; SA: Saudi Arabia; BR: Brazil; CA: Canada; CH: Switzerland; CN: China; DE: Germany; ES: Spain; FR: France; GB: United Kingdom; GR: Greece; HK: Hong Kong; IN: India; IL: Israel; IT: Italy; JP: Japan; KR: South Korea; MX: Mexico; NL: Netherlands; PK: Pakistan; SE: Sweden; SG: Singapore; TW: Taiwan; US: United States; LMWH: low-molecular-weight heparin.
Table 3 Clinical studies of combination therapies against SARS-CoV-2 and COVID-19 that showed efficacy against the disease
| Drug | Study identifier | Type of study/Status | Sponsor/Country of study | Phase, (Patients), Severity | Results | References |
|---|---|---|---|---|---|---|
| Baricitinib and LPV/R | RT, OL, NoR | Ministero "Ricerca corrente"/IT | I (24) Moderate | Reduced symptoms and disease progression | 42 | |
| Baricitinib and LPV/R | NCT04358614 | OB, RT, LN, MC | Hospital de Prato/IT | II (191) Moderate | Reduced symptoms, viral load, and disease progression | 43 |
| Baricitinib and corticosteroid | EUPAS34966 | OB, PR, SC | U. General Hospital of Albacete/ES | (112) Moderate - severe | Improved pulmonary function and reduced the need for oxygen | 41 |
| Danoprevir and ritonavir | NCT04291729 | IN, OG, OL /CM | The Ninth Hospital of Nanchang/CN | IV (11) Moderate | Suppressed viral replication in less than a week | 53 |
| HQ and oseltamivir | NCT04303299 | IN, R, PR, PL | Rajavithi Hospital/TH | III(320) Mild | At high doses, viral load decreased compared to the control | 65 |
| HQ and oseltamivir | NCT04349241 (-) | R, IN, OL, ST | Ain Shams University/ EG | III (100) Mild- moderate | Decreased viral load in 8 days | 64 |
| HQ, FVP, DRV, and ritonavir | NCT04303299 | IN, R, PR, ST | Rajavithi Hospital/TH | III(320) Moderate- severe | Together they had higher viral clearance | 65 |
| IV, AAS, DEX, and ENOX* | NCT04425863 | OB,PS/ CM | Eurnekian Public Hospital/AR | 167 Mild-severe | Reduced mortality and disease progression | 78 |
| LPV-R, IFNβ-1b, ribavirine | NCT04276688 | IN, MC, PR, OL | The University of Hong Kong/ HK | II (127) Mild- moderate | Significantly reduced viral load | 56 |
| Novaferon and or LPV/R | ChiCTR2000029496 | R, OL, PR, IN | The First Hospital of Changsha /CN | (89) Moderate- severe | Significantly reduced viral load | 46 |
| Remdesivir alone or with baricitinib | NCT04401579 | IN, R, PR, DB, MC, PL/ CM | I. Allergy and Infectious Diseases/ US, DK, JP, KR, MX, SG, ES, GB | III (1033) Moderate- severe | Baricitinib with remdesivir reduced recovery time and improved clinical status | 58 |
*Natural products or their semi-synthetic derivatives. COVID-19: coronavirus disease 2019; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; CM: completed; DB: double-blind; IN: interventional; LN: longitudinal; MC: multicenter; NoR: non-randomized; OB: observational; OG: one group; OL: open-label; PL: placebo; PR: parallel; PS: prospective; R: randomized; Rt: retrospective; SC: single center; ST: standard therapy. AR: Argentina; CN: China; DK: Denmark; EG: Egypt; ES: Spain; GB: United Kingdom; HK: Hong Kong; IT: Italy; JP: Japan; KR: South Korea; MX: Mexico; SG: Singapore; TH: Thailand; US: United States; ASA: Acetylsalicylic acid; DEX: Dexamethasone; DRV: Darunavir; ENOX: Enoxaparin; FVP: Favipiravir; HQ: Hydroxychloroquine; IFN: Interferon; IV: Ivermectin; LPV/R: Lopinavir with Ritonavir. (-): retracted article
Among the therapies that promoted clinical improvement, we highlight those that reduced the rate or probability of death by 50% or more in patients with severe, very severe, or critical illness. Aviptadil reduced mortality by 50% in critically ill patients. While in patients with very severe disease, the reduction in the rate of mortality was in first place bevacizumab (no mortality), in second place the combination of ivermectin, dexamethasone, aspirin, and enoxaparin (71%), and in third place pyridostigmine bromide (63.3%). In severe patients, Nigella sativa seeds with honey also reduced mortality (~70%), followed by low-molecular-weight heparin (64%), the combination therapy of hydroxychloroquine with favipiravir, darunavir, and ritonavir (16-4%), baricitinib (50% and 58% with corticosteroids) and anakinra (50%), as well as the combination of hydroxychloroquine, favipiravir, darunavir, and ritonavir (25%). We also highlight tocilizumab as it significantly reduced the time to clinical discharge (at day 4) (Tables 2 and 3).
However, given that the disease develops in two phases, an initial phase of high viral replication and a second phase of inflammation7,8, controlling the infection before the hyperinflammatory response is triggered is important. Therefore, we highlight the activity of those treatments that, in mild-to-moderate disease, promoted patient recovery before seven days or significantly reduced viral load, such as danoprevir with ritonavir, hydroxychloroquine with oseltamivir, the combination of baricitinib with lopinavir and ritonavir, multidrug therapy with lopinavir, ritonavir, interferon (IFN) β-1b, and ribavirin, in addition to, umifenovir, favipiravir, novaferon, tofacitinib, and colchicine (Tables 2 and 3).
