Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Archivos de cardiología de México
versión On-line ISSN 1665-1731versión impresa ISSN 1405-9940
Arch. Cardiol. Méx. vol.78 no.4 Ciudad de México oct./dic. 2008
Investigación clínica
A proposed functional clinical classification predicts in–hospital and long–term survival in the setting of acute right ventricular infarction
Propuesta de una clasificación funcional y clínica para predecir la sobrevida intrahospitalaria y a largo plazo de enfermos con infarto agudo del ventrículo derecho
Eulo Lupi–Herrera,* Eduardo Chuquiure–Valenzuela,** Héctor González–Pacheco,** Ursulo Juárez–Herrera,** Carlos Martínez–Sánchez,** Jorge Gaspar***
* Sub–Direction of Clinical Investigation.
** Coronary Care Unit.
*** Interventional Cardiology Department.
From the Instituto Nacional de Cardiología Ignacio Chávez, Mexico City, Mexico.
Correspondence:
Sub–Director Eulo Lupi–Herrera MD,
FACC. Sub–Dirección de Investigación Clínica.
Instituto Nacional de Cardiología Ignacio Chávez
(INCICH, Juan Badiano Núm. 1, Col. Sección XVI,
Tlalpan 14080. México D.F.).
Tel–fax: +525555732479,
E–mail: eulo.lupi@cardiologia.org.mx.
Recibido: 3 de marzo de 2008
Aceptado: 5 de junio de 2008
Abstract
Background: The objectives of the present investigation were to validate the prognostic role of a proposed Clinical Classification [CC], to evaluate the TIMI risk score [RS] and to establish whether the TIMI–RS should incorporate points for patients with acute right ventricular infarction [TIMI–RS–RVI].
Methods and results: A total of 523 RVI patients were classified on clinical and functional basis as: A, without right ventricular failure [RVF], B with RVF and C with cardiogenic shock. The CC was evaluated prospectively among 98 patients with RVI and retrospectively in 425 RVI patients. The TIMI–RS was evaluated prospectively among 622 patients with STEMI [anterior:277, inferior:247, RVI:98], and retrospectively in 425 RVI patients. The CC established differences among the 3–RVI Classes for in–hospital mortality [prospectively and retrospectively; p < 0.01, p < 0.001, respectively] that were maintained at 8 years [p < 0.001]. Patients with anterior and inferior STEMI, but not those with RVI revealed an association between outcome and TIMI–RS [p < 0.001]. Testing for TIMI–RS–RVI did not result a good prognostic tool [ROC = 0.9; excellent discrimination, but with a very poor "clinical calibration"].
Conclusions: The proposed CC allowed prediction of mortality at short– and long–term in the setting of acute RVI. The role of the TIMI–RS should be reevaluated prospectively as a prognostic tool in the scenario of RVI patients.
Key words: Right ventricular infarction. TIMI risk score. Clinical classification.
Resumen
Antecedentes: Esta investigación tuvo como metas validar el significado pronóstico de la clasificación clínica [CC] previamente propuesta, valorar el puntaje TIMI y tratar de establecer si es posible incorporar puntos clínicos/electrocardiográficos al puntaje TIMI en enfermos con infarto agudo del ventrículo derecho [TIMI–IAVD].
Métodos y resultados: Un total de 523 enfermos con IAVD se clasificaron con fundamento funcional y clínico en: Clase A, sin disfunción ventricular derecha [DVD], Clase B con DVD y en Clase C en estado de choque. La CC se sustentó con bases ecocardiográficas y hemodinámicas. Fue evaluada de manera prospectiva en 98 enfermos y de forma retrospectiva en 425 enfermos con IAVD. El puntaje TIMI fue valorado de manera prospectiva en 622 enfermos con SICA con elevación del segmento ST [anteriores: 277, inferiores: 247 y con IAVD: 98] y de forma retrospectiva en 425 con IAVD. La CC estableció diferencias en relación a la mortalidad intrahospitalaria en los tres grupos de enfermos [de manera prospectiva y retrospectiva; p < 0.01, p < 0.001, respectivamente], las que se mantuvieron durante 8 años de seguimiento [p < 0.001 ].
Los enfermos con infarto de la cara anterior e inferior, pero no en aquéllos con IAVD mostraron asociación entre la mortalidad y el puntaje TIMI [p < 0.001]. El intento de la creación del puntaje TIMI–IAVD no resultó un instrumento útil para ayudar a establecer el pronóstico.
