Introduction
Tropical montane cloud forests (TMCF) are a group of mountain communities characterized by the frequent presence of cloud and normally high levels of relative air humidity (Hamilton, Juvik, & Scatena, 1995). Their physiognomy, structure and composition depend on factors such as latitude, elevation, wind and precipitation patterns, as well as evolutionary history, all of which lead to the high beta diversity reported for this forest type (Bubb, May, Miles, & Sayer, 2004). Worldwide, TMCF cover a mere 0.14% of the land surface (Scatena, Bruijnzeel, Bubb, & Das, 2010) and are considered one of the most endangered ecosystems because of their limited distribution and the high rates of deforestation to which they are subjected (Toledo-Aceves, Meave, González-Espinosa, & Ramírez-Marcial, 2011).
Oaks (genus Quercus) are an important component of TMCF, since they are dominant canopy species of high importance values and can generate microenvironments that are suitable for the establishment of other species (Kapelle, 2006). Mexico has the highest richness of oaks in the world, with approximately 160 species (109 endemic species; Valencia, 2004; Valencia & Gual-Díaz, 2014). Of these, 38 have been reported in the TMCF of Mexico and 33 of these are at some degree of risk (González-Espinosa, Meave, Lorea-Hernández, Ibarra-Manríquez, & Newton, 2011).
Despite the risk of extinction of the oak populations that dominate TMCF canopies, little is known about their regeneration ecology (Valencia & Gual-Díaz, 2014). While they are considered intermediate or late successional species, some species have been reported to successfully regenerate in open areas and thus demonstrate potential for use in ecological restoration programs (López-Barrera, Manson, González-Espinosa, & Newton, 2006; Ramírez-Marcial, Camacho-Cruz, González-Espinosa, & López-Barrera, 2006). However, for other oak species, different limiting factors that affect regeneration have been documented in open areas and difficulties have been reported in terms of their conservation (Quintana-Ascencio, González-Espinosa, & Ramírez-Marcial, 1992; Ortega-Pieck, López-Barrera, Ramírez-Marcial, & García-Franco, 2011; Montes-Hernández and López-Barrera, 2013).
Several studies have analyzed early establishment in oaks and compared different habitats, including: a) forest successional stages (Li and Ma, 2003; Gómez, 2004; González-Rodríguez, Barrio, & Villar, 2012), b) light gradients within the forest (Gómez-Aparicio et al., 2008; Pérez-Ramos, Gómez-Aparicio, Villar, García, & Marañón, 2010), c) open sites vs. closed canopy forest (Negi, Negi, & Singh, 1996; Fan, Guo, Wang, & Duan, 2014) and different light environments under greenhouse and laboratory conditions (Khan and Shankar, 2001; Quero, Villar, Marañón, Zamora, & Poorter, 2007). Temperature, light and humidity have been identified as the most important factors for the germination and emergence of oak seedlings (Ashton and Larson, 1996; Puerta-Piñero, Gómez, & Valladares, 2007).
Other factors have been identified that affect germination and emergence in oaks, including maternal origin (González-Rodríguez et al., 2012), seed size (Tripathi and Khan, 1990), insect damage (Branco, Branco, Merouani, & Almeida, 2001; Yi and Zhang, 2008) and substrate characteristics (López-Barrera and González-Espinosa, 2001; Li and Ma, 2003; Flores-Cano, Badano, & Flores, 2012). These studies have demonstrated that the responses of the oaks to the evaluated factors are species specific; however, most of the study has been conducted with oaks of the temperate zone in forests dominated by a few species and little information is available about the effect of the light environment and seed quality on germination and emergence in the oak species that coexist in TMCF (Camacho-Cruz, González-Espinosa, Wolf, & De Jong, 2000).
Objective
The objective of this study was to evaluate and compare the germination and emergence of four endangered oak species in TMCF (Quercus germana, Q. insignis, Q. sartorii and Q. xalapensis) in two experiments carried out in (1) a secondary forest fragment (SFF) and (2) a greenhouse (GRE), each featuring two light environment treatments (sun vs. shade). Acorn weight variability was also evaluated for each species and examined in the context of germination and emergence.