Mechanism of action
Regarding the mechanism of action of the drugs, vitamins, and natural products that have shown efficacy in clinical trials have been found to act by inhibiting cytokine release, inflammation, tubulin polymerization, complement system, tyrosine kinases, viral replication, thrombosis, chemotaxis, or promote bronchodilation, or are regulators of innate and adaptive immunity. They are presented below according to their biological activity.
Anti-inflammatory drugs that regulate the release of proinflammatory cytokines
Aviptadil (Zyesami) is a vasodilator neuropeptide with anti-inflammatory and immunomodulatory activity. It binds to the vasoactive intestinal peptide (VIP) receptor type 1 in type II pneumocytes and blocks chromatin condensation and fragmentation by caspases, thus preventing cell apoptosis (Fig. 2)9; it also blocks the release of IL-6 and TNFα10. When used in COVID-19, Aviptadil reduced respiratory failure and mortality by ~50% when patients were treated with the maximum standard of care in tertiary care hospitals but not in regional secondary care hospitals10.
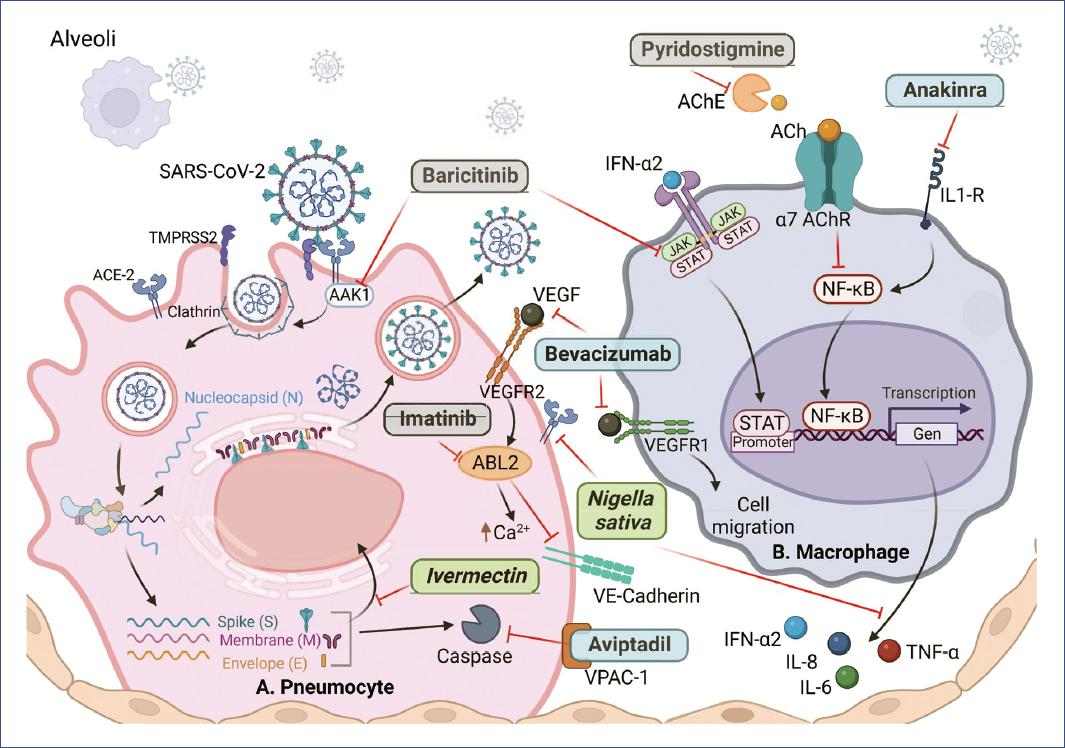
Figure 2 Drugs with greater potential against SARS-CoV-2 and COVID-19. A: type II pneumocyte. B: macrophage. ACE2: angiotensin 2 receptor; TMPRSS2: transmembrane serine protease 2; AAK1: AP2-associated protein kinase-1; VEGFR2/1: vascular endothelial growth factor receptor; ABL2: ABL tyrosine kinase; JAK: Janus kinase; STAT: signal transducer and activator of transcription; IFN-α2: interferon-alpha-2; AChE: acetylcholinesterase; ACh: acetylcholine; IL1-R: IL-1 receptor; NF-κB: nuclear factor enhancer of activated B-lymphocyte kappa light chains; VE-Cadherin: vascular endothelial cadherin. Natural products in green, biological in blue, synthetic in gray. Figure created on BioRender.com.
Anakinra (Kineret) is a recombinant form of the IL-1 receptor antagonist. It is a disease-modifying antirheumatic drug that inhibits nuclear factor-kappa B (NFκB) translocation (Fig. 2), thereby controlling the production of proinflammatory mediators11. It reduced the 30-day mortality by 50% and improved patients' ability to breathe. Pyridostigmine bromide (Mestinon) also inhibits NFκB because it is an acetylcholinesterase inhibitor, and its activity results in an increase in the half-life of acetylcholine, which is critical in the cholinergic anti-inflammatory pathway12-14 (Fig. 2); its administration reduced the need for mechanical ventilation by 2 days and reduced mortality by 63%15.