Conclusiones: La CC propuesta permite predecir la mortalidad a corto y a largo plazo en el escenario del IAVD. Debe revalorarse la utilidad del puntaje TIMI para el IAVD de forma prospectiva y en una cohorte más amplia.
Palabras clave: Infarto del ventrículo derecho. Puntaje TIMI. Clasificación clínica.
Introduction
The development of the TIMI [Throm–bolysis in Myocardial Infarction]–risk score [RS] has proved to be a useful tool to easily stratify patients with ST–elevation myocardial infarction [STEMI] and its usefulness has also been validated in a large STEMI registry.1,2 While several investigators have established the prognostic role of right ventricular infarction [RVI] in patients,3,4 only few have previously attempted to stratify risk among patients with RVI for in–hospital and long–term survival.5,6 Therefore, further studies are required to better define the prognostic importance of right–sided ST–elevation and to establish whether the TIMI–RS should incorporate points for right–sided electrocardiographic changes.7
Furthermore, the pathophysiologic mechanism of low output in RVI is quite different from that of left ventricular infarction. 3–6 Thus the criteria for inclusion in clinical classes of left ventricular STEMI can not be used for RVI. In a previous study we demonstrate that there is a wide "clinical spectrum of acute RVI", that three classes could be identified prospectively and that there are significant differences in early mortality rates between RVI classes.6 Accordingly, if RVI is documented, it must then be stratified using the clinical classification as has been proposed, because Class A RVI patients, those without right ventricular failure [RVF] was not found to be a predictor of mortality, Class B [with RVF] and class C [with cardiogenic shock] are strongest independent predictors of in–hospital mortality. Therefore, we recommend that the "spectrum of RVI" should always be considered in discussion clinical scenario of RVI.6
Methods
On the previous mentioned background, the aims of this study were: First, to ascertain the role of a proposed clinical classification [CC]6 in the prediction of short–term mortality and long–term survival for RVI patients. Second, to evaluate the TIMI–RS1 and if incorporating points for clinical RVI findings and right–sided electrocardiographic changes [TIMI–RS–RVI] could identify better the prognosis in patients with acute RVI.
Patient population
The study protocol was approved by the ethics review commission. We prospectively screened 1,402 consecutive patients admitted with a first inferior STEMI from 1990 to 2002, and identified 425 with extension to the right ventricle [Right ventricular infarction: RVI]. This RVI population underwent follow–up through July 2007. Patients were included in the study if they all had a complete clinical examination, an echocardiogram, or a coronary angiogram or a right heart catheterization. Isolated acute RVI, history of valve heart disease, previous heart failure, pulmonary arterial hypertension or renal failure were exclusion criteria for the enrollment to the study. We did not include in the shock category patients with hypotension related to hypovolemia, transient hypotension related to vasodilatation and bradycardia associated with spontaneous reperfusion [Bezold–Jarich reflex], or hypotension related to atri oven tricular heart block or cardiac arrhythmias or associated with mechanical complications [ventricular septal or myocardial wall rupture or cardiac tamponade] at entry.
Diagnosis for RVI, RVF and cardiogenic shock
The diagnostic criteria for inferior myocardial infarction with extension to the walls of the right ventricle, RVF and cardiogenic shock have been published previously.6 Briefly, in addition to standard ECG leads, right–sided thoracic ECG [leads V3R–V7R] was recorded in all patients immediately after admission. ST–segment elevation in lead V4R of greater than 0. lmV was used to diagnose RVI. The diagnosis of RVI was also based on clinical features that have been described previously as been associated with this variety of infarction, and also on echocardiographic findings. Echocardiograms were analyzed separated by two skilled cardiologist–echocardiographers who were blinded to the clinical characteristics, ECG findings, angiographic and hemodynamic information of RVI patients. RVF diagnosis, was based on clinical features [persistently systemic hypotension [systemic systolic pressure [SSP] < 100 mmHg, right–sided S3 and S4]] but without features of shock; echocardiographic evidence of ischemic RVF [for example RV wall motion abnormalities [WMA] associated with gross RV dilatation, findings that suggest global depress RV function and invasive hemodynamic monitoring identifying RVI by a combination of findings that suggest dysfunction of the RV [low cardiac output [CO] and a disproportionate elevation of the right atrial pressure[RAP] compared with the pulmonary wedge pressure[PWP]].7 The diagnosis for shock was made if all the following criteria were met: SSP persistently < 90 mm Hg or vasopres–sors required to maintain SSP > 90 mmHg; very low CO [cardiac index [CI] < 2.1L.min/m2]; evidence of end organ hypoperfusion.
