1 Introduction
The study of nanoparticles is a relevant area of research for the scientific community due to their magnetic, catalytic, electronic, and optical properties, which are very different from those of bulk materials. Nanomaterials composed of semiconducting nanoparticles, or quantum dots (QDs) are very promising in various applications, particularly ZnS QDs.
ZnS is an inorganic compound that is used as a pigment or as a semiconductor material [1] and is considered a suitable host matrix to form doped phosphors [2]. Great advances have been made in research and technological development to obtain luminescent materials in the different regions of the visible spectrum. ZnS nanoparticles doped with transition metals such as Manganese (Mn) [2] have been used as optical readers, diodes, handheld computers, personal assistants and optical storage [3].They are also suitable for applications as biological probes [4] and biosensors [1, 5] due to their luminescent characteristics in the visible spectrum, as well as their high biocompatibility [6].
Luminescence intensity is an important factor which have been improved modifying experimental conditions of synthesis, such as concentrations of reactants, pH, and temperature, among others [7- 9].
In the quest to obtain high luminescence intensity and near-infrared shift for possible medical and photonic applications, studies of semiconductors co-doped with transition metals and rare earth have also been carried out.
Lanthanide elements, also called rare earths, have relevant properties, which allows that semiconductor materials doped with these elements present convenient properties for their use in assay tests in biotechnology, bio imaging, bio-detection, diagnosis and treatment of diseases [10].
These properties include chemical stability [11], adjustable color emission [12], low toxicity, penetration into tissues with less damage, and allow selectivity [13]. From the reported research on rare earth, Europium (Eu) is a lanthanide material recognized as an efficient luminophore [14]; it presents a narrow and sharp bandwidth due to the transitions of the electrons in 4f6 orbitals, which are “protected” by the electrons in 5S2 and 5P6 orbitals, which means that the host keeps Eu without perturbing the emission states [15].
This exhibits an emission spectrum ranging from the early visible to the near-infrared. For these reasons, Eu-doped nanoparticles are an excellent alternative for many applications where long wavelength emission is required [16].
In this work, we present a method of synthesis and characterization of zinc sulfide nanoparticles doped with Mn and Eu, to obtain nanoparticles with sizes smaller than 100 nm and that produce high luminescence.
In the experimental process, two synthesis processes are carried out, increasing the concentration of the coating agent to evaluate the effect of the variation of the concentration on the size of the nanoparticles and the photo luminescent emission.
2 Experimental Procedure
2.1 Synthesis
The synthesis method used to obtain luminescent semiconductor nanoparticles is based on precipitation reactions by controlled-release processes of the precipitating cations or anions at room temperature. This is a simple and low-cost methodology [17]. In the present study, the precursors used to prepare the samples were: Zinc acetate (Zn (CH3COO)2 2H2O) (99.99%, Sigma Aldrich), Na2S 9H2O (99.98%, Sigma Aldrich), MnCl2 (99.99%, Sigma Aldrich), EuCl3 (99.99%, Sigma Aldrich) and Polysorbate 80 (Tween 80), Ethanol and Deionized Water. All materials were used without further purification.
2.1.1 ZnS Nanoparticles
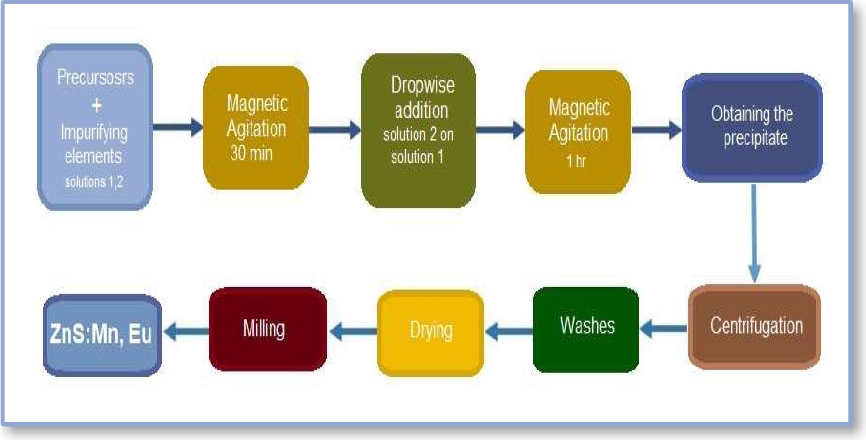
For the synthesis of zinc sulfide nanoparticles, two different solutions were prepared: a solution of 2.195 g of Zinc Acetate ((Zn(CH3COO)2 2H2O)), Polysorbate 80 as a capping agent or passivating agent with a concentration of 0.6% w/v, in 50 ml of ethanol. Another solution of 2.451 g of Sodium Sulfide (Na2S) in 50 ml of deionized water. The solutions were shaken vigorously for 30 min and the Na2S solution was incorporated dropwise into the zinc acetate solution with Polysorbate 80 and kept in agitation for 1 hr to obtain a white precipitate of ZnS nanoparticles (Fig 1).
