Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Archivos de cardiología de México
versión On-line ISSN 1665-1731versión impresa ISSN 1405-9940
Arch. Cardiol. Méx. vol.81 no.2 Ciudad de México abr./jun. 2011
Investigación clínica
Left and right ventricular power: outputs are the strongest hemodynamic correlates to allow identification of acute responders to vasodilator treatment in idiopathic pulmonary arterial hypertension
El poder del ventrículo derecho y del izquierdo: son parámetros hemodinámicos que correlacionan con la posibilidad de ser respondedor durante el reto agudo con vasodilatadores en la hipertensión arterial pulmonar idiopática
Eulo Lupi–Herrera,1 Julio Sandoval,2 Javier Figueroa,1 Arturo Carrillo,2 Rebeca Aguirre,3 Luis Efren Santos–Martínez,2,4 Tomás Pulido2
1 Sub–Direction of Clinical–Research.
2 Cardiopulmonary–Department, Instituto Nacional de Cardiología Ignacio Chávez.
3 Epidemiology–Department, School of Medicine, UNAM.
4 UMAE Cardiología Centro Médico Nacional Siglo XXI, IMSS.
Corresponding author:
Eulo Lupi Herrera. Director de la Línea de Servicio de l
División Cardiovascular Centro Médico ABC. Sur 136 N° 116.
Col. Las Américas, 01120,
México, D.F. México.
Telephone: 52 308 000 Extension: 3762.
E–mail: elupih@abchospital.com, eulolupiherrera@hotmail.com
Received on April 16, 2009;
Accepted on September 28, 2010.
Abstract
Introduction: Despite the prognostic importance of traditionally derived measurements, the significance of right heart catheterization (RHC) remains controversial. Thus, a continued search for hemodynamic markers that define better responsive patients is required. Since, right ventricular failure is the most fatal pathway, right (RVPO) and left (LVPO) ventricular power output are parameters that could provide input for a better understanding of the hemodynamics involved in idiopathic pulmonary artery hypertension (IPAH).
Method: We retrospectively analyzed how demographics and outcome correlate with hemodynamics to identify responders among IPAH patients.
Results: Ninety patients fulfilled the following criteria for inclusion in this study: (1) complete RHC at baseline; (2) an acute evaluation for vasodilators (AEFV, including a positive response, that is, an increase in CO, a decrease in both mPAP and pulmonary vascular resistance ≥ 20% from baseline, respectively); and (3) a long–term follow–up under accepted IPAH treatments. If RVPO decreased (p < 0.001) and LVPO increased (p < 0.012) during AEFV, it is considered that these findings reinforce our ability to identify responders; that is, patients that remained as responders after 6.4 ± 3 years under nifedipine treatment (37.7% of the studied IPAH population). After multivariate analysis, age, RVPO, and LVPO remained as independent variables (OR = 0.927, 95%CI: 0.87–0.98, p = 0.01; OR = 0.114, 95%CI: 0.00–0.91, p = 0.045; and OR = 171.5, 95% CI: 5.3–549, p = 0.004, respectively) when estimating the probability of being a responder. On this basis, an equation was derived to identify responders among IPAH patients, where the probability of being a responder = 1.0196–0.0631 (age) – 4.7693 (RVPO) + 3.8152 (LVPO), ROC: 0.76, 95% CI: 0.63–0.89; p = 0.001.
Conclusion:based on the proposed equation, LVPO and RVPO could be used for the identification of responders among IPAH patients.
Keywords: Idiopathic pulmonary hypertension; Right/left ventricular power output; Responders; Mexico.
Resumen
Introducción: A pesar de la importancia y del significado pronóstico que tienen las mediciones directas y las derivadas del cateterismo cardiaco derecho, éstas permanecen hasta el día de hoy en el terreno académico de la controversia. Por lo tanto, se requiere la continua búsqueda de marcadores hemodinámicos para estratificar a los enfermos con hipertensión arterial pulmonar idiopática. Particularmente, cuando la disfunción contráctil del ventrículo derecho es la vía final más común de esta patología. En esta circunstancia, la determinación del poder del ventrículo derecho y del ventrículo izquierdo representa parámetros que pudieran ser de utilidad para lograr un mejor entendimiento en la hemodinámica de la hipertensión arterial pulmonar idiopática.
Método: De manera retrospectiva, analizamos los aspectos demográficos, los hemodinámicos y la sobrevivencia, y si éstos se vieron asociados a la posibilidad de ser enfermos respondedores entre los portadores de hipertensión arterial pulmonar idiopática.