Clinical classification and the TIMI–RS
We risk–stratified RVI patients into 3–subsets based on clinical features and echocardiographic findings upon admission, that were further supported with hemodynamic findings as follows: Class A, comprised patients without evidence of systemic hypotension [SSP> 100 mmHg] orRVF [by clinical features, echocardiographic and/or hemodynamic findings]; Class B, were those with persistent systemic hypotension [SSP < 100 mm Hg] or RVF, but without other clinical features of shock; and Class C, were those in shock.
TIMI–RS was calculated according to previously published criteria.1 We tested retrospectively 2–schemes; TIMI–RS and the CC for 425 RVI patients; prospectively between January 2003 and July 2007 among 622 consecutive patients with STEMI [anterior: 277; inferior: 247; RVI: 98].
Reperfusion procedures
For RVI patients, considered for thrombolytic therapy [TT] or percutaneous coronary intervention [PCI]; TT was administered to all eligible RVI patients; inclusion criteria for classes A and B were defined before admission as follows: symptoms of MI lasting less than 6h; age < 75y; and absence of other well known accepted contraindications. TT was performed with either streptokinase or recombinant tissue plasminogen activator, preceded by a heparin bolus. Adjunctive treatment consisted of an intravenous heparin dose that was adjusted to keep the TPTa between 60–80s and aspirin. For PCI the same inclusion criteria for MI symptoms duration/age were applied; stent deployment was performed according to standard techniques and follow by the use of standard anti platelet therapy. Stents and glycoprotein lib/ Ilia platelet inhibitors became standard therapy in eligible patients in 1995 and 1996, respectively. Clopidogrel was used in stented patients [loading dose 300 mg] and continued [75 mg] for > 6 months in patients treated with bare metal stents and for 6 to 12 months in patients treated with drug–eluting stents.
For those RVI patients in shock considerer candidates for PCI: age < 75y and shock within < 24 hours of evolution. Conservative therapy for A and B classes was follow due to: symptoms of MI lasting more than 6h and/or to delay [> 6h] in patients arrivals for seeking for medical attention; for class C patients for those with age > 75y or to those who develop shock > 40h of the onset of MI.
Primary end point was in–hospital cardiac death at 20–30 days. Follow–up information after hospital discharge was obtained from the hospital records from the Instituto Nacional de Cardiología "Ignacio Chávez" registration database that is updated at each patient visit and upon patient death.
Statistical analysis
Continuous data are expressed as mean ± SD. Student t test, 1–way ANOVA [Bonferroni's test for multiple comparison], χ2, or Fisher exact test was used as appropriate. Univariate analysis based on the logistic regression model was used to examine the relation between selected demographic, medical history, clinical examination and ECG/ECHO data to add points in attempt to building–up the TIMI–RS–RVI model. Results are expressed as odds ratios 95% confidence intervals [CI]. At completion of the univariate analysis, any variable whose univariate test had a value of p < 0.25 was considered a candidate for the multivariate model. Inclusions into the multivariate logistic regression model was determined by the number of variables associated with a significance level of p < 0.05 in a stepwise elimination process were retained in the final model. After the predictor variables in the regression model had been finalized, the prognostic discriminatory capacity for the TIMI–RS–RVI was expressed as the c statistic, representing the area under the receiver operating characteristic [ROC] curve for prediction of in–hospital death. For the survival analysis we used the time of the initial ECHO/ RHC as an index for determining survival. The Kaplan–Meier method was used to estimate overall survival distribution [Log–rank test]. Analyses were performed by using of SPSS–13 and STATA–9 software.
Results
The diagnosis of RVI was made by ECG and either echocardiographic, hemodynamic or coronary angiographic criteria in 100% and by three criteria [ECG, echocardiographic and hemodynamic] in 85% of the patients.
Clinical and echocardiographic data
The frequency in the baseline clinical characteristics used as a predictors in the studied STEMI population and in the InTIME–II1 study are shown in Table I. Class A, B and C RVI–subgroups, had no differences in their baseline characteristics for both prospectively and retrospectively RVI studied patients. The echocardiographic data that give support to the CC for all studied RVI patients were documented as follows: no RV–dilatation, RV wall motion abnormalities [RVWMA] only for the inferior wall [IW = 100%] in addition to the free wall [FW = 14%], paradoxical ventricular septal motion [PVSM = 25%], tri–cuspid regurgitation [TR =17%] with a normal LVEF [> 0.5] in all class A patients. For class B and C patients in all of them was demonstrated RV–dilatation and RVWMA not only confined to the IW. TR and PVSM were present in 78 and 70% [respectively] and in 29% of Class C patients an abnormal LVEF was documented.