Thalidomide is an anti-inflammatory, immunomodulatory, and anti-angiogenic drug that inhibits the NFκB and interferon regulatory factor 3 (IRF3) pathways16. Thalidomide, in combination with glucocorticoids, reduced hospital stay and viral load by ~50%, decreased IFN-γ and IL-6 levels, and the need for mechanical ventilation17. In addition, chloroquine, an antimalarial and arthritis drug, inhibits the inflammatory response mediated by IL-6, IL-17, and IL-22 and prevents viral entry by interacting with ACE218. Chloroquine (400 mg, 2 times daily for 4 days) showed a mortality rate of 15% versus 39% in the high-dose group (600 mg, 2 times daily for 10 days)19. The mortality rate was similar to that of the thalidomide control group (16%), so its efficacy is questionable, and the use of high doses in critically ill patients is not recommended.
Fluvoxamine is an antidepressant that pharmacologically functions as a selective serotonin reuptake inhibitor. It inhibits the production of TNF-a and IL-6 through the S1R-IRE1 pathway20. Patients treated with fluvoxamine experienced no clinical worsening compared to 8.3% in the placebo group21, while tocilizumab (Actemra), an IL-6 receptor inhibitor, induced recovery of patients requiring supplemental oxygen in a median of 4 days in tertiary care22, compared to 10 days for patients treated with remdesivir23. However, several clinical trials have suggested that tocilizumab may not be effective against COVID-1911,24,25 because, unlike the drugs above, which inhibit the upstream inflammatory response, tocilizumab only inhibits IL-6 activity.
Treatment with N. sativa (Ranunculaceae) seeds and honey was evaluated in a study conducted at a tertiary care center in Pakistan. It reduced the time to symptom relief by 50%, accelerated viral clearance, and reduced the mortality rate fivefold (4% vs. 18.8% for placebo)26, while N. sativa seed oil increased the percentage of recovered patients (62%) compared to the control (36%) and reduced the recovery time in a study conducted at a tertiary care center in Saudi Arabia27.
In both studies, most patients were under 60 years of age, while the average age in the Saudi Arabian group was 36 years. Both studies showed a significant benefit from using N. sativa seeds, resulting in remission of symptoms. There were differences in the baseline characteristics of the patients in terms of comorbidities, as the Pakistani group had a higher percentage of hypertension and obesity. In addition, various concomitant treatments were used due to the greater severity of the disease compared to the patients in the Saudi Arabian study, where the use of other medications was not reported (Table 4).
Table 4 Characteristics of clinical studies with individually administered drugs
| Drug | Study identifier | Experimental treatment Dose/route/duration | Accompanying treatment | Demographic and clinical characteristics | Hospital/level | References |
|---|---|---|---|---|---|---|
| Acalabrutinib | NCT00001467; NCT01200953 | 100 mg PO, BID for 14 days or placebo | Steroids and/or hydroxychloroquine | Median age 61 years, 68% men, hypertension 84%, obesity 68%, and diabetes mellitus 37% | National Institutes of Health Clinical Center (CC). Tertiary | 47 |
| Anakinra* (Kineret) | NCT04357366 | 100 mg SC, QD, for 10 days or placebo | ND | Mean age 63 years, 62.3% men, diabetes 31.5%, hypertension 52.3% | 13 centers, tertiary | 11 |
| Aviptadil (Zyesami) | NCT04311697 | Aviptadil IV, 3 days in titrated doses of 50 pmol, 100 pmol, 150 pmol/kg/h or placebo | ND | 62.6% younger than 65 years, 64.9% men | Five centers, secondary and tertiary | 10 |
| Baricitinib | NCT04393051 | Italy: 4 mg/day for 14 days along with standard of care. Spain: 2 or 4 mg/day for 3-11 days or placebo | Hydroxychloroquine, antibiotics, protease inhibitors, enoxaparin, and steroids | Mean age 80 years, 65.2% men, 73.9% hypertension, 45.7% diabetes | Spain: Complejo Hospitalario Universitario de Albacete. Italy: University of Pisa. Tertiary | 38 |
| Bevacizumab* | NCT04275414 | Single dose of 500 mg dissolved in 100 mL of saline solution, IV or placebo | Lombardy and Wuhan: antivirals, hydroxychloroquine, antibiotics, steroids, antipyretics, and supportive care. Wuhan: Chinese herbal medicine in all patients | Mean age 62 years, 77% men, 50% hypertension, and 23% diabetes | China: Renmin Hospital of Wuhan University, Wuhan, Hubei Province. Tertiary Italy: Hospital S.p.A. Ospedale Generale di Zona Moriggia Pelascini. Secondary | 70 |
| Pyridostigmine bromide | NCT04343963 | 60 mg/day PO, 14 days or until hospital discharge | Dexamethasone 74.5% and tocilizumab (5.3%) | Average age 52 years, 59.6% men, diabetes 36.2%, hypertension 35.1%, and obesity 43.1% | Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán. Tertiary Instituto Nacional de Cardiología Ignacio Chávez (INCICh). Tertiary | 15 |