2.1.2 Doped ZnS Nanoparticles
Two syntheses were carried out by the precipitation method. For each synthesis two solutions were prepared. A solution of 2.195 g (Zn(CH3COO)2 2H2O), Polysorbate 80 as a capping agent or passivating agent with a concentration of 0.6% w/v, 0.05 g MnCl2 and 0.292 g EuCl3 as impurities, mixed in 50 ml ethanol. Another solution of 2.451 g Na2S in 50 ml of deionized water.
Both solutions were stirred vigorously for 30 min. Subsequently, the Na2S solution was added dropwise to the first mixture and stirred for 1 hr., obtaining a white precipitate of ZnS:Mn,Eu nanoparticles (Figure 2).
In the second synthesis, the same procedure and quantities of the precursors were carried out varying the concentration of the capping agent to 1% w/v.
After obtaining the precipitate from each synthesis, this was subjected to centrifugation and washed several times, then left to dry at room temperature and the resulting powder was milled to obtain the impurified zinc sulfide nanoparticles. The overall synthesis process is schematized in Fig. 2.
2.2 Characterization of Nanoparticles
The obtained nanoparticles were characterized by means of Dynamic Light Scattering (DLS), X-Ray Dispersion (XRD), Scanning Electron Microscopy (SEM), Photoluminescence (PL), and High-Resolution Transmission Electron Microscopy (HRTEM) techniques.
DLS was used to obtain the hydrodynamic diameter of the nanoparticles in dispersion and their Z-potential, thus obtaining their average size in suspension and the degree of repulsion between adjacent nanoparticles in the sample allowing at this way to determine the stability of the colloidal solution [17].
Peak broadening analysis by XRD is a basic tool for characterization to obtain information regarding the crystalline structure, its size, and lattice deformation.
The calculation of nanoparticle size was obtained from the main X-ray diffraction peak by measuring the non-defective region or coherent scattering zone of the peak.
In the SEM micrograph, it can be observed how the morphology of the crystalline structure is and if agglomeration and polydispersity are present. In this technique, the nanoparticles can be observed individually in some way and measured directly.
Whereas in HRTEM microscopy the crystal structure of the sample can be observed at the atomic scale. By means of photoluminescence spectroscopy we measured the electromagnetic radiation emission of doped and undoped ZnS nanoparticles.
3 Results and Discussion
3.1 DLS Analysis
Table 1 shows three measurements for the same sample with 0.6% w/v of the coating concentration where the average particle size is 6 nm hydrodynamic particle diameter. As the sample presents a single peak (monomodal) of narrow distribution amplitude (monodisperse) the result can be compared with the size measured by other techniques.
Table 1 Size and Zeta Potential
| Nanoparticles | Size (nm) | Zeta Potential pH4 | Zeta Potential pH6 | Zeta Potential pH8 |
| ZnS:Mn,Eu (0.6% w/v) | 6 | 10.9 | 13.8 | 12.4 |
| ZnS:Mn,Eu (1% w/v) | 11 | 23 | 23.2 | 15.6 |
Table 1 also shows that the nanoparticles have a zeta potential of 10.9 mV, indicating that the surface charges are positive and the magnitude indicates that the nanoparticles will prevent their aggregation among themselves, making them somewhat stable in storage time.
Being able to crowd. However, it is known that if nanoparticles are sterically protected with a surfactant or capping agent (passivator), they may experience less or no agglomeration and achieve stability at zeta potential values between ±30 mV. The positive or negative zeta potential and its magnitude allow us to determine the chemical changes on the surface of the nanoparticles.
The pH is an important factor on which the zeta potential depends. In this experiment, a pH of 4 was measured and the zeta potential presented positive values [18], thus having a homogeneous and stable solution. Nanoparticle sizes were smaller than at pH 6 and pH 8.