Resultados: Noventa enfermos llenaron los siguientes criterios para ser incluidos en el estudio: 1. Contar con cateterismo cardiaco derecho basal; 2. Tener valoración aguda con adenosina, en donde quedó definida una respuesta "positiva aguda" como: aumento del gasto cardíaco, disminución de la presión arterial pulmonar media y de la resistencia vascular pulmonar calculada (≥ 20% de la basal, respectivamente) y; 3. Contar con un seguimiento a largo plazo bajo la influencia de los tratamientos modernos aceptados para enfermos con hipertensión arterial pulmonar idiopática. Sí, el poder del ventrículo derecho disminuyó (p < 0.001) y el poder ventrículo izquierdo aumentó (p < 0.012) durante el reto vasodilatador agudo se consideró que éstos hallazgos reforzaban la habilidad para detectar a los sujetos respondedores con hipertensión arterial pulmonar idiopática; población de enfermos que guardó ese comportamiento hemodinámico durante 6.4 ± 3 años bajo la influencia de nifedipina (37% de la totalidad de la población con hipertensión arterial pulmonar idiopática). Después de efectuar un análisis multivariado, la edad, el poder del ventrículo derecho y del ventrículo izquierdo permanecieron como variables independientes (OR = 0.927, 95%IC: 0.87–0.98, p = 0.01; OR = 0.114, 95%IC: 0.00–0.91, p = 0.045; y OR = 171.5, 95%IC: 5.3–549, p = 0.004, respectivamente) para ser considerados "respondedores". Como resultado, se derivó una ecuación donde la probabilidad de ser respondedor = 1.0196–0.0631 (edad) – 4.7693 (poder del ventrículo derecho) + 3.8152 (poder del ventrículo izquierdo), ROC: 0.76, 95%CI: 0.63 – 0.89; p = 0.001.
Conclusión: Con fundamento en los hallazgos de este estudio, la ecuación propuesta, el poder del ventrículo derecho y el ventrículo izquierdo pueden ser utilizados para identificar "respondedores" entre los enfermos con hipertensión arterial pulmonar idiopática.
Palabras clave: Hipertensión pulmonar idiopática; Poder del ventrículo derecho; Poder del ventrículo izquierdo; Respondedores; México.
Introduction
The criteria to define a responder or non–responder based on changes in mean pulmonary artery pressure (mPAP) and pulmonary vascular resistance (PVR) during an acute vasodilation challenge have been questioned in recent years in idiopathic pulmonary artery hypertension (IPAH).1–8 Instead, a positive acute vasoreactive response is now defined as a reduction of the mPAP ≥ 10 mmHg to an absolute value of mPAP ≤ 40 mmHg, with increased/ unchanged cardiac output (CO).8 Accordingly, only about 6 to 15% of the IPAH population fulfills these criteria based on a retrospective experience, derived from hemodynamic characteristics of patients who benefited from long–term calcium channel blockers (CCBs) treatment.8,9
Despite significant advances in IPAH, right ventricular failure (RVF) remains the common fatal pathway and consequence of PAH.1–8 The principles used to determine the external work of the heart can be applied to measure either the hydraulic energy associated with blood flow at "any point in the circulation" or the energy dissipated by flow through a particular vascular bed.10 Thus, for the RV and pulmonary vascular circuit, it will be the product of flow output and pulmonary artery pressure, the rate of useful work done, or right ventricular power output (RVPO).10–12 Therefore, by coupling the mPAP and CO domains of the cardiopulmonary system, we will obtain a measure of the RV performance in IPAH. Besides, some studies have shown that left ventricular power output (LVPO) is a good indicator of cardiac function.10,11 On this functional basis,10–12 we previously found differences in RVPO in a small cohort of IPAH patients who were considered to be responders or non–responders.13 To extend this limited hemodynamic observation, the objective of our present study was to investigate the definitive role for RVPO and LVPO to identify better responders among IPAH patients.
