INTRODUCTION
Diatoms are eukaryotic algae with siliceous cell walls (frustules) that inhabit a wide range of lacustrine habitats, where they may occur as planktonic, benthic, or tychoplanktonic organisms (Battarbee et al., 2001; Cohen, 2003). Their frustules can be preserved when they are incorporated into lake sediments, making them one of the most valuable fossils for paleolimnological studies (Bradbury & Krebs, 1995; Cohen, 2003). Due to their specific preferences and tolerances for environmental conditions, sedimentary diatoms have been extensively used as bioindicators of water quality in freshwater ecosystems (Halland & Smol, 2010; Kireta et al., 2012) and have also been used to track long-term ecological perturbations such as deforestation, lake eutrophication, climate change, and surface water acidification (Winder et al., 2009; Quillen et al., 2013; Battarbee et al., 2014; Caballero & Vázquez, 2019; Liao et al., 2020), among others.
In recent decades there has been an increasing interest in using paleolimnological data sets to address long-term ecological processes in lakes (Gregory-Eaves & Beisner, 2011). This approach, however, arises questions about how faithful are sedimentary diatom assemblages reflecting changes in the diatom community of the water column, to allow for coupling studies of modern and fossil diatom assemblages (Battarbee et al., 2005), as it is well known that sedimentary diatoms may be altered by different processes occurring in the water column - sediment interface, such as zooplankton grazing, variable settling rates, lateral transport of sediments, and frustule fragmentation and dissolution (Kato et al., 2003; Ryves et al., 2003; Battarbee et al., 2005; Ryves et al., 2013). Furthermore, sedimentary diatom assemblages usually integrate not only planktonic diatoms but also taxa with different habitat types (Gregory-Eaves & Beisner, 2011), and therefore can give information on processes occurring not only in the water column, but also in other micro-environments within the lake. The potential of sedimentary diatoms to have a deeper time perspective of ecological processes justifies an in-depth comparative analysis of the diatom assemblages preserved in sediments compared to the diatom community present in the water column. This kind of comparison can be made using different sampling techniques such as phytoplankton, sediment traps, and surface sediment sampling (Battarbee et al., 2005; Ryves et al., 2013). These methods have their own advantages and limitations as each may present sampling or taphonomic biases (Behrensmeyer & Kidwell, 1985; Kato et al., 2003; Dubelaar et al., 2004; Battarbee et al., 2014; Hassan & Diaz, 2023; Hofmann et al., 2020) but, are these large enough so that they could provide antagonistic interpretations for the same ecosystem?
Most studies performed in inland waters comparing diatom assemblages from phytoplankton and sediments have been conducted in temperate zones. Only a few have been performed in tropical regions, where the lake mixing regime can lead to diatom populations dynamics that can be contrastingly different compared to temperate lakes (Caballero et al., 2006; Vázquez & Caballero, 2013; Caballero et al., 2016). Therefore, this work aimed to contribute to the evaluation and comparison of the diatom diversity and species composition observed from phytoplankton, sediment traps, and surface sediment samplings along two annual cycles in a deep tropical lake. We hypothesized that because surface sediment samples have a degree of temporal and spatial integration, their assemblages would include diatoms from different habits, leading to a higher species richness and Shannon diversity, but lower dominance when compared to the diatom assemblage from phytoplankton net samples or monthly sediment trap samples. The lake chosen for this study was Lake Alberca de Tacámbaro, a tropical crater in Western Mexico, which was sampled for two years. Previous studies have found that the diatom flora of this lake responds to changes in the environmental conditions and trophic status (Caballero et al., 2016; Caballero & Vázquez, 2019; Montero et al., 2021), making it an interesting lake to undertake comparative studies of data obtained from the living diatom community in the water column and from diatom assemblages in sediments.
MATERIALS AND METHODS
Study site. Lake Alberca de Tacámbaro is located in the south-west region of the Trans-Mexican Volcanic Belt (19° 12’ 40.56” N, 101° 27’ 28.59” W, 1460 masl; Fig. 1). This is a eutrophic, freshwater crater lake that has a maximum depth of 28 m and presents a warm monomictic stratification pattern. The lake shows a short mixing period in winter (January-February), an early shallow stratification period in spring (March-June), a full stratification period in summer (June-September), and a late, deep stratification period in autumn (September-December) (Caballero et al., 2016; Caballero & Vázquez, 2019; Montero et al., 2021). The main morphometric and environmental characteristics of this lake are summarized in Table 1.
Figure 1 Digital elevation model of Mexico and satellite imagery with topography highlighting the location of Lake Alberca de Tacámbaro (green star) at the south-west region of the Trans-Mexican Volcanic Belt.