3.2 X-ray Diffraction Analysis
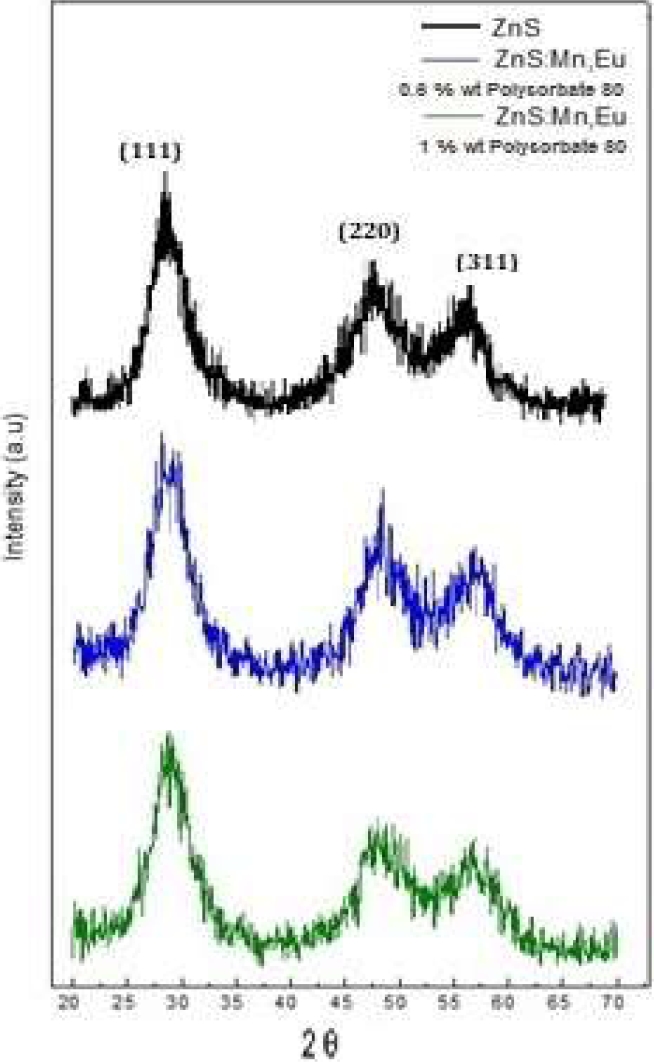
XRD spectra indicating the presence of NZnS impurified with transition metal ions and rare earth are shown. The identified planes correspond to cubic-phase ZnS (Fig. 3).
The black spectrum belongs to the undoped NZnS.The blue spectrum corresponds to the ZnS sample doped with Mn and Eu (0.6 % of the capping) and presents diffraction peaks at 28.5°, 48 °, and 57.5° (of 2θ), corresponding to the (111), (220) and (311) crystallization planes.
The spectrum in green color corresponds to the sample of ZnS doped with Mn and Eu (1 % of the capping), and the diffraction peaks are located at 29°, 48 °, and 56.5° (of 2θ), corresponding to the planes (111), (220) and (311), respectively [19].
The estimated size of the synthesized nanoparticles was obtained using the Debye-Scherrer equation (1):
D is the average diameter of the nanocrystals in the direction perpendicular to the related planes. The values of the full width at half peak maximum of pure and doped nanoparticles are presented in Table 2.
3.3 SEM Analysis
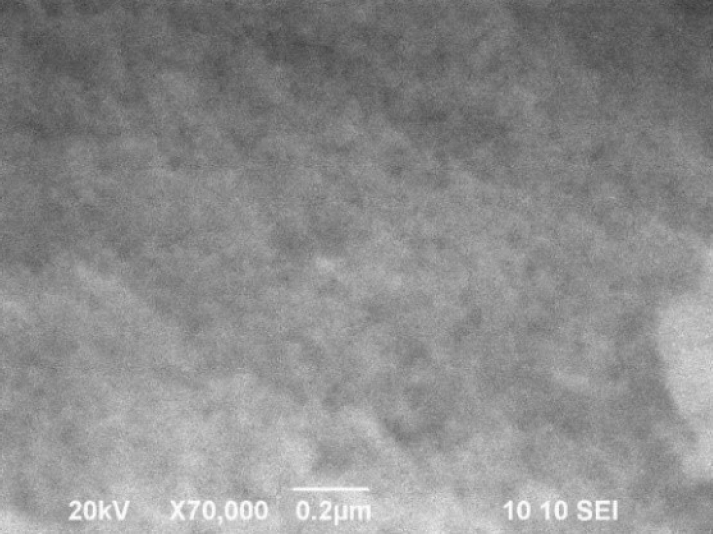
A SEM micrograph of the NZnS is present, showing their morphology. Nanometer-sized nanoparticles with agglomeration possibly due to the nature of the ZnS.