Method
Our protocol was approved by the institutional ethics review commission. All patients were born and raised at an altitude of 2240 m and were permanent residents of Mexico City. The IPAH diagnosis was based on both clinical and hemodynamic criteria and by excluding other conditions known to cause PAH: 1) PAH associated with anorexigens, connective tissue disease, congenital heart disease, portal hypertension, or HVI infection; 2) chronic thromboembolic pulmonary hypertension; and, 3) other chronic respiratory diseases.1–4,8 Pulmonary hypertension was defined by a resting mPAP ≥ 25 mmHg during RHC, with a mean pulmonary wedge pressure (mPWP) ≤ 15 mmHg and a PVR greater than three Wood units.14,15 We retrospectively studied the medical records of 134 consecutive adult patients hospitalized in our institution between 1997 and 2007, with a diagnosis of IPAH. A total of 90 patients fulfilled the following criteria for inclusion in the study: (1) a complete RHC at baseline, (2) an acute I.V. vasodilator–drug challenge to assess vasodilator response, and (3) chronic oral nifedipine therapy was initiated in patients who displayed significant acute pulmonary vasodilatation.
Hemodynamic measurements: Our procedure for RHC has been described previously.2,13,16,17 The following measurements were obtained: mean right atrial pressure (mRAP), mPAP, mPWP, CO (was measured by triplicate by the thermodilution method),2,13,16,17 and mean systemic arterial pressure (mSAP). The following parameters were derived using traditional equations: PVR, systemic vascular resistance (SVR), and pulmonary vascular–to–systemic resistance ratio (PVR/SVR). LVPO was calculated as mS–APxCO/451 and RVPO was calculated as mPAPxCO/451.12 As an important part of our catheterization protocol, we routinely assess the response to oxygen breathing to exclude the role of alveolar hypoxia in the genesis of PAH (our definition of IPAH at our moderate altitude includes the absence of a positive response to 99.9%O2 breathing).16,17
Acute Vasodilating Trial: For acute vasodilating testing we used adenosine, supported by the studies of Schrader18 and Sandoval,19 who observed a significant correlation between the reduction in PVR resulting from adenosine and that achieved by the administration of nifedipine. Furthermore, adenosine is considered today an acceptable alternative to the preferred vasodilator: inhaled nitric oxide (iNO).14,15
Based on the hemodynamic response, we separated the patients into two groups: responders and non–responders. Our criteria for an "acute positive response" to vasodilators included: 1) a decrease in mPAP, 2) an increase in CO; and, 3) a decrease in PVR (≥ 20% from baseline, respectively).2,16 When the above criteria were met, a patient was considered for treatment with nifedipine. "Responder" patients were administered oral doses of nifedipine (10 mg 3–4 times/day); then, daily doses were titrated to 20 mg, 3–4 times/day, provided the patient did not exhibit side effects.4 Long–term nifedipine responders were defined as patients with hemodynamic improvement (a sustained decrease in mPAP and an increase in CO ≥ 20%) with at least ≥ 12 months on nifedipine without addition of other modern modalities of therapy for IPAH.
Data analysis: We: 1) analyzed the clinical and hemodynamic characteristics of our patients for the whole group (n = 90) and separately for those considered to be responders (n = 34) or non–responders (n = 56); 2) evaluated the ability for LVPO, RVPO, and their ratio to identify responders; 3) compared the Task Force criteria (a decrease in mean PAP by at least 10 mmHg to an absolute of less than 40 mmHg without a decrease in CO)8,14,15 with our acute positive criteria to identify responders; 4) compared both criteria among responders; and 5) assessed the long–term response to nifedipine.
Data are expressed as mean ± SD, median (min,max) and frequencies (percentages) as appropriate. A Student t test, 1–way–ANOVA (Bonferroni's–test for multiple comparison), chi square, or Fisher exact test was used as appropriate. Univariate analysis based on the logistic regression model was used to examine the relation between responders or non–responders and selected demographics, medical history, and hemodynamic variables that were measured at initial RHC. Results are expressed as odds ratio with 95% CI. Upon completion of the univariate analysis, any variable whose univariate test produced a value of p < 0.25 was considered a candidate for the multivariate model. Inclusions in the final multivariate logistic regression model were determined by those variables associated with a significance level of p < 0.05 in a stepwise elimination process. After the predictor variables in the regression model had been finalized, two statistical tests were performed to assess the model performance: the Hosmer–Lemeshow statistics and the ROC curve. Analyses were performed using SPSS–13 software.
Results
Demographic/clinical characteristics: According to the NYHA/WHO classification, there were no class III–IV responders and no class–I non–responders (Table 1). Treatments, such as subcutaneous treprostinil (n = 12), sitaxsentan (n = 8), bosentan (n = 10), ambrisentan (n = 5) and sildenafil (n = 21), were only distributed in non–responders. Fourteen non–responders in classes III–IV were electively sent for graded atrial septostomy.17 Mortality associated to sudden death occurred in one responder and two non–responders and to refractory RVF in seven non–responders.