Table 1 Main morphometric and environmental characteristics of Lake Alberca de Tacámbaro. Environmental parameters are shown as average values along the water column for the mixing and stratification periods during 2018 and 2019.
| Morphometric parameter | |||||
| Crater major axisa | 0.7 km | ||||
| Crater minor axisa | 0.5 km | ||||
| Crater (catchment) areaa | 0.3 km2 | ||||
| Lake areaa | 8.2 ha | ||||
| Maximum deptha | 28 m | ||||
| Mean deptha | 13 m | ||||
| Environmental parameter | Period | ||||
| Mixing | Early stratification | Full stratification | Late stratification | ||
| Trophic statusb | Eutrophic - hypertrophic | ||||
| Euphotic zone depth (m)b | 7.2 | 7.8 | 8.0 | 5.6 | |
| Water temperature (°C)b | 18.1 | 19.7 | 20.6 | 19.7 | |
| Dissolved oxygen (mg L-1)b | 3.5 | 3.1 | 2.6 | 3.3 | |
| pHb | 7.1 | 7.2 | 7.4 | 7.1 | |
| Chlorophyll a (µg L-1)b | 11.7 | 10.1 | 12.5 | 10.8 | |
| Dissolved inorganic nitrogen (µM)b | 186 | 193 | 164 | 165 | |
| Soluble reactive phosphorus (µM)b | 1.4 | 2.2 | 8.3 | 1.9 | |
| Silica (SiO2 µM)b | 866 | 894 | 857 | 870 | |
aData obtained from Caballero et al., 2016. bData obtained from Montero et al., 2021.
Fieldwork. Phytoplankton, sediment traps, and surface sediment samplings were conducted during winter (mixing), spring (early stratification), summer (full stratification), and autumn (late stratification) in 2018 and 2019 (Table 2), at the zone of maximum depth (25 - 28 m) around 10:00 am (UTC -06:00), as previously described in Caballero & Vázquez (2020) and Montero et al. (2021).
Table 2 List of sampling dates and characteristics of samples from phytoplankton, sediment traps and surface sediments.
| Phytoplankton samples | |
|---|---|
| Sampling date | Sampling characteristics |
| 02/17/2018 | Average of 7 depths along the water column (0, 5, 8, 10, 15, 20 and 25 m). |
| 05/25/2018 | |
| 09/01/2018 | |
| 11/24/2018 | |
| 01/26/2019 | |
| 04/27/2019 | |
| 08/31/2019 | |
| 11/23/2019 | |
| Sediment trap samples | |
| Sampling date interval | Number of days |
| 11/19/2017 - 02/17/2018 | 90 |
| 02/17/2018 - 05/25/2018 | 97 |
| 05/25/2018 - 09/01/2018 | 99 |
| 09/01/2018 - 11/24/2018 | 84 |
| 11/24/2018 - 01/26/2019 | 63 |
| 01/26/2019 - 04/27/2019 | 91 |
| 04/27/2019 - 08/31/2019 | 126 |
| 08/31/2019 - 11/23/2019 | 84 |
| 09/01/2018 - 08/31/2019 | 364 |
| Surface sediment samples | |
| Sampling date | Sampling characteristics |
| 02/17/2018 | Collected from the top 1 cm of sediment. |
| 05/25/2018 | |
| 09/01/2018 | |
| 11/24/2018 | |
| 01/26/2019 | |
| 04/27/2019 | |
| 08/31/2019 | |
| 11/23/2019 | |
On each occasion, phytoplankton samples were collected at seven depths (0, 5, 8, 10, 15, 20 and 25 m depth) with a Van Dorn bottle, and then fixed with Lugol’s iodine solution. Also, a system with two tubular sediment traps (aspect ratio: 12) supported by a buoy and separated from the bottom of the lake by about 1 m, was installed at the zone of maximum depth of the lake (> 25 m). The buoy of the sediment traps was always left 2 to 3 m below the lake surface to avoid vandalism or theft by visitors. When the traps were installed, the GPS (GARMIN 64S) location of the buoy was recorded, and a “rodeo” sampling technique was developed to recover them. This method used two inflatable boats, one of which was anchored at the GPS location of the sediment traps while the second circled the first one with a long rope that had equally spaced weights. The loops trapped the buoy and allowed to recover it when pulling. The accumulated sediments were collected at time intervals ranging from 84 to 126 days from one of the two traps. In the second trap, the accumulated sediments were collected annually, from September 2018 to August 2019. Surface sediments from the central part of the lake were also collected on each occasion from the top 1 cm of the surface layer of sediments using an Ekman dredge. The dredge was recovered with a slow but constant upward movement to avoid sediment disturbance and/or washout. The information on the sampling dates and characteristics of samples from phytoplankton, sediment trap, and surface sediment, are shown in Table 1.
Diatom abundance (cells mL-1) in phytoplankton samples was estimated following Utermöhl’s method (Lund et al., 1958) by using sedimentation chambers (2.5 - 10 mL, 24 h) and an inverted microscope at 400x (maximum magnification available). When counting, there was no discrimination between living and dead diatoms. For each sampling, diatom abundances at the different strata of the water column were averaged to obtain an integrated phytoplankton sample that included all the sampled depths; cell densities were expressed as relative abundances (%).