Hemodynamic data
For the total RVI patients, invasive hemodynamic evaluation and coronary angiograms were performed in 81% and 76%, respectively. The hemodynamic data according to RVI Class was documented as follows: for class A patients [n = 235]: mean right atrial pressure [mRAP = 4.6 ± 2.1 mmHg], systolic pulmonary artery pressure [sPAP = 16.8 ± 4.4 mmHg], diastolic [d]PAP = 9.7 ± 3.7 mmHg, mean PWP [8.6 ± 3.1 mmHg], CI = 3.4 ± 0.71 L.min/m2, mean systemic arterial pressure [mSAP = 108.8 ±8.7 mmHg] were within normal hemodynamic limits. RAP/PWP ratio was found > 0.8 in 2% of the RVI class A patients. Hemodynamic measurements after volume loading with normal saline solution [increments of 200ml , range 200 to 1,400] until the mean PWP was 15–19 mm Hg were perform in 32% class A patients , where the volume loading maneuver ratify the assigned RVI classification at arrival for this patients.8 For class B patients [n = 119] an elevated mRAP [12.9 ± 3.6 mmHg], decreased CI [2.4 ± 0.21 L.min/m2] and mSAP [78.7 ± 12.3 mmHg] were documented with an increase in the RAP/PWP ratio [96% of the patients]; p < 0.01 between A and B RVI classes. For class C patients [n = 69] an elevated mRAP [21.4 ± 5.15 mmHg], sPAP [36.8 ± 9.3 mmHg], dPAP [22.2 ± 3.9 mmHg] andmPWP [19.9 ± 9.2 mm Hg], decreased CI [1.67 ± 0.5 L.min/m2] and mSAP [62.7 ± 9.5 mmHg] were documented with an increase in the RAP/PWP ratio [72% of the patients]; p < 0.01 between B and C RVI classes.
Angiographic results
The right coronary artery [RCA] was the infarct related artery in 93% of the studied RVI patients [n = 397]. The 3–CC–RVI subgroups had no differences in the number of diseased vessels. Complete RCA obstruction was found in 210 patients and 53% of these had coronary collateral circulation. Significant differences in coronary collaterals were found between A, B and C patients [52.4, 48.3 and 8.2%; respectively; p < 0.04], The combination of RCA and significant left anterior descending [LAD > 50% stenosis] disease was more commonly found in C patients [58.7%], which was significantly different from A [19.6%] and B [29.9%] patients [p < 0.03].
Reperfusion results
From the 523 RVI patients, 359 [68%] were considered eligible for thrombolytic therapy [TT] or percutaneous coronary intervention [PCI] .TT was performed with either streptokinase [32%] or recombinant tissue plasminogen activator [68%]. According to the current concepts of success by TT reperfusion9"11 or by PCI,12 this was achieved as a mean average in 73.6% of the patients. Mortality in these patients according to their class was as follows. When comparing with/without reperfusion for A, B and C classes, mortality was reduced when TT or PCI was administered [7.7% versus 3.5%, p < 0.05; 26% versus 11.%, p < 0.01; 89.% versus 50%, p < 0.01, respectively].
Morbidity and in–hospital mortality
Most class A patients had an uneventful clinical course [60.5%]. The remaining patients developed one or two of the following complications: sustained hypotension lasting less than 12h [18.7%] and cardiogenic shock . For class B patients, reversible RV dysfunction [defined as normalization of the SSP without volume or in–otropes and/or improvement or normalization of RVWMA and RV dilatation on ECHO] was documented in 82% patients, all of whom survived. Most of the remainder patients developed cardiogenic shock. For class C patients, it was observed a significant major incidence of third degree A V block [pacemaker requirement] when compared to A and B classes [57 versus 5 and 18%, respectively; p < 0.04].
The TIMI–RS analysis
Application of TIMI–RS retrospectively for RVI patients did not reveal an association between outcome and TIMI–RS, nor did it predict short–term mortality [Fig. 1]. The lack of association of mortality with TIMI–RS was observed in both the low [< 4 points] or higher score [> 5] groups. When the TIMI–RS was tested prospectively in 98 RVI patients, the score did not reveal a significant graded increase in mortality with rising score [Fig. 2].