Baseline hemodynamics: When we compared responders and non–responders, no differences in mPAP (p = 0.07), CO (p = 0.7), mPWP (0.87), PVR (0.08), and PVR/ SVR ratio (p = 0.08) were observed. Differences were observed for mRAP, LVPO, RVPO and LVPO/RVPO (p < 0.001; for all measurements, Table 2).
Acute response to vasodilating: The following changes in hemodynamic parameters were observed in the responders (34/90, 37.7%): a decrease from baseline mRAP, mPAP, and PVR values (–20%, p < 0.045; – 31.5%, p < 0.001; – 45%, p < 0.001, respectively), no change in mPWP (p = 0.901), an increase in CO (+ 36.9%, p < 0.001), and a decrease in PVR/SVR ratio (p < 0.001, Table 2). During the vasodilator trial, RVPO decreased (p < 0.001), LVPO (p = 0.012) increased and LVPO/RVPO increased. No differences from baseline mPAP (fall), RVPO (decrease), LVPO (increase), and LVPO/ RVPO (increase) reached during acute vasodilator testing among responders were observed when the Task Force8,14 and our criteria were compared (Table 3).
For the non–responders (56/90, 62.2%), mRAP (p = 0.275), mPAP (p = 0.222), PVR (p = 0.37), and mPWP (p = 0.81) did not change; CO increased (+ 12%, p < 0.001); mSAP decreased (p < 0.002); and the PVR/SVR ratio did not change (p = 0.243). During the acute vasodilator trial, RVPO increased (p < 0.001), LVPO (p = 0.125) and LVPO/RVPO (p = 0.467) did not change.
Hemodynamics and NYHA classes: mRAP was different among classes (class I: 5.5 ± 3.4, class II: 9.9 ± 4.2, class III: 11.4 ± 3, and class IV: 18 ± 0.1 mmHg; p < 0.001). For mPAP, CO, PVR, RVPO, LVPO, and LVPO/RVPO no differences were documented among classes.
Long–term evaluation: Was performed by a repeat RHC in 30 responders under chronic nifedipine (this cohort includes 16 patients who did not fulfill the Task Force criteria8,15 at the initial RHC) and in 42 non–responders treated with the other forms of therapy (Table 4). Long–term nifedipine–treated responders, according to the Task Force8,15 or our criteria, did not differ in terms of treatment regimen: the mean daily doses of nifedipine were 59 ± 15 mg (range 40–80 mg, n = 14), 62 ± 14 mg (range 40–80, n = 16), p = 0.27; and nifedipine therapy duration (6.4 ± 3.5 versus 6.3 ± 2.9 years, p = 0.85, respectively).
For responders, mRAP (p = 0.05) and mPAP (p < 0.001) remained low; CO was still increased (p < 0.002); the PVR/SVR ratio (p = 0.01) and RVPO (p < 0.04) remained decreased and LVPO increased (p < 0.001) after 6.4 ± 3.1 years of follow–up (Table 4). For the LVPO/RVPO, a further increase was documented in comparison to the acute evaluation among responders (p < 0.001). For this group, the patients remained in I–II classes.
For non–responders, mPAP (p = 0.473), mRAP (p = 0.494), CO (p = 0.121), RVPO (p = 0.1), LVPO (p = 0.193), and LVPO/RVPO did not change. The same initial abnormal hemodynamic profile was observed after 6.1 ±_3.2 years. In this group, the patients remained in classes II–III. When we compared the hemodynamic evolution over time of the responders versus the non–responders, the responders displayed a decreased mRAP (p < 0.001), mPAP (p < 0.001) and RVPO (p < 0.01) and an increased CO (p < 0.001), LVPO (p < 0.001), and LVPO/RVPO (p < 0.001, Figure 1).

Univariate analysis: Variables associated with an acute responder included: age (p = 0.03), estimated symptom duration (p = 0.02), mRAP (p < 0.001), mPAP (p < 0.001), RVPO (p = 0.007), and LVPO (p = 0.036) (Table 5).
