Introduction
The concept of cancer immunosurveillance establishes that the immune system can recognize and eliminate cancer cells1,2. However, cancer cells can escape from this immunosurveillance through several mechanisms, for example, creating an immunosuppressive environment in the tumor, which increases tumor survival or decreases its recognition by the immune system3-7. Defective death signaling has been associated with cancer immune escape through overexpression of antiapoptotic proteins, like Bcl-2 or c-Flip, and downregulation of proapoptotic proteins, like Bax or cell death receptors like Fas8,9.
Fas (also called Apo1 or CD95) is a member of the tumor necrosis factor receptor superfamily, and after its binding to Fas ligand (FasL), it triggers apoptosis in the cell10. Downregulation of Fas in tumors is associated with immune escape11-13. Evidence suggests that low levels of Fas are associated with inadequate treatment response in leukemia14.
Acute lymphoblastic leukemia (ALL) is a malignancy in the bone marrow characterized by the uncontrolled proliferation of lymphoid precursor cells15. Patients with ALL have a survival rate of around 80-90%16. However, patients with relapse have a much lower survival rate17-20. Some reports have provided evidence on which factors could be playing a critical role in relapse, including hypoxia and dysfunctional apoptosis21. Bone marrow biopsies of pediatric ALL overexpress hypoxia-inducible factor 1 a (HIF1-α) compared with healthy bone marrow22. A rat model of leukemia has provided evidence that the progression of this disease is associated with an expansion of hypoxic areas in bone marrow23.
Interestingly, hypoxia significantly reduces the effects of vincristine, methotrexate, and etoposide23,24. Moreover, another transcription factor increased under hypoxia is the Yin-Yang-1 (YY1) transcription factor, which increases significantly in lung microvascular endothelial cells (LMEC), and this increase is maintained for a long duration25. Using western blot analysis, Mojiri et al.25 found no differences in the levels of total YY1 protein under normoxic and hypoxic conditions; however, when nuclear fractions were analyzed, they found a significant increase of YY1 protein in hypoxia, which suggests that hypoxia induces nuclear translocation of this protein in LMEC25.
YY1 can play a dual role since it can repress or promote transcription depending on the cell circumstances26. YY1 is overexpressed in several types of cancer such as breast27, pancreatic28,29, prostate30, ovarian31, esophagus32, osteosarcoma33, brain34, melanoma35, and leukemia26. YY1 in cancer has been associated with cancer growth36, progression35,37, metastasis35,38, and poor prognosis36.
Recent studies have shown that YY1 is increased in patients with non-Hodgkins lymphoma and leukemia, and its high expression correlates with poor prognosis39. Recently, it was demonstrated that YY1 regulates the expression of multidrug resistance-1 (MDR1), and its over-expression is correlated with poor prognosis in ALL pediatric patients40. Furthermore, a computational analysis has shown that its expression correlates with poor survival in leukemia patients41. Importantly, it has been shown that YY1 negatively regulates the transcription of Fas42 in ovarian carcinoma43 and colorectal cancer cells44. However, the role of these transcription factors in the evasion of the immune system through the Fas/FasL pathway through the negative regulation of Fas by YY1 in ALL is not studied yet. Our first approach hypothesized that YY1 is upregulated under hypoxia, and this induces downregulation of Fas expression. This mechanism results in the evasion of apoptosis through the Fas/FasL pathway in ALL cells. A broader understanding of the regulatory mechanisms underlying YY1 expression and its implications in leukemia, as well as its relationship with Fas downregulation, is important for diagnostic and prognostic purposes. The study aimed to evaluate the effect of YY1 on Fas expression under hypoxic conditions in ALL.
Methods
Cell culture conditions
The human ALL cell line RS4; 11 was obtained from American Type Culture Collection (Manassas, VA) and cultured in Advanced RPMI Medium (Gibco) supplemented with 4% fetal bovine serum and 1% antibiotics. Cells under normoxic conditions were cultured in an incubator (Binder) with 5% of CO2. For hypoxic conditions, the cells were cultured in a hypoxia chamber (Bactrox), which created an atmosphere of 1% O2, 5% CO2, and 94% N2.