Materials and Methods
Study species
Species were selected based on seed availability and the following criteria: 1) species were co-dominants of the TMCF canopy, 2) species were included on the red list of Mexican tropical montane tree species (González-Espinosa et al., 2011), 3) species were white or red oaks, and 4) seed weight and size were highly variable between species. Quercus germana (Schltdl. & Cham.) and Quercus insignis (M. Martens & Galeotti) belong to the Quercus section (white oaks) and are classified as critically endangered species. Quercus sartorii (Liebm.) belongs to the Lobatae section (red oaks). It is classified as an endangered species but there is no information about its regeneration ecology. Quercus xalapensis (Bonpl.) is also a red oak and is classified as a critically endangered species. Quercus germana and Q. sartorii are endemic to Mexico, while Q. insignis and Q. xalapensis occur in Mexico and Central America (González-Espinosa et al., 2011).
Germination and emergence tests
This study was conducted in a secondary forest fragment, SFF (19° 27′ 59.7″ N and 96° 57′ 09.1″ W) and a greenhouse, GRE (19° 28′ 00.6″ N and 096° 57′ 08.1″ W) located in the municipality of Coatepec, in central Veracruz, Mexico (1250 m asl). The regional climate is tropical with a mean annual temperature of 17 °C - 20 °C (García, 1973) and mean annual rainfall of 1700 mm (Barradas et al., 2010). The SFF is dominated by several arboreal species, including Q. germana, Q. leiophylla, Alchornea latifolia, Clethra mexicana, Heliocarpus appendiculatus and Persea schiedeana, among others. This site was TMCF but was then converted into a coffee plantation (1942) and subsequently abandoned 15 years ago (2000). Two light conditions were selected in the SFF: a 20 m x 20 m canopy gap (sun) and a 20 m x 20 m area with a closed canopy (shade). In the GRE, two light conditions were created: sun or open conditions (under a transparent plastic cover) and partial shade (created using a black shade cloth that blocked 30% of the light). In each treatment, Photosynthetically Active Radiation (PAR) was recorded once a month (November and December 2012) using the average of 30 readings taken over an hour with a ceptometer (Decagon LP-80) connected to an external sensor for calibration. This sensor was also useful for taking reference PAR readings in open conditions when measurements were taken in the shade in SFF and in the GRE. To that end, the measurement units (mmol m-2 s-1) were expressed as percentages of the values measured in open conditions. Air temperature and relative humidity were measured in each treatment using sensors (HOBO Pro Series Onset); readings were taken every 10 minutes during 60 days.
In October 2012, approximately 800 acorns were collected from ten trees of each species. Acorns of Q. germana, Q. sartorii and Q. xalapensis were collected from aTMCF fragment in Xalapa, Veracruz, while those of Q. insignis were collected from a TMCF fragment in Huatusco, Veracruz. Damaged seeds were discarded using the float method (Gribko & Jones, 1995). Each acorn was weighed, measured (length and width) and individually labeled to facilitate individual monitoring during the germination trials. Prior to the experiment, an adhesive label was used on each acorn for identification. This label was removed when the acorns were sown and replaced with a plastic label indicating the position of each acorn, but not attached to it.
Five acorns per species were sown in black polyethylene bags (20 cm x 25 cm; 7850 cm3) filled with a substrate composed of sieved SFF soil and fine gravel (50:50) sterilized using the solar method. A total of 600 acorns were sown per species (a total of 2400 acorns in 480 bags). A total of 120 bags were randomly assigned to each light condition in the SFF (sun and shade) and GRE (sun and shade) experiments. The bags were rotated to minimize edge effects and watered to field capacity every seven days (600 ml). The number of acorns that germinated and emerged was recorded every 10 days for 60 days, after no further germination or emergence occurred. Germination was defined as the first visual appearance of the radicle, while development of the aerial shoot was taken to define an emergence event.