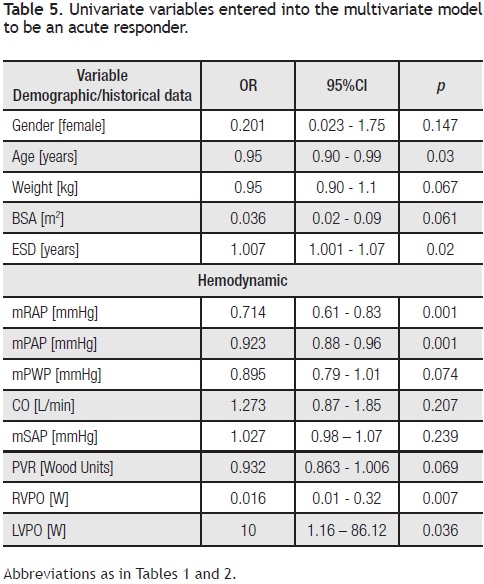
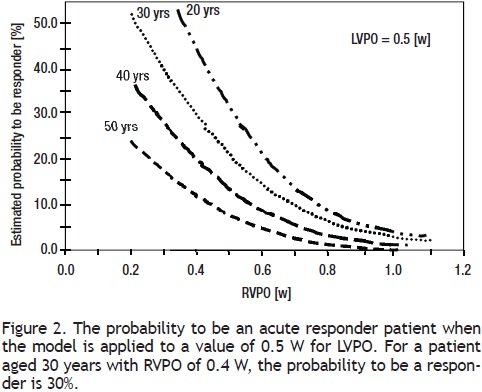
Multivariate analysis: Age, RVPO, and LVPO remained as independent variables (OR = 0.927, 95% CI: 0.87–0.98, p = 0.01; OR = 0.114, 95% CI: 0.00–0.91, p = 0.045; and OR = 171.5, 95% CI: 5.3–549, p = 0.004, respectively) for estimating the probability of being a responder. According to the logistic multivariate analysis, the following equation was derived for identifying responders among patients = 1.0196–0.0631 (age) –4.7693(RVPO), +3.8152 (LVPO), ROC: 0.76, 95% CI: 0.63–0.89; p = 0.001. According to the results of the Hosmer–Lemeshow test (X2: 9.234, DF = 8, p = 0.323) and the area value for the ROC curve (0.76, 95% CI: 0.63–0.89, p= 0.001), the derived model can be used to estimate the probability of being an acute responder among IPAH patients (Figure 2).
Discussion
A critical reason for considering an "acute positive" response in IPAH is to identify patients whose response at entry RHC appears to predict better long–term prognoses.1–5 However, this also has important economic consequences (low price oral vasodilators vs. other expensive modern therapies). Thus, a continued search for hemodynamic markers that define better responsive patients is required. By using the proposed criteria from the Task Force8,14 or our criteria to identify responders among IPAH patients, it is possible to achieve this important goal in this population. However, using the Task Force criteria,8,14 responders will be identified in 17.7%, whereas our criteria identify 37.7% of the total IPAH patients, and among responders the Task Force criteria8,14 would identify only 53% of them. Consequently, the question arises as to which hemodynamic criteria are the most appropriate for achieving this important goal. If analyzed as a group, the responders could reach 38.2 mmHg, although a stringent cut–off value of 40 mmHg was not always achieved in association with a >20% increase in CO. Although, we must emphasize that we also observed a decrease in ≥10 mmHg for mPAP in our responders.8,14 In addition, the different hemodynamic behavior of the responders and non–responders in regard to RVPO, LVPO, and LVPO/RVPO reinforces our ability to identify responders among IPAH population. In our study we demonstrated a decrease in RVPO associated with an increase in LVPO and LVPO/RVPO for responder patients.
NYHA/WHO–classification influence on the proportion of responders: In our study, the acute response rate appears higher than other reported for adult IPAH patients.9 This may be explained, in part, by differences in the proportional NYHA classes distribution of the studied populations. In the cohort studied by Sitbon9 (n = 557), only 19% (n = 70) were class I–II patients. Thus, a low proportion of classes I–II were included compared to classes III–IV patients in the Sitbon study.9 On the contrary, in our series, 78.8% (71/90) were in classes I–II at initial RHC.
Consequently, we should emphasize that whenever responder patients are examined in IPAH studies, we must take into consideration the total of class I–II patients who are evaluated in the acute vasodilator challenge. Thus, differences in the acute response rate not only could result from the applied criteria, but also from the number of class I–II patients tested in relation to the number of class III–IV patients tested in an acute trial.