It is observed that the nanoparticles are almost spherical in shape with average diameters of 10 nm and smooth and uniform surface.
The nanoparticle sizes are consistent with those estimated from the XDR patterns.
3.4 EDS Analysis
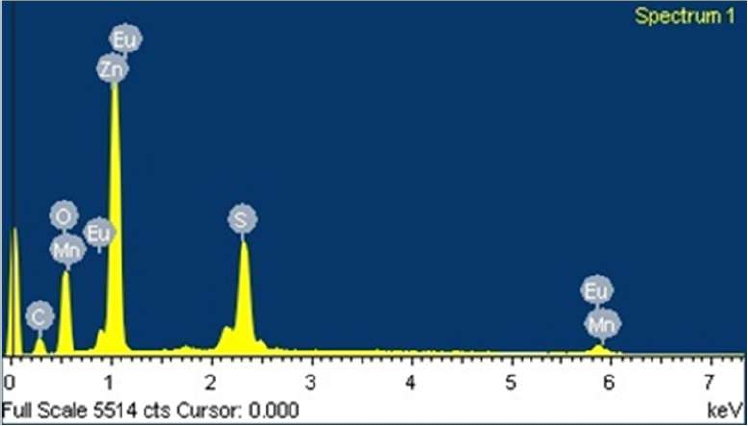
The energy dispersive spectroscopy analysis in Figure 5 shows the elemental chemical composition of the sample surface.
The presence of the precursor elements Zn, S, Mn, and Eu is observed.
A significant amount of oxygen and little carbon was found, possibly corresponding to the coating of the samples.
3.5 HRTEM Analysis
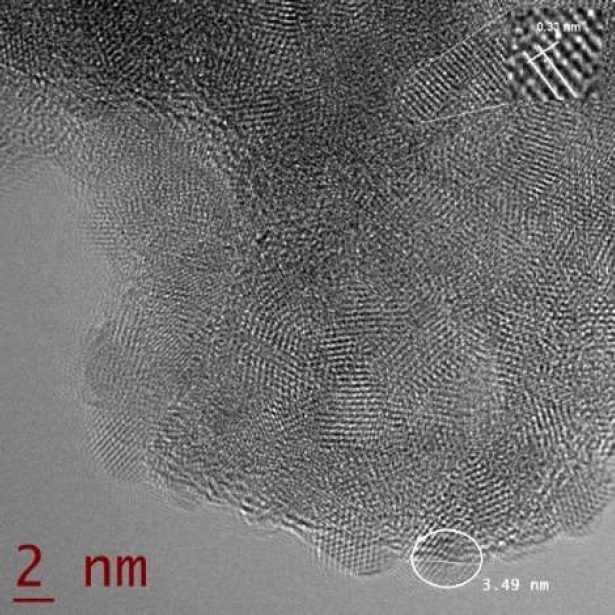
The micrograph obtained by means of HRTEM (Fig. 6) shows the formation of nanoparticles with sizes between 2 and 4 nm. The nanoparticle sizes observed in the image are in agreement with the sizes estimated from XRD spectrometry.
In the nanoparticles, lattice fringes are observed in the nanoparticles with the interplanar space assigned to the (111) planes of Zinc in the cubic phase.
3.6 Photoluminescence Studies
The obtained samples were irradiated with an ultraviolet light lamp, observing an emission in the red.
This is due to the recombination of Mn ions and the concentration of Mn used in the synthesis [20], in addition to the possible recombination of Eu ions with ZnS. (Fig. 7a).