Statistical analysis
The Pearson correlation coefficient (r) was used to analyze the relationships among morphometric acorn characteristics. The variable of final germination had a binary response (germinated or non-germinated), for which reason generalized linear models (logistic regression, link function, binomial family) were performed to determine the effects of the factors of species (Q. germana, Q. insignis, Q. sartorii and Q. xalapensis), light environment (sun and shade) and acorn weight, as well as the interactive effect of species and light environment on germination and emergence. Quercus germana was excluded from the emergence analysis because of the low emergence presented by the species. To compare the germination and emergence curves, a survival analysis was performed using the Log-rank statistic (Kaplan-Meier) to analyze the temporal behavior of the variables. Logistic regression analyses were performed using R studio environment (v.0.98.1091) while the survival analysis was performed with SPSS (v.15.0). Values presented in the text are means ± 1 standard error (SE), unless indicated otherwise.
Results
Light environment
Available PAR in the SFF was 78.56% ± 3.34% and 6.54% ± 2.21% in the sun and shade experimental treatments, respectively. In the GRE experiment, available PAR was 46.33% ± 4.36% and 4.24% ± 0.35% in the sun and shade conditions, respectively. In the SFF, relative humidity and temperature under sun conditions were 90.50% ± 0.16% and 16.04 °C ± 0.04 °C respectively. Under shade conditions, these values were 89.32% ± 0.12% and 15.70 °C ± 0.04 °C, respectively.
In the GRE, relative humidity and temperature values under sun conditions were 88.01% ± 0.19% and 16.91 °C ± 0.05 °C, respectively. Under shade conditions, these values were 91.01% ± 0.13% and 16.37 °C ± 0.04 °C, respectively.
Acorn characteristics
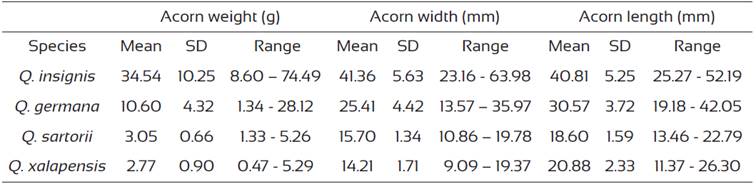
The morphometric characteristics of the acorns are presented in table 1. There was greater variation in the weight of white oak (Q. insignis and Q. germana) acornscompared to those of the red oaks (Q. xalapensis and Q. sartorii). The maximum values and range of variation in acorn width, length and weight were observed in Q. insignis (Table 1). Acorn weight was correlated significantly to acorn diameter in Q. insignis (r = 0.572, P < 0.0001), Q. germana (r = 0.122, P = 0.003), Q. sartorii (r = 0.530, P < 0.0001) and Q. xalapensis (r = 0.572, P < 0.0001). Acorn width/length ratio (±SD) was 0.85 ± 0.05 for Q. sartorii, with similar values in Q. insignis and Q. germana (0.83 ± 0.13) but lower values in Q. xalapensis (0.68 ± 0.06).
Germination
After 60 days, 62.17% of the acorns in both experiments had germinated. Germination was highest in Q. sartorii (71.83% ± 2.09%), followed by Q. germana (66.33% ± 2.06%), Q. insignis (60.83% ± 2.34%) and Q. xalapensis (53.50% ± 2.32%). The germination curves revealed significant differences among species (Log-rank = 18.38, df = 3, P < 0.001). In all species, acorn germination began within the first 10 days of the experiment. During this period, Q. germana had the highest number of germinated seeds (42.50%), followed by Q. insignis (29.17%), Q. xalapensis (24.17%) and Q. sartorii (14%; Fig. 1).

Figure 1 Proportion of a) germination and b) emergence in four Quercus species over a period of 60 days (n=600 acorns per species). Error bars indicate ±1 standard error. Quercus germana was excluded from the emergence analysis because of the low emergence presented by this species.