Long–term measurements: The significant differences noted between responders and non–responders for RVPO, LVPO, and LVPO/RVPO at the initial RHC were confirmed at the RHC long–term follow–up. For patients under chronic nifedipine therapy, the RVPO decreased further, and LVPO increased to 1.15 ± 0.27 W, which is close to the resting normal values.10 These data suggest a persistent predominant vasodilator effect in the pulmonary vasculature and translates a sustained good RV pumping performance. For non–responders, a persistent abnormal elevation in mPAP, decreased CO, increased RVPO, and a low abnormal resting LVPO (0.67 ± 0.15 W) were documented, resulting in a sustained hemodynamic profile that eventually must worsen the cardiac pumping ability. This condition could explain why a low long–term mortality was not associated in non–responders, but was related in the responder cohort (Table 1). The hemodynamic findings for the coupling of RV–pulmonary circulation observed at long–term RHC further underscores the validity of our criteria of "acute positive response" applied at the initial RHC for the IPAH patients studied. Stibon9 was able to retrospectively define more stringent criteria at baseline, which identified the responders who would have sustained long–term benefit from CCBs. We were unable to demonstrate that such criteria at baseline predict the change from responder to non–responder status in 58% (14/24) of patients; our responders remained as responders at least up to 6.4 ± 3 years under nifedipine (Table 4).
The model: To test the proposed model, we examined Rich's4 study. The rate of responders obtained by Rich4 after the vasodilating test was 26%. When we applied our model to estimate the proportion of responders, among IPAH patients, the derived value was close to 30%. Thus, our model to predict an acute positive response is in reasonable agreement with previous information on the proportion of responders who have been observed with a similar age, tested acutely for vasodilation with nifedipine and long–term treated with CCBs.
Overall evaluation: Based on our results, we propose that the hemodynamic behavior of RVPO and LVPO should be incorporated, in addition to the proposed criteria for the definition of a "positive" response to vasodilators. In this regard, there are several pathophysiological reasons to include RVPO for such clinical/hemodynamic stratification. As PAH develops, the distensibility of the elastic arteries decreases.8,20 Consequently, a larger amount of RV work must be expended to distend the stiff pulmonary arteriolar vessels. As a consequence, the pulsatile arterial power generated by the RV is an important proportion (35–40%) of the total external power.10 If we include only PVR, we are missing part of the RV load; whereas by determining RVPO, we obtain an approximate calculation of the pressure work generated by the RV, which is determined by flow output and the pulmonary arterial load.10
Our findings for the proposed markers support that RVF is the main resulting cause of death in these patients.1–3,5,8 In non–responders with lower likelihood of survival, the behavior of RVPO and LVPO indicates that these patients are hemodynamically characterized with a reduced reserve of the pulmonary circulation associated with decreased RV function, resulting in an inappropriate RV–pulmonary arterial coupling and an inappropriate ventricular interaction.1,3,5
Limitations: Are essentially associated with the fact that the information is from a single cardiovascular referral center, located at moderate altitude. Although, we should emphasize that alveolar hypoxia was ruled out (as part of our IPAH definition);14 thus, our findings could be generalized for IPAH patients. Moreover, the number of patients included for testing RVPO and LVPO can be considered large because IPAH is a rare disease.1,5 We are aware that the retrospective nature of the study potentially introduces a selection bias. However, the information collected at the first RHC for the RVPO and LVPO was reproducible for all the patients at long–term follow–up RHC. We are also aware that 1) instantaneous (pulsatile) data on pressure and flow are essential for an accurate calculation of external ventricular work and an error using mPAP in combination with CO (instead of measured flow) will underestimate the true RVPO–LVPO in our RHC studies.10 2) perhaps we cannot compare exactly the acute response to adenosine with that obtained with iNO or epoprostenol in class III–IV IPAH patients;14,15 and, 3) due to the small number of deaths in the studied population, a mortality prognostic value could not be derived for RVPO–LVPO. Mortality prognostic value for RVPO–LVPO that in the near future should be investigated in a larger population IPAH patients.
Conclusions
RVPO is a novel hemodynamic–derived measure that gives better insight into the RV–pulmonary arterial coupling nature and, in conjunction with LVPO and LVPO/RVPO, for the overall hemodynamics in IPAH. RVPO and LVPO result in good hemodynamic tools that should be added to the existing ones to identify responders among IPAH patients.