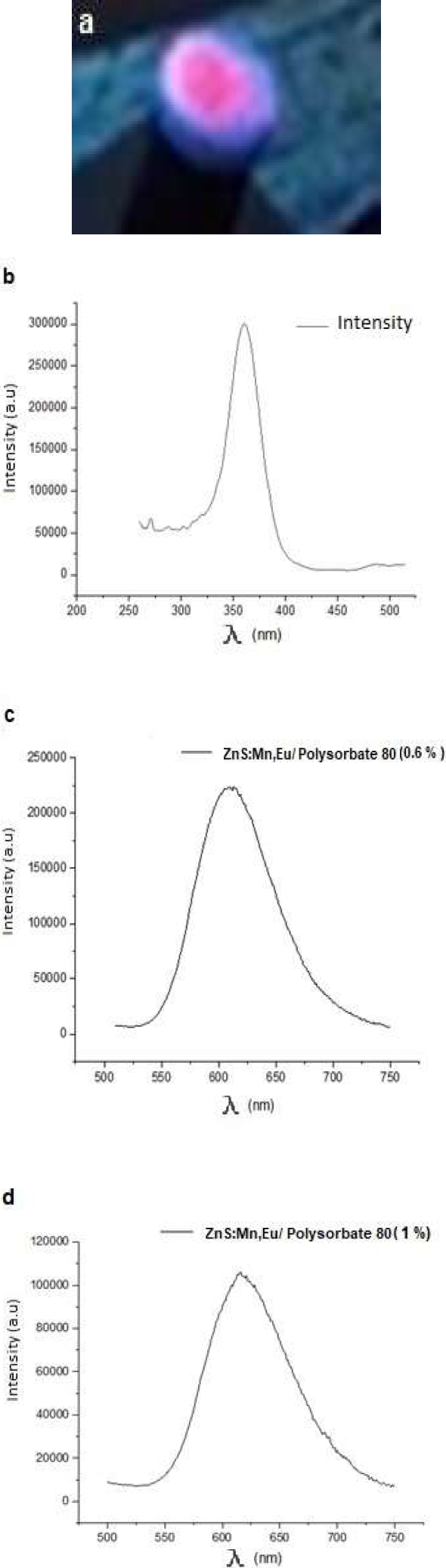
Fig. 7 a) Doped nanoparticle powder under UV light lamp, b) 364 nm excitation spectrum, c) Emission spectrum of ZnS:Mn, Eu nanoparticles with a lower concentration on the shell, d) Emission spectrum of ZnS:Mn, Eu nanoparticles with a higher concentration on the shell
It is observed from the PL spectra (Fig. 7c-d) that the visible light emitted by the doped nanoparticles was obtained with a broad emission peak with wavelengths of 612 nm at room temperature, with an excitation spectrum of 364 nm (Fig 7b).
The amplitude of the peak is characteristic of the Mn2+ ion. However, due to the concentration of Mn2+ used in the synthesis, an emission in the orange-red was achieved (Fig. 9a) [21]. A significant increase in luminescent intensity was observed in the ZnS:Mn,Eu nanoparticles with lower concentration of the passivating agent of about twofold.
This significant increase in intensity may be due to the passivation of the surface defects of the nanoparticles because this is where non-radiative recombination by the Mn 2+ ion centers [22] and Eu III occurs.
So also, the increase in surface area/volume influences, since the nanoparticle size decreases as the concentration of the coating agent decreases.
4 Conclusions
The nanoparticles of ZnS were obtained by precipitation of a homogeneous suspension of zinc sulfide, doped with transition metals and rare earths, and Polysorbate 80 as a passivation agent.
The synthesized ZnS nanoparticles have an average size of about 6 nm. (hydrodynamic diameter). The measured zeta potential indicates that the sample is homogeneous and stable.
From the technique to obtain the size of the nanoparticles by XRD spectra, it was possible to estimate a size between 2 to 3.63 nm with a cubic structure according to the planes that define the crystal lattice and this is corroborated with what was observed by SEM and the interplanar space of the HRTEM micrograph.
Also, the PL experiments show a strong luminescent intensity, which is approximately doubled due to the lower concentration of the coating agent. As the concentration of the coating agent decreases, the size of the nanoparticle is smaller and the luminescent intensity increases.
In our experimentation, due to the concentration of Mn added in the synthesis, and the incorporation of Eu III ions we obtained an increase in the emission wavelength, towards red, which is very important and favorable for biomedical applications.
ZnS nanoparticles doped with transition metals and rare earths can be considered to form a new unique luminescent material with strong and stable visible emission. With the doping of Mn and Eu in ZnS nanoparticles, an enhancement of luminescence efficiency is observed.
The micrograph results suggest that metal and rare earth ions were incorporated into the network of the new nanomaterial.











 nueva página del texto (beta)
nueva página del texto (beta)