In the GRE experiment, overall acorn germination was 62.83% ± 1.66% and was affected by species (Table 2), as well as by the interaction between species and light conditions. Quercus insignis germination was higher under sun than under shade conditions (P < 0.05; Table 3). In the SFF experiment, overall acorn germination was 63.42% ± 1.58% and germination was affected by species (Table 2). Neither light environment nor acorn weight had a significant effect on germination. Quercus xalapensis presented the lowest germination value compared to the other species (P < 0.001; Table 3).
Table 2 Results of the logistic regression model applied to Quercus species germination and emergence as a function of light environment (sun vs. partial shade), species (Q. germana, Q. insignis, Q. sartorii and Q. xalapensis) and acorn weight (continuous variable) in two experiments: Secondary Forest Fragment (SFF) and Greenhouse (GRE).

Table 3 Percentages of germination and emergence (± 1 S.E.) for each of four Quercus species in two experiments.

1. Secondary Forest Fragment experiment (SFF): sun treatment (under a canopy gap) and shade treatment (under closed canopy). 2. Greenhouse experiment (GRE): sun treatment (under transparent plastic cover) and partial shade treatment (under a shade cloth that reduced light by 30%). Quercus germana was excluded from the emergence analysis because of the low emergence presented by this species.
Emergence
After 60 days, 37.79% of the seedlings of all four species had emerged in both experiments. Quercus sartorii presented the highest degree of emergence (56.16% ± 2.59%), followed by Q. xalapensis (53.50% ± 2.32%), Q. insignis (40.8% ± 2.27%) and Q. germana (1.33% ± 0.45%). According to observations made at the end of the experiment, the low proportion of emergence in the latter species was the result of an apparent fungal attack on the germinated acorns. The emergence curves revealed significant differences among species (Log-rank = 301.90, df = 3, P < 0.001). In the first emergence count, Q. xalapensis had the highest value (24.33%), followed by Q. insignis (21.17%), Q. sartorii (11.17%) and Q. germana (0.83%; Fig. 1).
In the GRE experiment, overall emergence was 38.33% ± 2.13% and differed among species. Quercus insignis was the species with the lowest emergence and differed (P< 0.001) from Q. sartorii and Q. xalapensis. In the SFF experiment, overall seedling emergence was 37.58% ± 1.96% and differences were found between species only. Neither light environment nor acorn weight had a significant effect on emergence (Table 2). Quercus sartorii presented the highest emergence, compared to Q. insignisand Q. xalapensis (P < 0.001).
Discussion
Acorn weight
Contrary to our expectations, intraspecific variability in acorn weight did not affect acorn germination or seedling emergence. Species with considerable intraspecific variation in seed size may present enhanced establishment in a heterogeneous environment (Quero et al., 2007). Some studies have reported that heavier and larger acorns also present higher germination (Bonfil 1998; Purohit, Tamta, Nandi, Rikhari, & Palni, 2003), while others report no such correlation (Tilki & Alptekin 2005; Yi, Wang, Liu, Guoqiang, & Zhang, 2014). These differences can be attributed to specific-species responses, variation in maternal origin, differences in provenance and intraspecific temporal and spatial variability in the range of seed size and weight (Tilki & Alptekin 2005; Koenig, Knops, Dickinson, & Zuckerberg, 2009; González-Rodríguez et al., 2012). Methodological factors such as acorn storage prior to the experiment, dry vs. fresh acorn weight and exclusive consideration of extreme seed size categories may also affect comparability between results (Seiwa 2000; Khan & Shankar 2001; Quero et al., 2007). There is a clear need for long-term studies that more accurately reflect the variation in seed weight for each species, covering years of high and low seed production in order to accurately determine the intra and interspecific effect of acorn weight on germination and seedling establishment.
Species differences
The results of this study demonstrate that some of the evaluated species differ in terms of their rates and final values of germination and emergence. The studied white oak species (Q. germana and Q. insignis) mostly germinated within the first 10 days, which was more quickly than the red oaks (Q. sartorii and Q. xalapensis). This concurs with other reports of white oaks, which have been documented to germinate faster than red oaks in response to greater attack and browsing by predators (Fox 1982). White oak acorns are consumed more than those of the red oaks due to the larger seed size, greater cotyledon content and lower tannin content of the former. Tannins function as inhibitory toxins that deter predatory attack (Weckerly, Sugg, & Semlitsh, 1989; Smallwood, Steele, & Faeth, 2001).