Sediment samples (traps and dredges) were freeze-dried, and 0.5 g of dry sediment (gds) were cleaned with HCl (10%) and H2O2 (30%) until all carbonates and organic matter were eliminated. Diatom slides were made using Naphrax® as the mounting medium and minimum counts of 400 valves were made under the optical microscope (Olympus BX50) at 400x. Based on these counts, diatom valve concentration (valves per gram of dry sediment [v gds-1]) and diatom species relative abundances were estimated.
Diatom species were identified based on specialized literature (Krammer & Lange-Bertalot, 1997; 1999; 2000); taxonomic classification was based on AlgaeBase (Guiry & Guiry, 2022). Due to the limited magnification in the inverted microscope, Aulacoseira granulata var. angustissima (O. Müller) Simonsen could not be distinguished from the nominal variety (A. granulata (Ehrenberg) Simonsen), and Discostella pseudostelligera (Hustedt) Houk & Klee could not be distinguished from D. stelligera (Cleve & Grunow) Houk & Klee; therefore, these forms were counted together and treated as species groups: A. granulata and D. stelligera/pseudostelligera. All diatom species were classified according to their habit into planktonic (centric taxa and needle shaped arraphids), tychoplanktonic (short, arraphid taxa) and benthic (mono and biraphid taxa).
Diversity metrics. Alpha diversity was evaluated using the effective number of species method, which is equivalent to Hill’s numbers (Hill, 1973). The diversity of order 0 (0D) is insensitive to species frequencies and represents species richness. The diversity of order 1 (1D) is equivalent to the exponential of Shannon’s entropy and accounts for the most common species in a community (Shannon’s diversity). The diversity of order 2 (2D) is equivalent to the inverse of Simpson’s index and gives more weight to dominant species (Jost, 2006). The sample completeness for each sampling (and sampling method) was evaluated with the iNEXT package in R studio (Chao et al., 2014; Hsieh et al., 2016; R Core Team, 2019). Additionally, dominance-diversity curves were constructed to analyze and compare diatom abundance distribution patterns in phytoplankton, sediment trap, and surface sediment samples. Dominance-diversity figures were based on the eight most-abundant diatoms for each sampling.
Beta diversity (variation in species composition) for the different diatom assemblages was measured as the overall dissimilarity among sites using the multiple-site Sørensen dissimilarity index (βSOR). This dissimilarity index ranges from 0 to 1, where 0 indicates a null dissimilarity among sites, and 1 indicates a total dissimilarity among sites. The βSOR index can be partitioned into two components (Baselga, 2012): dissimilarity due to species replacement (or Simpson dissimilarity, βSIM) and dissimilarity due to nestedness (βNES); both dissimilarity components were calculated. Beta diversity was also evaluated using the Whittaker’s method, which represents the number of different communities in the dataset (Whittaker, 1960). Beta diversity analyses were computed based on presence/absence datasets in the betapart package of R studio (Baselga & Orme, 2012; R Core Team, 2019).
Sediment characterization. Total carbon (TC) was determined using a Thermo ScientificTM Flash 2000 soil analyzer. The remaining percentage represents the silicate fraction composed of diatom frustules and terrigenous material and is used here to describe the sediment composition.
Statistical and numerical analysis. Analyses of variance (ANOVA) were performed to determine whether diversity measures (0D, 1D, 2D) were significantly different in phytoplankton, sediment trap, and surface sediment samples; when significant differences were found, multiple comparison tests (Tukey test) were performed to identify the differences. Wilcoxon tests (95 % confidence level) were computed to determine whether the percentage of the silicate fraction, and valve concentration were statistically different between trap and surface sediments. A cluster analysis was conducted to compare the diatom assemblages from phytoplanktonic, sediment trap, and surface sediment samples. A dissimilarity matrix was calculated using the percentage similarity method, and the dendrogram was generated by the unweighted pair-group method using arithmetic averages (UPGMA) (Borcard et al., 2018). Singletons were removed from the analysis (Legendre & Legendre, 1998). The cluster analysis and dendrogram were performed using the packages ‘vegan’ and ‘ggdendro’ in R Studio version 4. 1. 0 (R Core Team, 2019).
RESULTS
Diatom diversity, habit, and species composition. A total of 40 diatom taxa were recorded in all the samples, 11 were planktonic, 28 were benthic, and one was tychoplanktonic. Thirty-one of these taxa (80%) were present in the three kinds of samples (see Table 3 for taxa names and authors). Sample coverage was higher than 0.9 in all samples, which indicates that more than 90% of the individuals in the diatom community were represented in the samples.