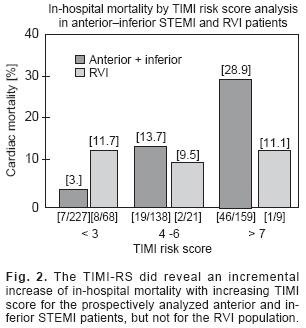
TIMI–RS showed a direct relationship with mortality in the 524 STEMI patients without–RVI that were evaluated prospectively [Fig. 2, range 3%–28.9%; p < 0.001 for trend].
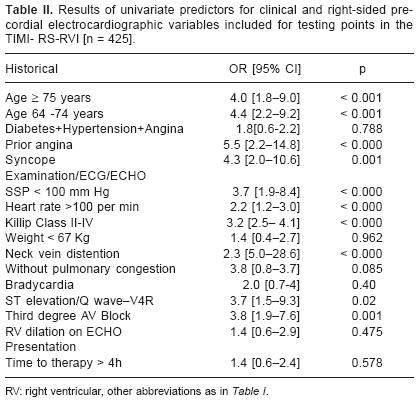
To establish whether TIMI–RS should incorporate clinical points for right–sided electrocardiographic changes the following variables were tested: preinfarction angina, syncope, elevated neck venous pressure, Kussmaul's sign, right–sided S3/S4, ST–segment elevation [<or>1 mm], new Q wave in V3R/V4R, RBBB, bradycardia, third degree AV block, ventricular arrhythmias, clear lung fields on chest X–rays and echocardiographic evidence of RV dilatation. This process yielded the following independent variables: age > 75y, age 64–74y, SSP < 100 mmHg, heart rate > 100 min, Killip class II–IV, preinfarction angina, ST–segment elevation > 1 mm in V4R, syncope, third degree AV block and elevated neck venous pressure [Table II]. When submitted to stepwise logistic regression: age 64–74y, age <75y, SSP < 100 mmHg, heart rate > 100 min, Killip class II–IV remained as a significant predictors of mortality in the multivariate model [Table III]. For the derived model to predict the probability for mortality [TIMI–RS–RVI] a c statistic = 0.9 was found, value that indicate a model with an excellent discrimination, but with a "very poor calibration".
Prognostic impact of the classification in RVI on mortality
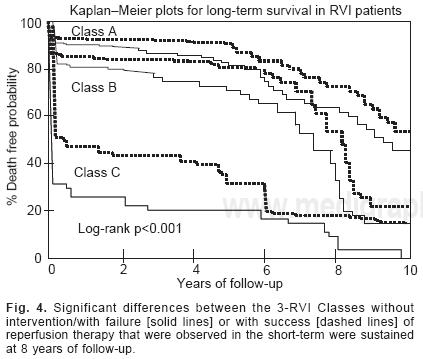
The retrospective application of the clinical classification revealed a strong association between the short– and long–term mortality risk [Fig. 3]. The in–hospital mortality rates were 6% in patients within Class A, 19.1% in those within Class B and 76.4% in those within Class C [A versus B, p < 0.001; B versus C, p < 0.001], A progressive rise for long–term mortality was noted for RVI patients. The classification helped to risk–stratify the majority of RVI patients with a low–risk of in–hospital mortality and was also able to stratify those with a high–risk of in–hospital mortality. When the classification was tested prospectively in RVI patients [Fig. 3] a progressive rise in mortality was seen: A: 5.4%, B: 16% and C: 50% [A versus B, p < 0.01; B versus C, p < 0.01]. The number of RVI patients alive at 8 years was for A class: 118, for B:60 and for C: 10 patients. The significant differences between the RVI Classes that were observed in the short–term analysis were sustained at 8 years of follow–up [p < 0.001]. At 8 years, total mortality without intervention or failure in reperfusion in Class A patients was 35%, in B patients 55% and in C patients 90% [Fig. 4, solid lines]. When reperfusion therapy at admission was successful, total mortality at 8 years was lower than the previously mentioned for each RVI Classes [for A: 19%, for B: 32% and for C: 78%; p < 0.001, dashed lines].