In spite of the high germination values of Q. germana, in this study, emergence of this species was almost null. Q. germana seeds were exposed to the same biotic and abiotic conditions as the other species and experimental replicates within each treatment were randomly determined and were also rotated during the study period. The lack of emergence of Q. germana acorns could be attributed to the synergistic effect of several factors, such as insect and fungal damage to the endosperm and/or the plumule (Leiva & Fernández-Alés 2005). Acorn quality varies between species but also presents temporal and spatial intraspecific variation (Yu, Zhou, & Luo, 2003; González-Rodríguez et al., 2012). While we did employ the float method to identify and discard damaged seeds, some of the apparently undamaged acorns may have been infested with larvae but with minimal levels of damage to the cotyledon at the beginning of the experiment. Higher cotyledon damage might have occurred during the course of the study but, due to the large seed reserves of Q. germana, its germination rate was unaffected. This pattern has been documented for other species with large acorn reserves (Xiao, Harris, & Zhang, 2007; Yi & Yang 2010). However, this insect damage may have exposed the Q. germana acorns to subsequent fungal attacks and thus early embryo and cotyledon rot and decay. Fungal attack has been reported as a factor in mortality during emergence in some oak species (Yamazaki, Iwamoto, & Seiwa, 2009). It is therefore necessary to conduct further emergence trials for Q. germana that take these factors (fungal and insect damage) into account, in order to fully understand how variability in emergence is related to the maternal origin of the seeds and intraspecific temporal and spatial variation of insect-infected acorns (Yu et al., 2003; González-Rodríguez et al., 2012).
Light environments
In this study, Q. insignis was the only species affected by light, with higher germination presented under the sun condition. Quercus insignis seedlings are capable of establishment in open sites; however, seedling survival is higher under partial vegetation cover since mortality is increased in open sites due to herbivory by gophers as well as competition with exotic grasses and bracken (Pteridium aquilinum; Montes-Hernández & López-Barrera 2013; Avendaño-Yáñez, Sánchez-Velásquez, Meave, & Pineda-López, 2014)
Conclusions
We conclude that, in general, variation in the light environment and in acorn weight had no significant effect on germination or emergence in either experiment. These species can therefore be propagated in nurseries for the purposes of introduction into degraded sites. It is necessary, however, to test whether this plasticity in the studied species continues into the following stages of regeneration, under both controlled and field conditions. Similarly, there are other factors that were not controlled in this experiment, such as differences in evapotranspiration rates in the two experiments, and this remains to be quantified in future studies.
Management implications
In this study, the germination and emergence values did not differ between the experiments conducted in the SFF and the GRE. This suggests that there is no need for a substantial investment in infrastructure in order to reproduce these species, but rather that the local inhabitants or owners of deteriorated forests could propagate them in sites close to their own homes. These sites could be equipped with moderate shading that would act to protect the seeds and seedlings from variations in temperature and humidity.
The oaks studied here are classified as endangered species, owing to the high rate of deforestation to which the TMCF is subjected and which has caused decreased populations. However, the germination and emergence values reported here for Q. insignis, Q. sartorii and Q. xalapensis are within the ranges published for other non-endangered species. While emergence failed in Q. germana, the germination was high and it is a species that has been successfully propagated in local nurseries (Carlos Iglesias, pers. comm.). Considering this, there are opportunities to reproduce these species in nurseries and to test their survival and growth under field conditions. Plantations including these species would have a two-fold objective: to encourage their reintroduction in order to increase populations and to restore the structure and function of TMCF, since such plantations can accelerate secondary succession and thus the recuperation of environmental interactions and the services these provide.











 nueva página del texto (beta)
nueva página del texto (beta)