Table 3 List of diatom species observed in samples from phytoplankton, sediment trap, and surface sediments in Lake Alberca de Tacámbaro. Diatom species are classified by their habit into planktonic, benthic, and tychoplanktonic species. Question marks indicate that the taxon identification is still uncertain.
| Phytoplankton | Sediment trap | Surface sediments | |
| Planktonic species | |||
| Aulacoseira granulata (Ehrenberg) Simonsen | ✓ | ✓ | ✓ |
| Aulacoseira granulata var. angustissima (O. Müller) Simonsen | ✓ | ✓ | ✓ |
| Cyclotella sp. | ✓ | ✓ | ✓ |
| Cyclotella meneghiniana Kützing | ✓ | ✓ | ✓ |
| Discostella stelligera (Cleve & Grunow) Houk & Klee | ✓ | ✓ | ✓ |
| Discostella pseudostelligera (Hustedt) Houk & Klee | ✓ | ✓ | ✓ |
| Fragilaria crotonensis Kitton | ✓ | ✓ | ✓ |
| Pantocsekiella ocellata (Pantocsek) K. T. Kiss & Ács | ✓ | ✓ | ✓ |
| Ulnaria delicatissima (W. Smith) Aboal & P. C. Silva | ✓ | ✓ | ✓ |
| Ulnaria delicatissima var. angustissima ? (Grunow) Aboal & P. C. Silva | ✓ | ✓ | ✓ |
| Ulnaria ulna (Nitzsch) Compère | ✓ | ✓ | ✓ |
| Benthic species | ✓ | ✓ | ✓ |
| Achnanthidium minutissimum (Kützing) Czarnecki | ✓ | ✓ | ✓ |
| Amphipleura pellucida (Kützing) Kützing | ✓ | ✓ | ✓ |
| Brachysira vitrea (Grunow) R. Ross | ✓ | ✓ | ✓ |
| Cocconeis placentula Ehrenberg | ✓ | ✓ | ✓ |
| Cymbella affiniformis Krammer | ✓ | ✓ | ✓ |
| Cymbella lanceolata C. Agardh | ✓ | ✓ | ✓ |
| Cymbella mexicana (Ehrenberg) Cleve | ✓ | ✓ | ✓ |
| Denticula kuetzingii Grunow | ✓ | ✓ | ✓ |
| Encyonema silesiacum (Bleisch) D. G. Mann | ✓ | ✓ | ✓ |
| Encyonopsis microcephala (Grunow) Krammer | ✓ | ✓ | ✓ |
| Epithemia adnata (Kützing) Brébisson | ✓ | ✓ | ✓ |
| Epithemia turgida (Ehrenberg) Kützing | ✓ | ✓ | ✓ |
| Gogorevia exilis (Kützing) Kulikovskiy & Kociolek | ✓ | ✓ | ✓ |
| Gomphonema sp. | ✓ | ✓ | ✓ |
| Gomphonema acuminatum Ehrenberg | ✓ | ✓ | ✓ |
| Gomphonema lagenula Kützing | ✓ | ✓ | ✓ |
| Iconella linearis ? (W. Smith) Ruck & Nakov | ✓ | ✓ | ✓ |
| Navicula cryptocephala ? Kützing | ✓ | ✓ | ✓ |
| Navicula veneta Kützing | ✓ | ✓ | ✓ |
| Nitzschia amphibia Grunow | ✓ | ✓ | ✓ |
| Nitzschia amphibioides Hustedt | ✓ | ✓ | ✓ |
| Pinnularia subcapitata ? W. Gregory | ✓ | ✓ | ✓ |
| Pinnularia viridis (Nitzsch) Ehrenberg | ✓ | ✓ | ✓ |
| Planothidium rostratum (Østrup) Lange-Bertalot | ✓ | ✓ | ✓ |
| Psammothidium helveticum (Hustedt) Bukhtiyarova & Round | ✓ | ✓ | ✓ |
| Rhopalodia gibba (Ehrenberg) O. Müller | ✓ | ✓ | ✓ |
| Sellaphora pupula (Kützing) Mereschkovsky | ✓ | ✓ | ✓ |
| Surirella elegans Ehrenberg | ✓ | ✓ | ✓ |
| Tychoplanktonic species | ✓ | ✓ | ✓ |
| Staurosirella pinnata (Ehrenberg) D. M. Williams & Round | ✓ | ✓ | ✓ |
Diversity metrics in phytoplankton samples indicated that diatom species richness (0D) ranged from 14 to 19 (average 16) and Shannon’s diversity (1D) indicated that the number of equally common diatoms ranged from 1 to 6 (Fig. 2A). Simpson’s diversity (2D) highlighted that the number of dominant diatoms in phytoplankton varied from 1 to 3. Beta diversity (measured as βSOR) was 0.55, and species replacement (or turnover) accounted for 85 % of the overall dissimilarity (βSIM = 0.47). Beta diversity using Whittaker’s method was 2.01, which indicates that two main alternating species associations were present in the phytoplankton. Regarding species composition, phytoplankton samples evidenced the seasonal dynamics of the planktonic diatom community, which was highly dominated by Aulacoseira granulata in the mixing and early stratification periods, where its abundance and the associated chlorophyll a concentration (>1000 cells mL-1 and > 20 µg L-1, data not shown) were characteristics of algal blooms (Alcocer & Lugo, 2003; Chorus & Welker, 2021). On the other hand, Discostella stelligera/pseudostelligera, in association with Pantocsekiella ocellata and Brachysira vitrea, were the dominant diatoms in full and late stratification, and Achnanthidium minutissimum dominated once, during late stratification (Fig. 2B). The diatom assemblages from phytoplankton samples were dominated by planktonic species (82% on average), but they also included benthic taxa (17% on average of total abundance).