Immunocytochemical analysis of HIF-1α, YY1, and Fas expression
After treatment, 20,000 cells were placed on slides by duplicate. When dried, the cells were fixed with 4% paraformaldehyde for 20 min at 4°C. Cells were washed twice with phosphate-buffered saline (PBS), and the immunostaining protocol was performed. Briefly, antigen retrieval was performed using sodium citrate (0.01 M, pH 6.0). Endogenous peroxidase activity was inhibited with methanol and 3% hydrogen peroxide. The antibody-tissue non-immune binding was blocked by immersing the samples in a universal blocking solution and 1% albumin bovine serum for 60 min. The samples were incubated overnight at room temperature with an anti-YY1 antibody (dilution 1:2000, Novus Biologicals NBP2-20932), anti-Fas antibody (dilution 1:500, Novus Biologicals NBP1-41407), and anti-HIF-1α antibody (dilution 1:2000, Novus Biologicals NB100-479). The slides were washed and incubated with a universal link biotinylated antibody (dilution 1:1000) (Dako K069011, California, USA) and then with streptavidin conjugated to horseradish peroxidase; the signal was generated by the addition of diaminobenzidine (DAB) (Dako K346811, California, USA). When the reaction was stopped, samples were counterstained with hematoxylin. The samples were dehydrated, and the preparations were covered with resin and dried at room temperature.
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was isolated from 2 × 106 cells using Trizol (Life Technologies) according to the manufacturers instructions. cDNA synthesis was performed with 1 mg of purified RNA using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) according to the manufacturers instructions. Subsequently, the expression of Fas and YY1 was determined by qRT-PCR amplification in a QuantStudio 3 qPCR System (Applied Biosystems) using the TaqMan probes for Fas (HS00236330), YY1 (HS00998747), and TBP (HS00427620) as an endogenous control. PCR conditions were at 50°C for 2 min, 95°C for 10 min, and 40 cycles at 95°C for 15 s followed by 60°C for 1 min. The relative expression levels were calculated using the 2-DDCt method.
Apoptosis induction
An apoptosis test was performed to evaluate the relationship between Fas and YY1, in which 300,000 cells were incubated with 2 μg of Fas agonist antibody (D × 2, BioLegend) for 9 h at 37°C under normoxic and hypoxic conditions.
Assessment of Fas and active caspase 3 by flow cytometry
For the assessment of Fas by flow cytometry, cells were cultured under normoxic or hypoxic conditions for 3, 6, and 9 h. Cells were incubated with anti-Fas antibody (0.5 mg/million cells, BioLegend) for 1 h at 4°C in darkness. After incubation, cells were washed twice and resuspended in Fix/FACS solution. The cell fluorescence was analyzed by flow cytometry on a CytoFlex cytometer (Beckman Coulter). A minimum of 10,000 events was analyzed for the generation of each plot. The experiments were performed in triplicate independently.
After apoptosis induction, the percentage of active caspase 3 in cells under normoxia and hypoxia was quantified by flow cytometry. Briefly, cells were incubated for 1 h at 4°C in Cytofix solution (Cytofix/Cytoperm Kit, Biosciences BD) and washed with Perm/Wash solution (Cytofix/Cytoperm Kit, Biosciences BD). Cells were incubated with anti-caspase 3 antibody (dilution 1:50, Bioscience BD) for 1 h at 4°C in darkness. After incubation, cells were washed twice and resuspended in Fix/FACS solution. Cell fluorescence was quantified by flow cytometry on a CytoFlex cytometer (Beckman Coulter). A minimum of 10,000 events was analyzed for the generation of each plot. The experiments were performed 3 times.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
After treatment, 20,000 cells were placed on slides by duplicate. When dried, cells were fixed with 4% paraformaldehyde for 20 min at 4°C and washed 2 times with PBS, then proceeded to TUNEL protocol. Briefly, antigen retrieval was performed using sodium citrate (0.01 M, pH 6.0). Endogenous peroxidase activity was inhibited with methanol and 3% hydrogen peroxide. The antibody-tissue non-immune binding was blocked by immersing the samples in a 10% milk-PBS solution for 60 min. Slides were incubated with enzyme solution dilution 1:400 (In situ cell death kit, ROCHE) for 1 h at 37°C in a wet chamber. After three washes with PBS, slides were incubated with TUNEL reaction mixture (In Situ Cell Death Kit, ROCHE) for 1 h at 37°C in a wet chamber. Slides were washed 3 times, and the signal was generated by the addition of DAB (Dako K346811, California, USA). When the reaction was stopped, samples were counterstained with hematoxylin. The samples were dehydrated, and the preparations were covered with resin and dried at room temperature.