Discussion
The studied RVI patient population where the CC and the TIMI–RS was tested is in accordance to cases described by other authors with STEMI for RV location, as defined by validated diagnostic criteria.12,13 Also our RVI patients were similar in regard to baseline demographics, treatment strategies and mortality to those described in the majority of previous studies.3,4,14,15 From 523 patients with RVI, 65% had silent hemodynamic RVI, 22% had RVF and 13% were in shock. Therefore, it should be emphasized that we covered all the "clinical spectrum of acute RVI" in the studied cohort. The TIMI–RS predictive value has been validated in a large, non–selected registry of STEMI patients.1,2 This observation was confirmed in our prospectively studied STEMI population with an anterior and inferior STEMI location. According to Gumina et al,5 their retrospective observations extended the utility of the TIMI–RS for RVI patients. Nonetheless, in the Gumina study5 the TIMI–RS helped mainly to stratify the risk in the minority of RVI patients with low scores, but failed to further stratify a large group of RVI patients with scores > 4. Also, when the mortality rate of RVI patients in their study is stratified by the TIMI–RS and compared with the mortality rate in patients with STEMI in the InTIME–II trial, large differences were observed.1,7 The analysis of both our whole RVI cohort [three–fold size of Gumina's],5 or the prospective RVI cohort [similar in size of Gumina's; 98 versus 102 patients, respectively]5 demonstrates a poor prognostic performance of the TIMI–RS. Accordingly, the lack of its applicability is particularly relevant in a cohort that comprises the wider clinical/ hemodynamic spectrum for RVI. The divergent findings on the TIMI–RS for RVI patients could be due to the following explanations: 1– In TIME–II TIMI–RS was derived among patients with left ventricular STEMI [42.7% anterior, 56.9% inferior, others 0.4%]; therefore a small proportion of RVI patients where included for testing risk stratification, none were in shock and all were fibrinolytic–eligible patients.1 On the contrary, in our study all patients had RVI, no more than 68% underwent reperfusion therapy and 13% were in shock. Thus, differences in infarct location, area of myocardium at risk, time to therapy and hemodynamic status, could account for the different results derived from the TIMI–RS when applied to a non–selected population of RVI patients in clinical real life. 2– An arbitrary dichotomous cut–off value of SSP < 100 mmHg which is used as a one of the major constituents in the TIMI score1 model–leave little room for further risk stratification of a population of RVI that covers the whole clinical spectrum and where the maj ority of RVI patients have a SSP > 100 mmHg [86%]. On the contrary, for our proposed classification, while using the same SSP–cut–off value it allows for the risk stratification of RVI patients in those without RVF, those with RVF or with cardiogenic shock. Although, we found a low performance for the TIMI–RS in RVI patients we could not categorically denied the utility of this well proved tool on the basis of the following reasons [limitations]: 1.– it has been shown previously that the TIMI–RS has diminished performance in patients not treated with reperfusion therapy [31 % of the studied population]. 2.– due to the mainly retrospective nature of the study we could also rise the question as to whether the TIMI–RS was always correctly determined and 3.– the information presented is coming from a single– cardiovascular center study.
Several investigators demonstrated that age, ST elevation in V4R, shock, ventricular arrhythmias and AV–block are associated with an increased risk of death for RVI patients.3,4,12,17 When we incorporate these variables for the construction of the TIMI–RS–RVI model in addition to echocardiographic abnormalities used for the diagnosis of RV dilation in the multivariate analysis; none except age [> 64y] and those hemodynamic conditions related to shock, remain as predictors of short–term mortality. The addition of these variables result in a TIMI–RS–RVI model with an excellent discrimination, but with a very poor calibration;18 therefore, the derived model resulted in a measure of "non goodness–of–fit" and therefore could not be applied in clinical practice for RVI risk stratification. It is also seems the reason why, shock in this model failed to predict mortality when shock as part of the CC scheme did.
The long–term prognosis of RVI patients has been defined by several investigators.3–5,19–22 Our data reiterate that patients with RVI in the categories of RVF and cardiogenic shock have the high in–hospital morbidity and mortality 3,4,19,21,23–25 Accordingly, our information underscores the importance of making the correct stratification of RVI, with particular emphasis to consider at all times the "clinical spectrum of RVI " in its discussion.