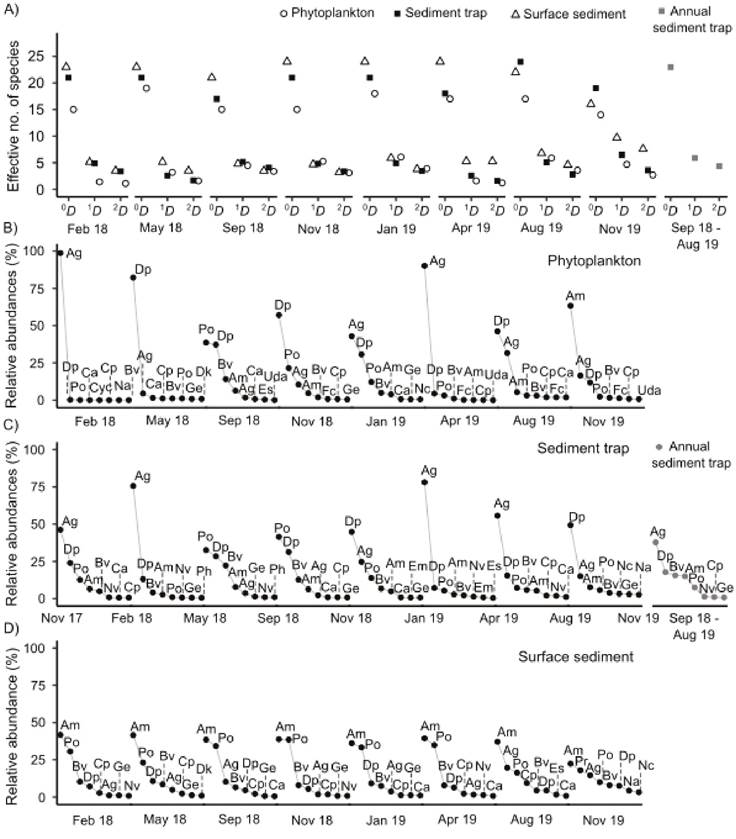
Figure 2 Diatom diversity and species composition in Lake Alberca de Tacámbaro during the period February 2018 - November 2019. A) Diversity measures: species richness (0D), Shannon’s diversity (1D), and Simpson’s diversity (2D). Dominance-diversity curves for the diatom assemblage in samples from B) phytoplankton, C) sediment trap and D) surface sediments. Ag = Aulacoseira granulata; Am = Achnanthidium minutissimum; Bv = Brachysira vitrea; Ca = Cymbella affiniformis; Cp = Cocconeis placentula; Cyc = Cyclotella sp.; Dk = Denticula kuetzingii; Dp = Discostella pseudostelligera; Es = Encyonema silesiacum; Em = Encyonopsis microcephala; Fc = Fragilaria crotonensis; Ge = Gogorevia exilis; Na = Nitzschia amphibioides; Nc = Navicula cryptotenella; Nv = Navicula veneta; Ph = Psammothidium helveticum; Po = Pantocsekiella ocellata; Pr = Planothidium rostratum; Ud = Ulnaria delicatissima; Uda = U. delicatissima var. angustissima.
In sediment traps, diatom species richness (0D) ranged from 17 to 24 (average 20), including the annual trap where 23 taxa were present. The number of equally common diatoms (Shannon’s diversity 1D) in sediment trap samples ranged from 1 to 6 (including the annual trap where it was 6), and the number of dominant diatoms (Simpson’s diversity 2D) ranged from 2 to 4 (including the annual trap where it was 4) (Fig. 2A). Beta diversity (βSOR) was 0.49, with species replacement accounting for 83 % of the overall dissimilarity (βSIM = 0.41). The beta diversity calculated with Whittaker’s method (1.7) was slightly lower than in the phytoplankton. Diatom assemblages in samples from sediment traps were similar to those observed in the phytoplankton, with A. granulata being the dominant species in the intervals that included the mixing and early stratification periods, and with D. stelligera/pseudostelligera, in association with P. ocellata, and B. vitrea having high abundances during the intervals that included the full and late stratification period. Achnanthidium minutissimum was always present in these samples but it never reached the highest abundances (Fig. 2C). The annual sediment trap showed an assemblage where A. granulata was the most abundant species, closely followed by D. pseudostelligera, B. vitrea, A. minutissimum, and P. ocellata, which together contributed to approximately 95% of total valve concentration (Fig. 2C). The diatom assemblages in sediment trap samples were mostly constituted by planktonic species, which accounted on average for 80%; benthic and tychoplanktonic species were also present and accounted for 19% and 0.5% respectively.