Digital image analysis
Stained slides were digitized at a 40 × magnification using an Aperio ScanScope CS (Aperio, Vista, CA). The Aperio ScanScope CS obtains 40 × images with a spatial resolution of 0.45 μm/pixels. The images were reviewed using an ImageScope (Aperio). Once the areas were annotated, they were sent for automated image analysis using Spectrum Software (Aperio). The output from the algorithm returns a quantitative percentage of positive staining.
Bioinformatic analysis of the correlation between Fas and YY1 gene expression in ALL
An analysis of Fas and YY1 expression levels in ALL patients was performed using a public data set of microarrays retrieved from the Oncomine database and Gene Expression Omnibus, derived from a published analysis reported by Andersson et al.45 and Coustan-Smith et al.46 The microarray data of Andersson et al. came from 121 childhood leukemias (87 B-linage ALL, 11 T-cell ALL, and 23 acute myeloid leukemia)45. In the Coustan-Smith et al. study, the authors analyzed the genome-wide gene expression of bone marrow samples from 270 patients with newly diagnosed childhood ALL versus normal samples46. In both studies, the results of HIF-1α, Fas, and YY1 expression were analyzed.
Data processing
All analyses were processed using the statistics package GraphPad Prism 6 (San Diego, CA.). Data were presented as the arithmetic mean and standard error of each group. The mean differences were determined by two-tailed t-test or by two-way ANOVA followed by Bonferronis multiple comparisons test. A two-tailed correlation analysis was performed using Pearsons correlation calculations. A p ≤ 0.05 value was considered statistically significant.
Results
Hypoxia reduces Fas expression in RS4; 11 cells and is associated with an increase in the expression of HIF-1α and YY1
Since both HIF-1α and YY1 are overexpressed in hypoxia, we first analyzed their expression in RS4; 11 cells in both normoxic and hypoxic conditions using immunocytochemistry. As shown in figures 1A and B, HIF-1α is significantly overexpressed in RS4; 11 when the cells are under hypoxic conditions (p = 0.001) and reached its highest level after 9 h of hypoxia. A low basal level of HIF-1α was detected in normoxic conditions. Even when a high baseline level of YY1 was observed in normoxia, its expression increased significantly (p = 0.03) under hypoxic conditions and reached its highest level after 9 h of hypoxia (Fig. 1C and D). Interestingly, Fas is expressed inversely: in hypoxia, Fas expression decreased significantly compared to normoxia (p = 0.0003) (Fig. 1E). Since we are interested in Fas membrane expression, we performed a flow cytometry assay (Fig. 1F). The highest decrease of Fas was observed after 9 h of hypoxia, where we found the lowest number of Fas positive cells compared with normoxia (Fig. 1F). Due to this finding, the responses were evaluated at 9 h in later experiments.