The Global Use of Strategies to Open Occluded Coronary Arteries [GUSTO–I] risk model uses a graded scoring system for hemodynamics parameters that might be better for classification of patients at varying risk such as those with and without RVI.22 This hypothesis will need to be tested in RVI patients;7 in relation to, we should mention that we clearly separated RVI patients from those with only inferior–STEMI wall, and the classification for RVI patients was supported by hemodynamic findings in 80% RVI patients. In the present study, we report that long–term mortality continues to increase after the first year and is different for the 3–RVI Classes. The differences noted for long–term survival after RVI among the 3–Classes in the present study could be attributed to coronary artery disease progression, in part to the association of complete RCA obstruction significant LAD disease and a poorly developed collateral coronary circulation, conditions most frequently observed on admission for B and C RVI patients; and also according to our results, to the lack of indication and to the impact at admission of reperfusion success or failure.13,17,20
The proposed Classification for RVI patients, which is based on the pathophysiology of RVI, is a simple, intuitive to most clinicians, non–invasive tool that can easy be applied at the bedside, has been supported by RV hemodynamic measurements that separate hemodynamically stable from unstable patients in the acute setting of RVI.
Conclusions
Although, the proposed Classification for short– and long–term survival is derived from retrospective and prospective data of a single cardiovascular center, our findings seems to support the use of a "Clinical Classification" on admission for risk stratification for acute RVI patients. The performance of the TIMI–RS in the acute setting of RVI should be investigated and reevaluated prospectively in a large cohort of RVI patients to ascertain its definitive role.
References
1. MORROW DA, ANTMAN E, CHARLESWORTH A, CAIRNS R, MURPHY SA, DE LEMOS JA, ET AL: TTMI risk score for ST elevation myocardial infarction: A Convenient, Bedside, Clinical Score for Risk Assessment at Presentation. An Intravenous nPAfor treatment of lnfarcting Myocardium Early II trial substudy. Circulation 2000; 102: 2031–7. [ Links ]
2. MORROW D, ANTMAN E, PARSONS L, DE LEMOS JA, CANNON C, GIUGLIANO RP, ET AL: Application of the TIMI risk score for ST–elevation MI in the National Registry of Myocardial Infarction 3. JAMA.2001; 286: 1356–9. [ Links ]
3. ZEHENDER M, KASPER W, KAUDER E, SCHÖNTHALER M, GEIBEL A, OLSCHEWSKI M, HANJÖRG J: Right ventricular infarction as an independent predictor of prognosis after acute inferior myocardial infarction. N Engl J Med 1993; 328: 981–98. [ Links ]
4. MEHTA SR, EIKELBOOM JW, NATARAJAN MN, DÍAZ R, YI CHEELONG, GIBBONS RJ, YUSUF S: Impact of right ventricular involvement on mortality and morbidity in patients with inferior infarction. J Am Coll Cardiol 2001; 37: 37–43. [ Links ]
5. GUMINA RJ, WRIGHT RS, KOPECKY SL, MILLER WL, WILLIAMS BA, REEDER GS, MURPHY JG: Strong predictive value of TIMI risk score analysis for inhospital and long–term survival of patients with right ventricular infarction. Eur Heart J 2002; 23: 1678–83. [ Links ]
6. LUPI HE, LASSES LA, COSSIO–ARANDA J, CHUQUIURE VE, MARTÍNEZ SC, ORTIZ P , ET AL: Acute right ventricular infarction: clinical spectrum, results of reperfusion therapy and short—term prognosis. Coron Artery Dis 2002; 13: 57–64. [ Links ]
7. WONG C–K, WHITE HD: Risk stratification of patients with right ventricular infarction: is there a need for a specific risk score ? Eur Heart J. 2002; 23: 1642–5. [ Links ]
8. DELL'ITALIA LJ, STARLING MR, CRAWFORD MH, BOROS BL, CHAUDHURI TK, O'ROURKE RA, ET AL: Right ventricular infarction: Identification by hemodynamic measurements before and after volume loading and correlation with noninvasive techniques. J Am Coll Cardiol 1984; 4: 931–9. [ Links ]