Finally, surface sediments had the highest species richness (p < 0.05), ranging from 16 to 24 (average 22) (Fig. 2A). Shannon’s diversity (1D) varied from 4 to 7 and these values were statistically similar (p > 0.05) to those found in phytoplankton and sediment traps. The number of dominant species was also significantly higher (p < 0.05) in surface sediment samples ranging from 3 to 7 (Fig. 2A). A lower beta diversity was observed in these diatom assemblages (βSOR = 0.45), where species replacement accounted for 77 % of the overall dissimilarity. Whittaker’s beta diversity was also the lowest (1.5). The diatom assemblage was dominated by A. minutissimum closely followed by P. ocellata, which together contributed to 50 - 70% of the total valve counts (Fig. 2D). Aulacoseira granulata was always present in the surface sediment assemblage, but it reached higher abundances in the late stratification periods, following the early stratification blooms recorded in the phytoplankton and trap sediments. Discostella stelligera/pseudostelligera and B. vitrea were also constantly present in the surface sediment assemblages, and less frequently, Cocconeis placentula and Planothidium rostratum. The diatom assemblages in surface sediment samples were characterized by a higher proportion of benthic species (57% on average), but with an important presence of planktonic taxa (42% on average).
Trap and surface sediment characteristics. The average diatom valve concentration in trap sediments was 1000 ± 530 x106 v gds-1 and the silicate fraction was 88% (Fig. 3). The valve concentration of the most abundant diatoms in the sediment samples is shown in figure 4 (diatoms with valve concentration ≥ 5% of total valve concentration in at least one sample). It can be observed that the sediment traps evidenced the seasonal dynamics of A. granulata, D. stelligera/pseudostelligera, and P. ocellata recorded in the phytoplankton samples. As mentioned above, the annual sediment trap was a compilation of the seasonal trap samples between September 2018 and August 2019, A. granulata was the most abundant diatom, followed by D. pseudostelligera, B. vitrea, A. minutissimum, and P. ocellata.
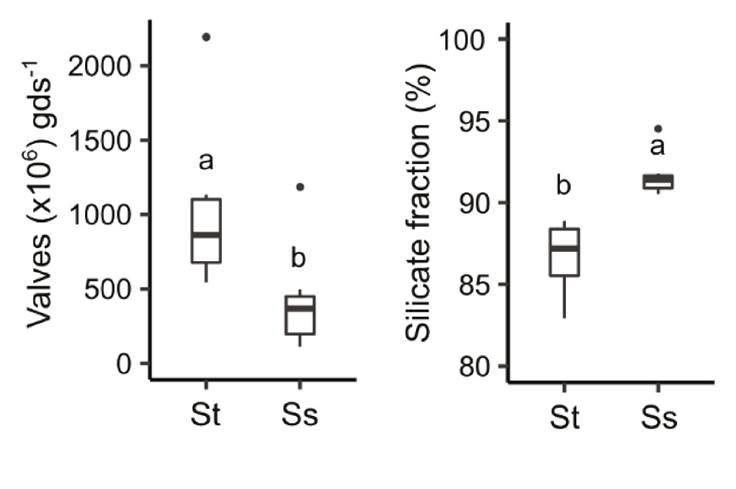
Figure 3 Diatom valves (x106 gds-1), and silicate fraction in trap (St) and surface sediments (Ss). Superscript letters (a, b) indicate significant differences.
Surface sediments presented lower (p < 0.05) average diatom valve concentration (415 ± 340 x106 v gds-1) than trap sediments but higher (p < 0.05) silicate fraction (92%) (Fig. 3). In contrast to trap sediments, high concentrations of A. granulata and D. stelligera/pseudostelligera were not observed in the surface sediment samples. Instead, A. minutissimum showed the highest valve concentration followed by P. ocellata. Other taxa such as B. vitrea, C. placentula, and P. rostratum had similar valve concentrations in trap and surface sediments (Fig. 4).