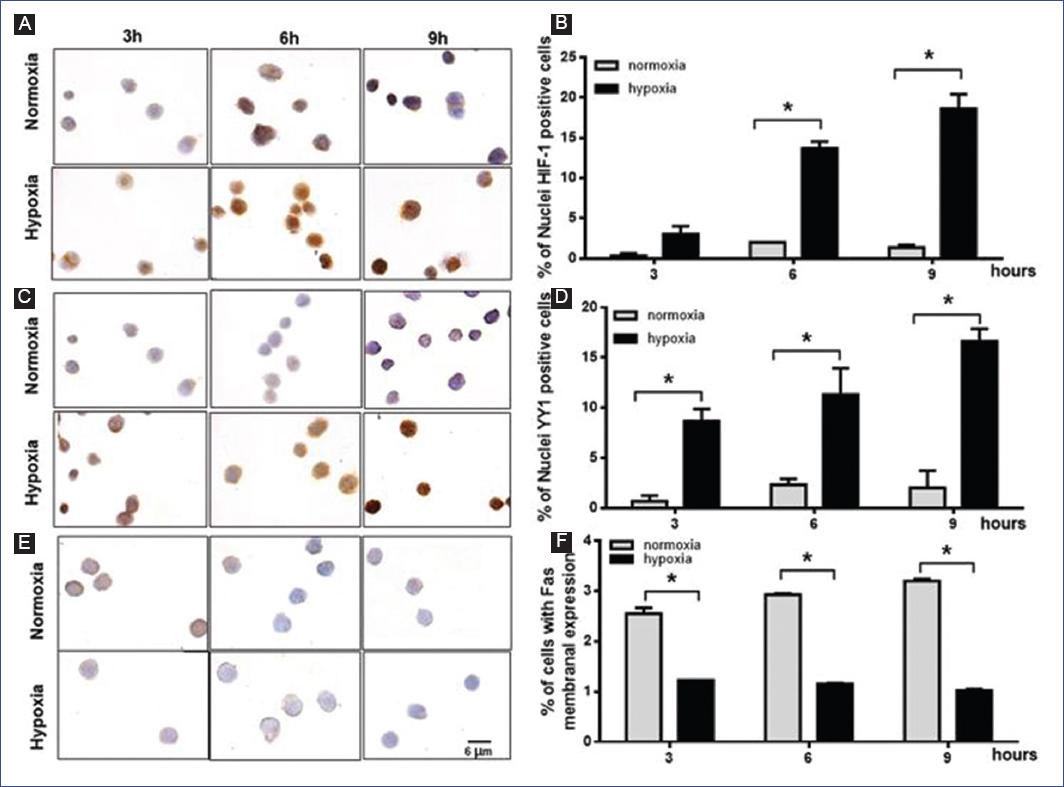
Figure 1 Hypoxia reduces Fas expression in RS4; 11 cells and is associated with an increase in the expression of hypoxia-inducible factor (HIF)-1α and Yin-Yang-1 (YY1). RS4; 11 cells were incubated under normoxic or hypoxic conditions for 3, 6, and 9 h, and the expression of HIF-1α, YY1, and Fas was analyzed by immunocytochemistry or flow cytometry. Representative microphotographs of HIF1-α A: YY1 C: and Fas E: expression; positive cells are shown in brown. Graphs represent the mean percentage and standard deviation (SE) of nuclei positive cells of HIF1-α B: YY1 D: and Fas membrane expression E: A representative result of three independent experiments is shown. Statistical differences were analyzed by two-way ANOVA followed by Bonferronis multiple comparisons test. *p < 0.05.
To corroborate our immunocytochemistry results, we analyzed the expression of Fas and YY1 by real-time PCR. Our results show that YY1 doubles its expression in hypoxia compared with normoxia (p = 0.0001) (Fig. 2A), while Fas decreases four times in hypoxia compared with normoxia (p = 0.0361) (Fig. 2B). Our data suggest that YY1 may control Fas expression under hypoxia in the RS4; 11 cell line.
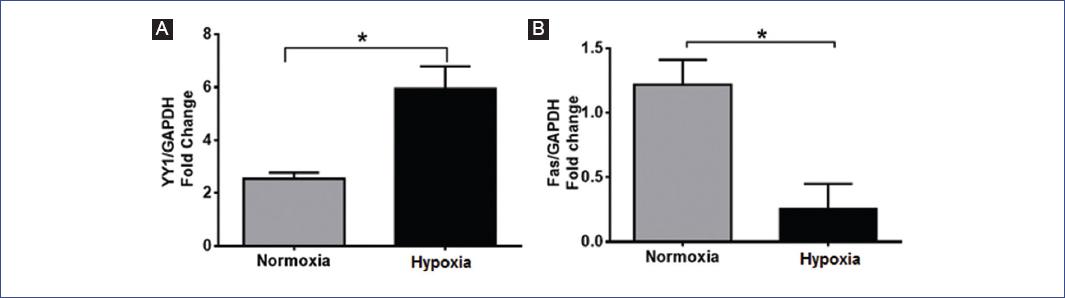
Figure 2 Reverse transcriptase-polymerase chain reaction (RT-PCR) analyses of Yin-Yang-1 (YY1) A: and Fas B: expression. RS4; 11 cells were cultured for 9 h in normoxia or hypoxia. Graphs represent the mean and standard deviation (SE) of the fold change expression in each condition. A representative result of three independent experiments is shown. Statistical differences were analyzed by Students t-test. *p ≤ 0.03.