| Chloroquine | NCT04323527 | High dose: 600mg BID for 10 days Low dose: 450 mg BID on day 1 and QD for 4 days | Ceftriaxone IV (1g 2 BID for 7 days), azithromycin (500 mg QD for 5 days), oseltamivir (75 mg BID for 5 days) in case of influenza | Mean age 51.1 years, 75.3% male, hypertension 45.5%, alcohol use disorder (27.5%), and diabetes (25.5%) | Hospital e Pronto Socorro Delphina Rinaldi Abdel Aziz. Tertiary | 19 |
| Colchicine† | NCT04322682 | 0.5 mg oral, twice a day for 3 days and then once a day for 27 days | Hydroxychloroquine (0.5%), oral anticoagulant (2.1%), aspirin (8.7%), other platelet agents (1.4%) | Mean age 54 years, 53.9% women. Hypertension (34.9%), respiratory disease (26.1%), diabetes (19.9%) | Eight centers. Secondary and tertiary | 31 |
| Eculizumab* | NCT04346797 | 900 mg every week, 900 mg every 4 days and 3 doses of 1,200 mg on days 1, 4 and 8 showed the most satisfactory results. Intravenous | Enoxaparin, unfractionated heparin, dexamethasone | Hypertension (87.5%), diabetes (37.5%) | Hôspital saintlouis aphp, ND | 32 |
| Favipiravir | CTRI/2020/05/025114 | 1800 mg twice daily 1; maintenance dose of 800 mg twice daily thereafter, maximum 14 days | Antipyretics, cough suppressants, antibiotics, and vitamins. | 73.5% men, 77.6% aged 30 to 60 years, 25.9% with diabetes mellitus, hypertension and/or obesity | AIIMS India. Tertiary. Breach Candy Hospital Trust Secondary. Dr. Balabhai Nanavati ND Hospital. Fortis Hospital Limited, primary to quaternary | 61 |
| Favipiravir versus umifenovir (Arbidol) | ChiCTR2000030254 | 1600 mg, twice on the first day followed by 600 mg, twice daily, for the next few days. Or Arbidol (200 mg, 3 times a day) plus standard care for 7 days | Traditional Chinese herbal medicines, antibiotics, additional antiviral treatment, immunomodulatory drugs, steroids, antipsychotic drugs, nutritional support, cardiovascular drugs, oxygen support, non-invasive positive pressure ventilation (NPPV), or invasive ventilation | In favipiravir group 50.86% men, 75% < 65 years, 31.05 hypertension, 12.07% diabetes. In Arbidol group 42.50% men, 65.83% < 65 years, 25% hypertension, 12.07% diabetes | Zhonghan Hospital of Wuhan University. Tertiary Leishenshan Hospital, field hospital Hospital of Hubei Province, Wuhan, Tertiary | 62 |
| Fluvoxamine | NCT04342663 | 50mg once, 100 mg 2 times a day for 2 days, then 100 mg 3 times | ND | Diabetes 11%, hypertension 19%, 30% male | BJC Belleville. Primary. Washington University | 21 |
| a day as tolerated until day 15 | School of Medicine. Tertiary | |||||
| Nitric oxide gas | NCT04305457 | NO inhaled at 160 ppm for 30 min, twice daily for 14 days or until discharge | ND | Hypertension 41%, diabetes 34.5%, 65.2% male | Massachusetts General Hospital. Tertiary | 72 |
| LMWH | NCT04323761 | Subcutaneous enoxaparin 40-60 mg daily, or therapeutic 40-60 mg subcutaneously twice daily | Hydroxychloroquine LPV/r or DRV/r, doxycycline dexamethasone, prednisone, methylprednisolone, hydrocortisone), macrolides, baricitinib tocilizumab remdesivir | Hypertension 46.03%, 19.68% diabetes, 76.2% male, mean age 70 years old | University Hospital of Pisa, Italy. Tertiary | 24 |
| Imatinib mesylate | EudraCT 2020-001236-10 | 800 mg oral on day 0 followed by 400 mg daily on days 1-9 | ND | Median age 64 years, diabetes 25%, cardiovascular disease 22%, hypertension 37.6% | VU University Medical Center. Tertiary Erasmus MC (Rotterdam). Tertiary, Spaarne Ziekenhuis (Haarlem). Secondary. Haaglanden Medical Center (the Hague) Secondary. Isala Clinic (Zwolle) Primary | 37 |
| Nigella Sativa oil‡ | NCT04401202 | 500 mg of Nigella sativa oil in capsule, 2 times a day for 10 days | ND | Average age 36 years old, 36% male, 8% diabetes, and 9% hypertension | King Abdulaziz University Hospital. Tertiary | 27 |
| Nigella sativa and honey‡ | NCT04347382 | Honey 1 g/kg/day and Nigella sativa seeds 80 mg/kg per day for 13 days | Panadol, azithromycin, montelukast, LMWH, hydrocortisone, multivitamins, tazobactam+ piperacillin, ivermectin, meropenem, at physician's consideration | 56.86% men, 49.84% ≤ 40 years, hypertension 31.62%, and diabetes 36.74%. | Shaikh Zayed Post-Graduate Medical Complex, Services Institute of Medical Sciences, Doctor's Lounge, and Ali Clinic. Tertiary | 26 |
| Opaganib | NCT04414618 | 2 × 250 mg of oral Opaganib in capsules or placebo every 12 h for up to 14 days | Remdesivir and/or corticosteroids | Mean age 58 years, 64.3% male | Honor Health Research Institute, Miami Cancer Institute, Tertiary. Oregon Health & Science University, Albany Medical Center, Henry Ford Hospital, Ziv Medical Center, Tertiary | 51 |
| Ravulizumab* | NCT04369469 | Day 1: 2400 mg, 2700 mg, or 3000 mg if they weighed ≥ 40- < 60 kg, 60- < 100 kg, or ≥ 100 kg, respectively. On days 5 and 10 600 mg if they weighed < 60 kg and 900 mg if they weighed ≥ 60 kg, then 900 mg on day 15 | Antivirals such as remdesivir | 54.5% men, mean age 66 years, diabetes 50%, hypertension 45.5% | Brigham and Women's Hospital Tertiary, Houston Methodist Hospital, Quaternary, King's College Hospital Tertiary, Washington University School of Medicine. Tertiary | 34 |