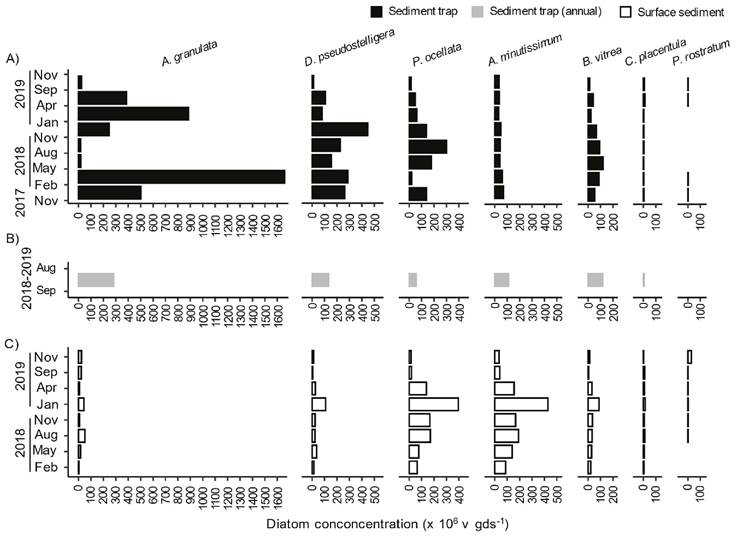
Figure 4 Diatom concentration (x 106 v gds-1) through time of the most abundant diatoms (abundance ≥ 5 % in at least one sample) in sediment samples. Diatom concentration in sediments from seasonal trap (A), annual trap (B) and surface sediments (C).
Diatom assemblage comparison. The cluster analysis based on diatoms relative abundance displayed four groups constituted by the combination of samples from phytoplankton and sediment traps, and one with a large majority of samples from surface sediments (Fig. 5). The first group was comprised by phytoplankton and sediment trap samples in which A. granulata was the dominant species. The second group included all the surface sediment samples and one phytoplankton sample, characterized by the high relative abundance of A. minutissimum and P. ocellata. The third group was represented by phytoplankton and trap samples (including the annual trap) in which A. granulata, and D. stelligera/pseudostelligera, were the main components of the diatom assemblage. Finally, the fourth group was formed by phytoplankton and sediment trap samples with dissimilarities ranging from 15 to 40 %, where D. stelligera/pseudostelligera was the main component of the diatom assemblage, with an important presence of P. ocellata and B. vitrea.
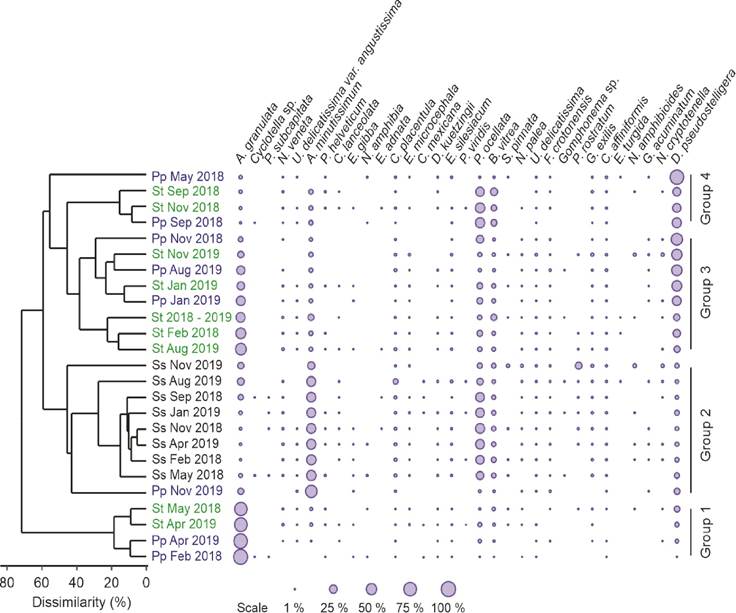
Figure 5 Dendrogram from the cluster analysis based on quantitative data (percentage similarity, UPGMA) of the diatom community observed in samples from phytoplankton (Pp in blue), sediment trap (St in green), and surface sediment (Ss in black). Bubble plot represents the relative abundance of diatom species in the different samples.
DISCUSSION
The comparative assessment of the diatom assemblages in Lake Alberca de Tacámbaro allowed to determine that phytoplankton samples, sediment traps, and surface sediments samples provided a consistent spectrum of the diatom species composition and diversity in the study site. During the studied interval (2018 - 2019), 80% of the diatom species were present in the samples collected by the three sampling methods. Furthermore, the same five main taxa (A. granulata, D. stelligera/pseudostelligera, B. vitrea, P. ocellata, and A. minutissimum) were present in the three kinds of samples. These results showed that despite the intrinsic differences between the three sampling methods, these will not provide antagonistic results on the ecological condition of the lake.