Hypoxia inhibits Fas-related apoptosis
Due to the decrease of Fas expression under hypoxic conditions, we evaluated cell death by induction of apoptosis with a Fas agonist. First, we treated cells with 2 mg/ml of Fas agonist (DX2) under normoxic and hypoxic conditions and we found that DX2-induced higher apoptosis in normoxia (Fig. 3) compared with hypoxia (p = 0.0009). To corroborate our results, we analyzed apoptosis by TUNEL in situ and immunocytochemistry of active caspase 3. We observed a similar effect as mentioned above: cells under normoxia have a higher number of active caspase-3 positive cells as well as TUNEL positive cells when compared with hypoxia (p = 0.004 and p = 0.019, respectively) (Fig. 4). These results show that Fas-related apoptosis is inhibited when cells are under hypoxic conditions, and strongly suggests that hypoxia induces inhibition of Fas/FasL pathway.
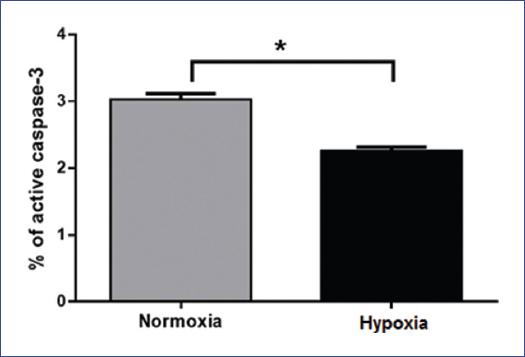
Figure 3 Hypoxia inhibits Fas-related apoptosis. RS4; 11 cells were incubated under normoxia and hypoxia for 9 h and treated with the Fas agonist, DX2 (2 μg/ml). Analyses of active caspase-3 were performed by flow cytometry. The graph represents the mean and standard deviation (SE) of the percent of active caspase-3 positive cells in each condition. A representative result of three independent experiments is shown. Statistical differences were analyzed by Students t-test. *p = 0.0009.
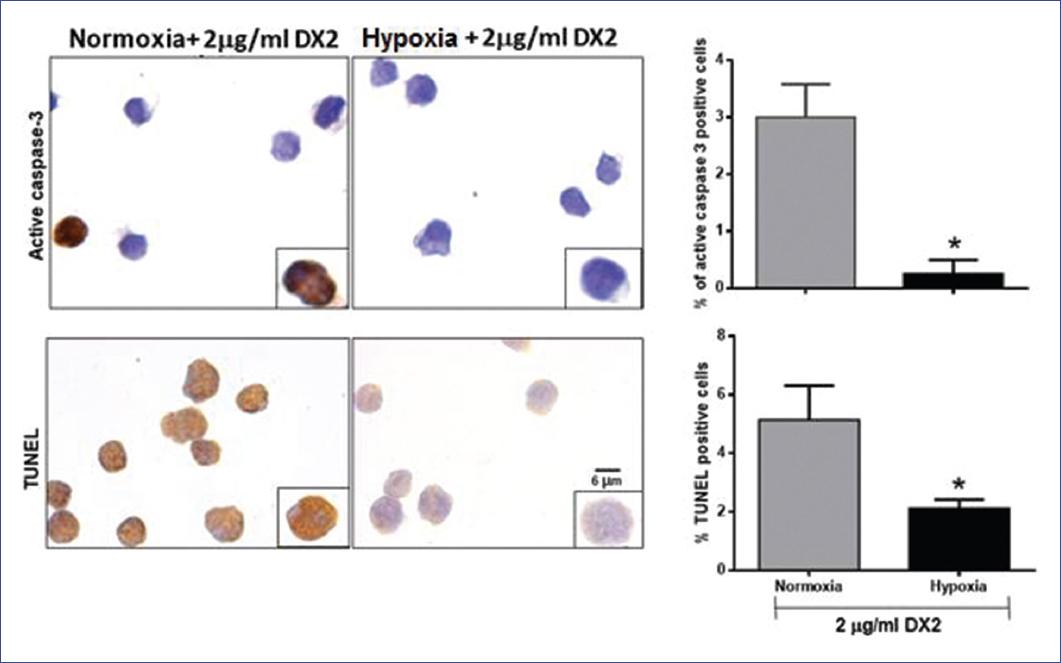