| Remdesivir | NCT04292730 | Intravenous remdesivir 200 mg on day one, followed by 100 mg/day For 5-10 days or standard care | Steroids, hydroxychloroquine/chloroquine, lopinavir-ritonavir, tocilizumab, azithromycin, aspartate aminotransferase, alanine aminotransferase | Average age 57 years old, 61.13 male, 42.46% hypertension, 39.72% diabetes | 105 hospitals in the US, Europe, Asia: Secondary and tertiary | 57 |
| Remdesivir | NCT04280705 | Intravenous 200 mg on day one and 100 mg daily for up to 9 additional days | Hydroxychloroquine and glucocorticoid | Average age 58.9 years, 64.4% men, 50.2% hypertension, 44.8% obesity, and 30.3% diabetes mellitus | 60 centers ND | 23 |
| Thalidomide | NCT04273529 | 100 mg per day for ≥ 7 days, with a median duration of 12 days | Dexamethasone in low doses (40 mg IV every 12 h for 3 days, then every 24 h for 5 days) | 66.7% male, median age 65.5 years 50% with comorbidities | First Affiliated Hospital of Wenzhou Medical University. Tertiary | 17 |
| Tocilizumab* | NCT04331795 | Range of 40, 80, 120 and 200 mg, with possible repetition at 24 or 48 h | Hydroxychloroquine or azithromycin, lopinavir-ritonavir, or systemic corticosteroids | Median age 69 years, 50% men, 62% two or more comorbidities | University of Chicago Medicine. Tertiary | 22 |
| Tofacitinib | NCT04469114 | 10 mg oral or placebo 2 times daily for up to 14 days or until discharge | It may have included glucocorticoids, antibiotic agents, anticoagulants, and antiviral agents | Mean age 56 years, 65.1% men, 50.2% with hypertension, and 23.5% with diabetes | Multicenter, 17 locations, Secondary and Tertiary | 44 |
| Umifenovir (Arbidol) | 2 pills 0.2 g 3 times daily | Interferon, asmeton (compound methoxyphenamine), limonene and pinene, moxifloxacin, ibuprofen, and ambroxol | 54.8% men, 17.7% hypertension, 11.3% diabetes. Most between 48 and 63 years old | First Hospital of Jiaxin Tertiary | 63 | |
| Vitamin D3† | NCT04560608 | Group 1: 50,000 IU per month, 80,000 IU or 100,000 IU every 2-3 months the year before infection. Group 2: 80,000 IU within a few hours of COVID-19 diagnosis | Antibiotics, corticosteroids, and pharmacological treatments for respiratory disorders | Mean age 88 years, 50.6% men. 63.6% hypertension, 54.5% cardiomyopathy | Angers University Hospital Tertiary | 75 |
*Biologicals.
†Natural products or their semi-synthetic derivatives.
‡Herbal remedy. SA: Saudi Arabia; BR: Brazil; CA: Canada; CH: Switzerland; CN: China; DE: Germany; ES: Spain; FR: France; GB: United Kingdom; GR: Greece; HK: Hong Kong; IN: India; IL: Israel; IT: Italy; JP: Japan; KR: South Korea; MX: Mexico; NL: Netherlands; PK: Pakistan; SE: Sweden; SG: Singapore; TW: Taiwan; US: United States; LMWH: low molecular weight heparin; IV: intravenous; PO: oral; SC: subcutaneous; QD: once daily; BID: twice daily; TID: three times daily; ND: Not described. NO: Nitric oxide.
The main phytopharmaceutical of this seed is thymoquinone, which has anti-inflammatory effects by suppressing expression of enzymes that produce prostaglandins and leukotrienes and also blocks ACE2 (Fig. 2)28. This species also contains nigellone, which blocks histamine release29. The oil showed antiviral activity in a murine cytomegalovirus and H9N2 model and decreased proinflammatory cytokines in a murine allergic asthma model28.
Antimitotics
Colchicine, an alkaloid derived from the plant Colchicum autumnale (Colchicaceae), used in the treatment of gout, inhibits tubulin polymerization in leukocytes and acts on the NLRP3 inflammasome30. Colchicine-reduced mortality and hospitalization by 24% compared with placebo in patients over 40 years of age with COVID-19 confirmed by polymerase chain reaction (PCR)31.
Complement system inhibitors
Eculizumab (Soliris) is a monoclonal antibody used to treat autoimmune diseases; it is an inhibitor of complement protein C5b-932 and reduces the levels of IL-1, IL-6, and TNFa33. In one study, it contributed to the improvement of 6 out of 8 patients32. Ravulizumab (Ultomiris), on the other hand, is a complete inhibitor of C5 convertase, which is the initiator of the terminal phase of the complement system34, so in addition to preventing complement-mediated inflammation, it also blocks cell activation and lysis35.
Tyrosine kinase inhibitors
Imatinib mesylate, a c-ABL kinase inhibitor used to treat certain cancers, inhibits VE-cadherin dissociation (Fig. 2), preventing capillary leakage and alveolar edema; it also reduces IL-6 and IL-8 secretion36. Imatinib mesylate reduced the median duration of mechanical ventilation from 12 to 7 days and reduced the likelihood of death in patients by 49%37.