9. GORE JM, ROBERTS R, BALL SP, MONTERO A, GOLDBERG RJ, DALEN JE: Peak Creatine Kinase as a measure of effectiveness of thrombolytic therapy in acute myocardial infarction. Am J Cardiol 1987; 59: 1234–8. [ Links ]
10. DE LEMOS JA, ANTMAN E, GIUGLIANO RP, MCCABE CH, MURPHY SA, VAN DE WERF F, ET AL: for the Thrombolysis in Myocardial Infarction [TIMI] 14 Investigators: ST—segment resolution and infarct–related artery patency and flow after thrombolytic therapy. Thrombolysis in Myocardial Infarction [TIMI]I4 Investigators. Am J Cardiol. 2000; 85: 299–304. [ Links ]
11. LUPI HE: Impacto de la fibrinólisis en la reperfusión del IAVD. In: Lupi HE, Férez SM, Ed. De la Isquemia a la Reperfusión del Ventrículo Derecho. Los Síndromes Isquémicos Coronarios Agudos. México DF; Editorial Intersistemas. 2007, p.511–41. [ Links ]
12. BOWERS TR, O'NEILL W, GRINES C, PICA MC, SAFIAN RD, GOLDSTEIN JA: Effect of reperfusion on biventricularfunction and survival after right ventricular infarction. N Engl J Med 1998; 338: 933–40. [ Links ]
13. GOLDSTEIN JA: Pathophysiology of hemodynamic severe right ventricular infarction. Coron Artery Dis 1990; 1: 314–22. [ Links ]
14. DELL'ITALIA LJ, STARLING MR, O'ROURKE RA: Physical examination for exclusion of hemodynamically important right ventricular infarction. Ann lntern Med 1983; 99: 608–11. [ Links ]
15. LOPEZ–SENDÓN J, COMA–CANELLA I, GAMALLO C: Sensitivity and specificity of hemodynamic criteria in the diagnosis of acute right ventricular infarction. Circulation.1981; 64: 515–26. [ Links ]
16. GISSI [Gruppo Italiano per lo Studio della Streptochinasi nell'Infarto Miocardico]: Effectiveness of intravenous thrombolytic treatment in acute myocardial infarction. Lancet 1986; 1: 1397–401. [ Links ]
17. ISIS–2 [Second International Study of Infarct Survival] Collaborative Group: Randomized trial of intravenous streptokinase, oral aspirin, both or neither among 17,187 cases of suspected acute myocardial infarction:ISIS–2. Lancet 1988; 2: 2349–60. [ Links ]
18. DIAMOND GA: What price perfection? Calibration and discrimination of clinical prediction models. J Clin Epidemiol 1992; 45: 85–89. [ Links ]
19. HANZEL GS, MERHI WM, O'NEILL WW, GOLDSTEIN JA: Impact of mechanical reperfusion on clinical outcome in elderly patients with right ventricular infarction. Coron Artery Dis 2006; 17: 517–21. [ Links ]
20. BERGER PB, RUOCCO NA, RYAN TJ, JACOBS AK, ZARET BL, WACKERS FJ, ET AL and the TIMI Research Group. Frequency and significance of right ventricular dysfunction during inferior wall left ventricular myocardial infarction treated with thrombolytic therapy [results from the Thrombolysis in Myocardial Infarction [TIMI] 11 trial]. Am J Cardiol 1993; 71: 1148–52. [ Links ]
21. PFISTERER M: Right ventricular involvement in myocardial infarction and cardiogenic shock. Lancet 2003; 362: 392–4. [ Links ]
22. CALIFF RM, WOODLIEF LH, HARRELL FE, LEE KL, WHITE HD, GUERECI A, ET AL for the GUSTO–I Investigators. Selection of thrombolytic therapy for individual patients: Development of a clinical model. Am Heart J 1997; 133: 630–9. [ Links ]
23. HOCHMAN JS, BULLER CE, SLEEPER LA, BOLAND J, DZAVIK V, SANBORN TA, ET AL for the SHOCK Investigators. Cardiogenic Shock complicating Acute Myocardial Infarction–Etiologies, Management and outcome: A report from the SHOCK Trial Registry. J Am Coll Cardiol 2000; 36: 1063–70. [ Links ]
24. JACOBS AK, LEOPOLD JA, BATES E, MENDES LA, SLEEPER LA , WHITE H, ET AL: Cardiogenic Shock caused by Right Ventricular Infarction. A report from the SHOCK Registry. J Am Coll Cardiol 2003; 41: 1273–9. [ Links ]
25. BUENO H, LOPEZ–PALOP R, BERMEJO J, LÓPEZ SENDÓN JL, DELCAN JL: In–hospital outcome of elderly patients with acute inferior myocardial infarction and right ventricular involvement. Circulation 1997; 96: 436–41. [ Links ]
26. GUMINA RJ, MURPHY JG, RIHAL CS, LENNON RJ, WRIGHT RS: Long–term survival after right ventricular infarction. Am J Cardiol 2006; 98: 1571–3. [ Links ]