Figure 4 Analysis of apoptosis in RS4; 11 cells. Cells were incubated under normoxia and hypoxia for 9 h and treated or untreated with the Fas agonist, DX2 (2 μg/ml). Apoptosis was evaluated by terminal deoxynucleotidyl TUNEL in situ and immunocytochemistry of active caspase-3. Representative microphotographs of positive apoptotic cells are shown in brown. Graphs represent the mean and standard deviation (SE) of the percent of positive cells in each condition. A representative result of three independent experiments is shown. Statistical differences were analyzed by Students t-test. *p ≤ 0.01.
Bioinformatics analysis of the correlation between HIF-1α-YY1 and YY1-Fas gene expression in ALL
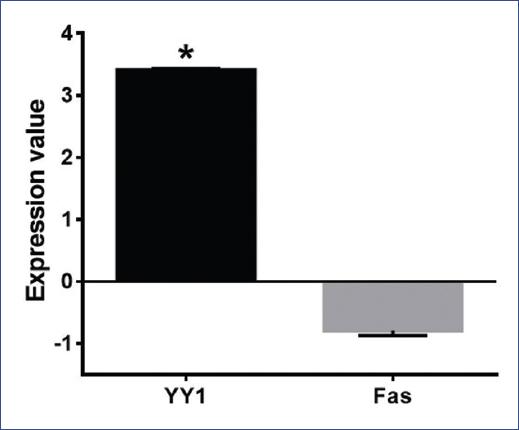
The correlation analyses between HIF-1α versus YY1 and YY1 versus Fas in ALL patients were performed using the microarrays data of Andersson et al.45 The analysis of Fas and YY1 expression levels in ALL patients was performed using the microarrays data of Coustan-Smith et al.46 Our bioinformatics analysis showed a significant positive correlation for HIF-1α and YY1 (p = 0.0005, r = 0.3279) (Fig. 5A). No negative correlation between Fas and YY1 was found (p = 0.421, r = −0.07695) (Fig. 5B). We observed that patients with high YY1 expression had low Fas expression; however, the difference in expression between Fas and YY1 was statistically significant (p = 0.0001) (Fig. 6).
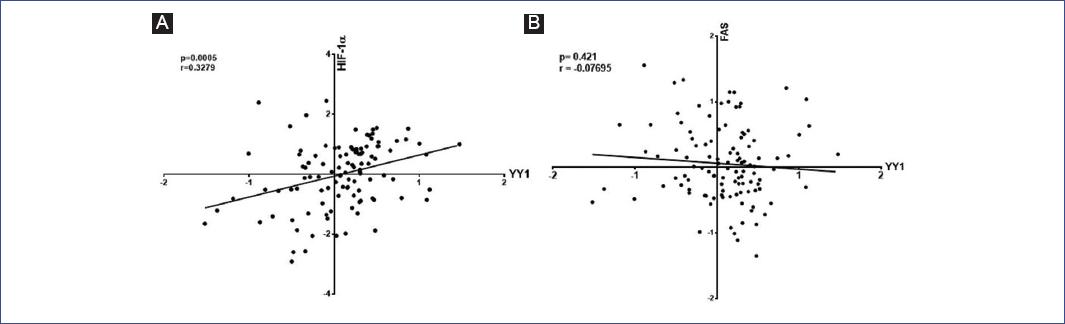
Figure 5 Correlation analyses of hypoxia-inducible factor (HIF-1α)- Yin-Yang-1 (YY1) and YY1-Fas in leukemia patients. Bioinformatics analysis was done using Oncomine and Gene Expression Omnibus Data Bases. A: Pearson correlation analyses of HIF-1α and YY1 (p = 0.0005, r = 0.3279). B: Pearson correlation analyses of YY1 and Fas (p = 0.421, r = −0.07695). Each point represents one leukemia patient.