Baricitinib (Olumiant), an inhibitor of Janus kinase (JAK) 1 and 2 kinases used in the treatment of rheumatoid arthritis, decreases the expression of ACE2 in human liver cells and the expression of proinflammatory cytokines induced by IFN-α2 through the JAK/signal transducer and activator of transcription (STAT) pathway38,39. In addition, it has affinity for the AP2-associated protein kinase-1 and reduces SARS-CoV-2 endocytosis (Fig. 2)40. Baricitinib reduced respiratory failure, mortality, and disease progression by 50%38, and in another study where it was administered with corticosteroids, supplemental oxygen requirements were reduced (25.8% vs. 62% in the methylprednisolone control group), and mortality was ~4% in both groups41, suggesting that corticosteroid activity reduced mortality. Baricitinib in combination with lopinavir and ritonavir (Kaletra) improved patient status and prevented disease progression42, and in another study, inhibited mortality, reduced disease progression by 95% (0.88% vs. 17.9% control), and increased hospital discharge rate (9.7% vs. 1.3%)43.
Tofacitinib as baricitinib, is an inhibitor of JAK1 and 3 kinases and the JAK/STAT pathway used to treat rheumatic diseases. Tofacitinib alone reduced mortality at day 28 by 49% (2.8% vs. 5.5% for placebo) and the cumulative incidence of death or respiratory failure by 37.6% (18% vs. 29% for placebo)44. Meanwhile, Novaferon, an antitumor/antiviral protein that interacts with the IFN2 receptor, a JAK/STAT45 signaling pathway45, with or without lopinavir and ritonavir, had a higher viral clearance rate at day 6 than the lopinavir/ritonavir group (50.0% vs. 24.1% and 60.0% vs. 24.1%, respectively)46.
Acalabrutinib improved lung function and reduced inflammation by targeting BTK tyrosine kinase; BTK is important in activating the innate immune response of blood monocytes47 by promoting inflammasome and phagocytic receptor activation48. In addition, BTK inhibition also blocks nuclear translocation of NFκB, which results in a reduction of the synthesis of proinflammatory cytokines. Also, the activity of opaganib, which is an inhibitor of sphingosine kinase-2. This kinase has been proposed to be a factor in viral replication49,50, and its inhibition also decreases TNF-α and IL-6. In patients, it reduced the need for supplemental oxygen (61.6% vs. 46.7% placebo) and accelerated hospital discharge51.
Antivirals
Danoprevir is an inhibitor of hepatitis C virus NS3/4a protease, which has high structural similarity to Mpro of SARS-CoV-252, while ritonavir increases danoprevir exposure by inhibiting cytochrome P450 isoenzyme 34A. Danoprevir with ritonavir suppressed viral replication in < 1 week and reduced ground glass opacity53.
Lopinavir and ritonavir are HIV-1 protease inhibitors; ribavirin inhibits normal viral replication, and IFN β-1b induces the synthesis of antiproliferative and immunomodulatory proteins54. Meanwhile, lopinavir and ritonavir had no clinical benefit when administered alone24 or with Arbidol55. Treatment with lopinavir and ritonavir with ribavirin and IFN β-1b, administered 7 days after symptom onset, reduced the median time for negative nasopharyngeal swab results compared to the control group (from 12 to 7 days), suppressed viral load at 8 days, relieved symptoms at 4 days, and reduced IL-6 levels56.
Remdesivir reduced the median recovery time by 10 days (vs. 15 days for placebo) and also reduced mortality (6.7% vs. 11.9% for placebo) in a study conducted in 60 tertiary care hospitals in Europe, Asia, and the Americas in patients with moderate to severe illness23. In another study conducted in 105 secondary and tertiary care hospitals in the U.S., Europe, and Asia in patients with moderate illness, 5 days of remdesivir improved the condition of patients compared to placebo. However, the clinical significance was uncertain due to the study design57. In another study conducted in 71 centers in eight countries in Europe, Asia, and the Americas, remdesivir was administered alone or in combination with baricitinib, and in the second case, the median recovery time was reduced from 8 to 7 days58.
The protocols of the three studies were the same in terms of doses. However, there was a difference in the duration evaluated, as the first study found that the 10-day treatment was sufficient to shorten patients' recovery time. In contrast, the second study found that a 5-day treatment was more beneficial than a 10-day treatment. From these data, it can be concluded that a 10-day course provides greater benefit in patients with more advanced diseases (Tables 4 and 5).