References
1. D'Alonzo GE, Barst RJ, Ayres SM, et al. Surv'val in patients with primary pulmonary hypertension: Results from a national prospective registry. Ann Intern Med 1991;115:343–349. [ Links ]
2. Sandoval J, Bauerle O, Palomar A, et al. Survival in primary pulmonary hypertension: validation of a prognostic equation. Circulation 1994;89:1733–1744. [ Links ]
3. Rich S, Levy PS. Characteristics of surviving and non–surviving patients with primary pulmonary hypertension. Am J Med 1984;76:573–578. [ Links ]
4. Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium–channel blockers on survival in primary pulmonary hypertension. N Eng J Med 1992;327:76–81. [ Links ]
5. Weir EK, Rubin LJ, Ayres SM, et al. The acute administration of vasodilators in primary pulmonary hypertension. Experience from the National Institutes of Health Registry on primary pulmonary hypertension. Am Rev Respir Dis 1989;140:1623–1630. [ Links ]
6. Rich S, Martínez J, Lam W, et al. Reassessment of the effects of vasodilator drugs in primary pulmonary hypertension: Guidelines for determining a pulmonary vasodilator response. Am Heart J 1983;105:119–127. [ Links ]
7. Galie N, Ussia G, Passarelli P, et al. Role of pharmacologic tests in the treatment of primary pulmonary hypertension. Am J Cardiol 1995;75:55A–62A. [ Links ]
8. Guidelines on diagnosis and treatment of pulmonary arterial hypertension. The Task Force on diagnosis and treatment of pulmonary arterial hypertension of the European Society of Cardiology. Eur Heart J 2004;25:2243–2278. [ Links ]
9. Sitbon O, Humbert M, Jaïs X, et al. Long–term response to calcium channel blockers in Idiopathic Pulmonary Arterial Hypertension. Circulation 2005;111:3105–3111. [ Links ]
10. Milnor WR. Cardiac Dynamics. In: Hemodynamics. Baltimore/ London: Williams & Wilkins 1982;pp:244–271. [ Links ]
11. Cotter G, Williams SG, Vered Z, et al; for the SHOCK Investigators. Role of cardiac power in heart failure. Curr Opin Cardiol 2003;18:215–222. [ Links ]
12. Fincke R, Hochman JS, Lowe AM, et al.; for the SHOCK Investigators. Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. J Am Coll Cardiol 2004;44:340–348. [ Links ]
13. Lupi HE, Sandoval JZ, Chuquiure VE, et al. El análisis del poder cardíaco "pulmonar" en hipertensión arterial pulmonar primaria y secundaria. Observaciones iniciales, su posible utilidad en la clínica. Arch Cardiol Méx 2006;76:140–150. [ Links ]
14. Expert Consensus Document ACCF/AHA 2009 Expert Consensus Document on Pulmonary Hypertension. A Report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association. Developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc, and the Pulmonary Hypertension Association. Circulation 2009;119:2250–2294. [ Links ]
15. Guidelines for the diagnosis and treatment of pulmonary hypertension. The Task Force for the diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology [ESC] and the European Respiratory Society [ERS] endorsed by the International Society of Heart and Lung Transplantation [ISHLT]. Eur Resp J 2009;34:1219–1263. [ Links ]
16. Lupi HE, Sandoval JZ, Seoane M, et al. The role of hydralazine therapy for pulmonary artery hypertension of unknown cause. Circulation 1982;65:645–650. [ Links ]
17. Sandoval J, Gaspar J, Pulido T, et al. Graded balloon dilatation atrial septostomy in severe primary pulmonary hypertension. A therapeutic alternative for patients nonresponsive to vasodilator treatment. J Am Coll Cardiol 1998;32:297–304. [ Links ]
18. Schrader BJ, Inbar S, Kaufmann L, et al. Comparison of the effects of adenosine and nifedipine in pulmonary hypertension. J Am Coll Cardiol 1992;19:1060–1064. [ Links ]
19. Sandoval J, Pulido T, Suarez J, et al. Adenosine as a predictor of the hemodynamic response to nifedipine in Primary Pulmonary Hypertension (abstract). Am J Respir Crit Care Med 1997;155:A443. [ Links ]
20. Champion HC, Michelakis ED, Hassoun PM. Comprehensive Invasive and Noninvasive Approach to the right ventricle –pulmonary circulation unit. State of the Art and Clinical and Research Implication. Circulation 2009;120:992–1007. [ Links ]