Discussion
The immune system can eliminate damaged cells by the cytotoxic effect of T-cells and natural killer (NK) cells through the interaction of the FasL present in their membrane and Fas present in the target cell. Cancer cells with low Fas expression could evade immune surveillance because they would be insensitive to death signals mediated by NK and T-cells. Perabo et al. showed that bladder cancer cells (BCC) with low Fas expression had higher cell viability and lower apoptotic rate when they were cultured with Fas agonists, as compared with cells with high Fas expression47. Furthermore, co-culture experiments of BCC with low Fas expression and Jurkat cells showed that BCC was not killed even by high amounts of Jurkat cells; similar results were obtained in the co-culture of BCC with activated peripheral blood mononuclear cells47. In leukemia, low levels of Fas are associated with poor prognosis48 and a poor response to the treatment in pediatric ALL14. Our results show that low expression of Fas results in a low rate of apoptosis when cells are treated with a Fas agonist, and interestingly, we show a correlation between low Fas levels and YY1 overexpression under hypoxic conditions in leukemia cells. Similar results were obtained from a computational analysis of public microarrays available in Oncomine and Gene Expression Omnibus of bone marrow samples.
Hypoxia is a state of low oxygen resulting in the stabilization of HIF1-α, which triggers the transcription of many downstream hypoxia-inducible gene targets. Pediatric22 and adult24 patients with ALL, overexpress HIF1-α, and the expansion of hypoxic areas in bone marrow is associated with leukemia progression23. In this study, we show that overexpression of HIF1-α is correlated with nuclear overexpression of YY1 in ALL cells, suggesting that YY1 regulation could be associated with HIF1-α expression. Mojiri et al.25 showed that YY1 mRNA levels increase in response to hypoxia in LMEC. Furthermore, LMEC increased the nuclear translocation of YY1 under hypoxic conditions25. Nuclear expression of YY1 in cancer has been associated with a shorter survival49, poor prognosis33,49, and metastasis33,38.
Nuclear translocation of YY1 is crucial since YY1 acts as a transcription factor and can regulate the transcription of 10% of human genes50. In this study, we show a high nuclear expression of YY1, which could downregulate Fas expression in leukemia. It has been previously reported that YY1 suppresses Fas transcription in ovarian cancer43 and colorectal cancer cells44. ALL patients with low expression of proapoptotic proteins like caspase 3, caspase 8, and Fas have low induction treatment response14, and this expression is an independent predictor for poor prognosis48. Interestingly, the downregulation of YY1 resensitizes cancer cells to Fas-induced apoptosis44. Taking all these observations together, we propose that hypoxia could be a way to evade the immune system, as this condition produces low Fas expression through YY1. This suggestion could partially explain the poor prognosis observed in cancer patients with high YY1 levels.
Patients with high YY1 expression have low Fas levels, as shown by our results and the results of others45,46. A possible reason as to why we did not find a negative correlation between YY1 and Fas may be due to the low sample size of our study. More studies should be done to establish a possible negative correlation between Fas and YY1, in addition to other factors that could affect Fas expression in leukemia.
Our results show that low Fas expression due to hypoxia generates resistance to apoptosis in leukemic cells, which was determined by the lower number of dead cells after treatment with a Fas agonist in comparison with cells under normoxic conditions, which may be a way to evade the immune system.
In conclusion, hypoxia is a critical condition for the immune escape of LLA cells since this condition allows Fas downregulation through YY1 and confers apoptosis resistance.











 nueva página del texto (beta)
nueva página del texto (beta)