Table 5 Characteristics of clinical studies of combination therapies
| Drug | Study identifier | Experimental treatment Dose/route/duration | Accompanying treatment | Demographic and clinical characteristics | Hospital/level | References |
|---|---|---|---|---|---|---|
| Baricitinib and LPV/R | Baricitinib 4 mg QD, PO for 14 days. LPV/R 250 mg, PO, BID, 14 days | Hydroxychloroquine 400 mg/QD/PO, LPV/R | 83% men, mean age 63.5 years, 20.83% hypertension, 29.16% diabetes | Hospitals in Prato and Alessandria. ND | 42 | |
| Baricitinib and LPV/R | NCT04358614. | Baricitnib 4 mg, PO, QD, 14 days. LPV/R 250 mg PO BID, 14 days | HQ, LPV/R | 62.30% men, mean age 65.5 years, 27.74% hypertension, 16.23% diabetes | 7 care centers, ND | 43 |
| Baricitinib and corticosteroid | EUPAS34966 | Baricitinib 4 mg PO the 1st day, then 2 mg QD or 4 mg QD Methylprednisolone 80, 125 or 250 mg/QD | LPV/R, two tablets PO 200/50 mg BID, 7-10 days HQ 200 mg, PO, BID | Mean age 63 years, 69.6% men, 28.8% hypertension, 18.8% diabetes | Hospital General Universitario de Albacete, Spain, Tertiary | 41 |
| Danoprevir and ritonavir | NCT04291729 | Danoprevir 100 mg PO, BID, 4-12 days, Ritonavir 100 mg PO, BID | α-interferon, 5 million units, IN BID | Mean age 44 years, 36.4% men, 18.2% hypertension | The Ninth Hospital of Nanchang Tertiary | 53 |
| HQ and oseltamivir | NCT04303299 | Oseltamivir 300 mg/ QD O 4-6 mg/kg QD; HQ 800 mg QD | Supportive care without experimental treatments | Mean age 32 years, 46.6% male, 6.6% obese | Rajavithi Hospital Tertiary | 65 |
| HQ and oseltamivir | NCT04349241 (-) | Oseltamivir PO 75 mg BID 10 days; HQ 800 mg PO day 1, followed by 200 mg BID, days 2-10 | ND | Mean age 36.4 years, 50% male, 18% with comorbidities ND | Ain Shams University Hospital, Tertiary y Assiut University Hospital, Tertiary | 64 |
| HQ, FVP, DRV yand ritonavir | NCT04303299 | HQ 400 mg QD; FVP 6000 mg 1st day, then 2400 mg QD; DRV 1200 mg, QD or 4-6 mg/kg, QD; R 200 mg, QD or 2.5 mg/kg QD | Supportive care with no experimental treatments | Mean age 42 years, 52% men, 30% obese | Rajavithi Hospital Tertiary | 65 |
| IVE, AAS, DEX and ENOX* | NCT04425863 | IVE PO 24, 36 and 48 mg days 0 and 7; DEX 4 mg IM; ENOX SC; ASA 250 mg PO | ND | Average age 55.7 years, 51.5% men | Hospital Eurnekian Secondary | 78 |
| LPV-R, IFNβ-1b, Ribavirine | NCT04276688 | LPV 400 mg; R 100 mg BID, 14 days; ribavirin 400 mg BID; IFNβ-1b 8 million IU, TID every other day | Amoxicillin, azithromycin, ceftriaxone, doxycycline, levofloxacin, corticosteroids | Average age 52 years old, 54% men, 13% diabetes, 27% hypertension | 6 centers | 56 |
| Novaferon and/or LPV/R | ChiCTR2000029496 | Novaferon 40 µg TID, IN. LPV/R 200 mg/50 mg PO TID | ND | Average age 46, 50, 37 years in the different groups, 9% diabetes, 10% hypertension | First Hospital of Changsha city, ND | 46 |
| Remdesivir alone or with baricitinib | NCT04401579 | Remdesivir 200 mg IV day 1, 100 mg day 2-10. Baricitinib 2mg BID 14 days | Standard support with no experimental drugs | Mean age 55 years, 63.1% male, 57% 2 or more coexisting conditions | 71 centers | 58 |
*Natural products or their semi-synthetic derivatives. ASA: Acetylsalicylic acid; DEX: Dexamethasone; DRV: Darunavir; ENOX: Enoxaparin; FVP: Favipiravir; HQ: Hydroxychloroquine; IFN: Interferon; IVE: Ivermectin; LPV/R: Lopinavir with Ritonavir; PO: oral; IV: intravenous; SC: subcutaneous; IN: intranasal; IM: intramuscular; QD: once daily; BID: twice daily; TID: three times daily; ND: not determined; (-): retracted article.
We also found two articles that are not included in the results tables. In one, remdesivir was administered for 5 or 10 days and produced a clinical improvement of 2 points or more on the 7-point ordinal scale at day 14 in 64% of patients; however, they found no significant differences between the two groups, in patients with severe disease who did not require mechanical ventilation59. In another study in China with critically ill patients, remdesivir did not affect clinical improvement60. Therefore, the benefit of remdesivir is uncertain; it appears that its individual activity may not be sufficient to improve clinical status significantly.
Favipiravir is an influenza virus RNA polymerase inhibitor. It has been evaluated in various studies in patients with mild to severe disease in which it did not reduce viral load; it only showed a reduction in the recovery time of patients from 5 to 3 days in a study conducted in India61 and in a study conducted in China it contributed to 71% of patients recovering before 7 days versus 55% in the group with umifenovir62. The latter study used lower doses, with a difference of 200 mg. Both studies were conducted in tertiary care hospitals, and the patients evaluated had similar characteristics in terms of disease status. However, the standard of care differed in that the Chinese study used herbal medicines and additional antiviral treatment. Both studies concluded that favipiravir therapy accelerated patient recovery but had no effect on viral load reduction (Table 4).
On the other hand, umifenovir, a broad-spectrum antiviral61,63 that prevents the hemagglutinin from switching to its fusion state, thereby inhibiting the entry of the virus, also reduced the recovery time of symptoms such as fever (4.9 vs. 6 days) and dry cough (4.3 vs. 5 days)63.
Oseltamivir inhibits influenza virus neuraminidases, limiting the spread of infection and inflammation. Patients who received hydroxychloroquine with oseltamivir had a negative reverse transcriptase-PCR (RT-PCR) test in an average of 8.1 days64 (article retracted due to questionable results). In another study, patients had a negative RT-PCR test 4 days earlier than the control group (7.5 vs. 11.5 days)65. This is important because it reduces the period of infectiousness and the risk of disease progression.
Antithrombotics
Low-molecular-weight heparin is an anticoagulant that enhances antithrombin III activity, reduces the risk of coagulopathy and thromboembolism, and interacts with NF-kB by mediating the production of proinflammatory cytokines66. It reduced the likelihood of death by 64% and the risk of disease progression and death by 39%24.
Chemotaxis inhibitors
It is possible that the effect of bevacizumab is related to the secretion of vascular endothelial growth factor (VEGF) by leukocytes in response to a hypoxic environment67 and that inhibition of VEGF (Fig. 2) prevents its function as a chemoattractant for monocytes and macrophages through its receptor VEGF receptor 168. Bevacizumab reduced fever in the first 72 h, improved respiration in populations from China and Italy, and no patients died. However, a decrease in respiratory capacity was observed only in the control group in Italy throughout the study69. This is probably due to the use of Chinese herbal medicine, including Jinhua Qinggan, Lianhua Qingwen, and Xuebijing70, in the treatment of all control patients in Wuhan, unlike those in Italy69 (Table 4 for more data on concomitant treatments).
Bronchodilators
Inhaled nitric oxide reduced respiratory rate, improved oxygenation, and prevented hospital readmissions after discharge71. In addition to its bronchodilator activity, it is thought to have antiviral activity, as it reduced viral replication by 99% in in vitro studies by inhibiting the Mpro protease of SARS-CoV-272.
Vitamins
Regular supplementation with Vitamin D3 during the year before illness significantly reduced mortality; the clinical benefit was not significant in patients who received it after diagnosis73. In an extension of this trial, Vitamin D3 supplementation before, during, or after hospitalization reduced mortality in geriatric patients74. Vitamin D regulates innate and adaptive immunity, as its hormonal metabolite (calcitriol) mainly suppresses the production of inflammatory cytokines, induces regulatory T lymphocytes, and has a possible antiviral effect75.
Multi-target combination therapies
The combination of hydroxychloroquine with favipiravir, darunavir, and ritonavir reduced mortality by 25% compared with patients receiving oseltamivir, lopinavir, and ritonavir (4% vs. 16%)65. The individual effect of the drugs in this regimen against COVID-19 is inconclusive. However, he efficacy of this regimen is related to the inhibition of different stages of the viral cycle, as it includes antiviral agents, protease inhibitors, polymerase inhibitors, and an antiparasitic agent that may interfere with viral entry.
The combination of ivermectin, dexamethasone, acetylsalicylic acid (aspirin), and enoxaparin covers different aspects of the disease, as ivermectin blocks the IMPa/b1 import heterodimer on which nuclear trafficking of RNA virus proteins depends (Fig. 2)76. Aspirin is an antithrombotic agent in mild cases, enoxaparin is an anticoagulant77, and dexamethasone inhibits transcription factors such as AP-1, NF-kB, and IRF78, prevents the synthesis of cytokines and chemokines, and induces apoptosis of T lymphocytes and neutrophils79. This therapy inhibited disease progression and reduced mortality by 71% compared to the mortality rate in Argentina and 87% compared to the mortality rate in Spain and Italy for hospitalized patients77.
Fig. 3 shows the molecular structure of the drugs included in clinical trials that were shown to inhibit the processes involved in SARS-CoV-2 infection.
Discussion
The global pharmaceutical industry has relied on developing prophylactic vaccines to reduce transmission through large-scale mass vaccination programs worldwide, aiming to achieve herd protection in the short term. Although highly effective vaccines have been developed, there is still significant mortality, as well as limitations in vaccine availability and the emergence of SARS-CoV-2 variants.
Drug repurposing is an alternative to the traditional drug development and discovery process to address emerging diseases such as COVID-19. In this study, we identified drugs, an herbal remedy, and a vitamin that were administered orally (23), intravenously (6), subcutaneously (3), intramuscularly (2), and inhalational (2). Twenty-four of these were administered alone, and 12 were in combination therapies that were shown to be effective against COVID-19 during the 1st year of the pandemic. Several of these drugs were recommended by the Instituto Mexicano del Seguro Social (Mexican Social Security Institute) for their use in patients with COVID-19 in the Clinical guide for the treatment of COVID-19 in Mexico (Guía clínica para el tratamiento de la COVID-19 en México).
The drugs that showed the best results in reducing mortality and the need for mechanical ventilation in critically ill, very severe and severe patients were those that reduced inflammation even though they were not specifically anti-inflammatory, such as the kinase inhibitors imatinib mesylate and baricitinib, the proinflammatory cytokine regulators aviptadil, anakinra, and pyridostigmine bromide, the monoclonal antibody bevacizumab, the herbal remedy based on N. sativa seeds with honey, and the combination of ivermectin, aspirin, dexamethasone, and enoxaparin; also, hydroxychloroquine in combination with favipiravir, darunavir, and ritonavir; and the anticoagulant low-molecular-weight heparin.
In mild-to-moderate cases, the drugs that reduced viral load, mortality, or prevented progression to severe disease were synthetic antivirals such as danoprevir with ritonavir, the combination of lopinavir with ritonavir, IFN β-1b and ribavirin, also umifenovir, favipiravir, the recombinant IFN novaferon, the kinase inhibitor tofacitinib, the natural product colchicine, and the semisynthetic derivative hydroxychloroquine in combination with the antiviral oseltamivir.
We highlight the remarkable responsiveness of the drug repurposing strategy, which allowed clinical trials to be conducted early in the pandemic, reducing mortality, and recovery time; however, it is essential to continue to dedicate efforts to research and development of effective drugs and vaccines to have better, affordable, and easily accessible therapeutic options to face this pandemic and increase preparedness for future epidemics fully.











 nueva página del texto (beta)
nueva página del texto (beta)