Furthermore, the diatom assemblages obtained by the three sampling methods did not show significant differences in their Shannon’s diversity, even though, as was hypothesized, the species richness (0D) and Simpson´s diversity (2D) were significantly higher in the surface sediment samples. Relating to the habit of the diatom taxa in each kind of samples, according to what was expected, the surface sediment samples had a relatively equitable split between planktonic (42%) and benthic (57%) forms, however, it was an unexpected result to find a relatively high proportion of benthic taxa (of nearly 20%) in the plankton and sediment trap samples. These included taxa such as A. minutissimum (particularly in Nov 2019) and B. vitrea (particularly in Sep 2018). The presence of these species in phytoplankton samples has been documented previously in this lake (Caballero et al., 2016) and in other deep, warm-monomictic lakes in Mexico (Vázquez & Caballero, 2013). It is possible that these species are re-suspended from the bottom sediments where they live, but in this lake the photosynthetically active radiation penetrates to a maximum of about 12 m (Montero et al., 2021). Therefore, the vast majority of the lake bottom (>70%) is inhospitable for diatoms, and strongly suggests that in spite of being raphe bearing taxa generally considered to have a benthic habit, these species can adopt a tychoplanktonic form of live, with floating strategies that allow them to survive in the plankton (Cantonati & Lowe, 2014; Cvetkoska et al., 2018).
In addition, some studies have pointed out that benthic species usually dominate diatom assemblages from surface sediments because the concentration of planktonic species is frequently diluted by the horizontal transport of sediments (Buchaca & Catalan, 2007; Hofmann et al., 2020). Considering that diatom valve concentration was higher in the trap sediments than in surface sediments, it is reasonable to assume that terrigenous materials account mostly for this difference pointing to the existence of lateral sediment transport and sediment focusing on Lake Alberca de Tacámbaro, bringing terrigenous and organic sediments from the perimeter of the lake to the central regions. Given that A. minutissimum and P. ocellata are the dominant diatoms in the sediment of this lake, it is reasonable to expect that this material could include a high number of diatom valves of A. minutissimum and P. ocellata, which may contribute to a decrease in the proportion of more recent planktonic diatoms. The loss of frustules by fragmentation or dissolution in the surface sediments was discarded in this study because there was no evidence in our material to support this assumption, as we did not find redissolved valves or abundant fragments that might suggest preferential breaking or dissolution of A. granulata or D. stelligera/pseudostelligera.
The beta diversity of the plankton and sediment trap samples as well as the results of the cluster analysis showed species replacement related with water column mixing and stratification which resulted in at least two main seasonal diatom assemblages in this lake. Aulacoseira granulata was favored by the winter mixing processes when there was a higher turbulence and higher total phosphorus availability in the lake (Montero et al., 2021). In contrast, P. ocellata and D. stelligera/pseudostelligera flourished during times of warmer temperatures, a stratified water column, and lower nutrients concentration associated with the stratification process, even though in at least one sampling this “stratification” assemblage was dominated by A. minutissimum. In addition, phytoplankton and sediment trap samplings often displayed a coupled temporal variability in their diatom assemblages; for example, phytoplankton blooms of A. granulata were visible in the trap sediments collected at the same time or in the following sampling date. In relation to the annual sediment trap, it accurately reflected the average species abundances in the phytoplankton.
The pattern of seasonal species replacement along the year was attenuated in the diatom assemblage from surface sediments, in agreement with results from other studies comparing planktonic and sedimentary diatom assemblages (Kato et al., 2003; Ryves et al., 2003; Pla-Rabés & Catalan, 2018). Surface sediment samples showed a richer, more equitable and more homogeneous diatom assemblage throughout the year. This assemblage was dominated by A. minutissimum and P. ocellata in association with B. vitrea, D. stelligera/pseudostelligera and A. granulata; small peaks of A. granulata were recorded during late stratification, following the winter and early stratification blooms recorded in the phytoplankton and trap sediments. These samples showed a much smaller temporal variability in their diatom assemblages, and species replacement occurred only among diatoms with low abundance (e.g., C. placentula, Cymbella affiniformis, Gogorevia exilis, Encyonema silesiacum, Navicula veneta and P. rostratum). These diversity patterns are common in surface sediments because these samples often represent a time-average window larger than the trap or the phytoplankton samples (Dong et al., 2008), which may explain why the high abundances of A. granulata and D. stelligera/pseudostelligera observed in phytoplankton and trap samples were attenuated in the surface sediment samples. This time window depends on the sediment accumulation rate in the lake, and even though this is a variable parameter, in a previous study in this lake it was established to be around 7 mm/year (Caballero et al., 2016). In this way, the surface layer of sediments (~1cm) in Lake Alberca de Tacámbaro is likely to represent around two years of sedimentation (Caballero et al., 2016) and could therefore have a memory of a recent past where A. minutissimum and P. ocellata were more abundant in the phytoplankton. In fact, these species were the dominant taxa in the annual sediment trap deployed in this lake from April 2015 to April 2016 (Caballero, unpublished data).
From previous work in this lake (Caballero et al., 2016) we also know that a diatom assemblage dominated by A. minutissimum and P. ocellata has been present in the surface sediments since the 2010 “El Niño” event when P. ocellata became abundant in the phytoplankton. Previously, from 1988 to 2010, the surface sediments were dominated by A. minutissimum in association with needle-shaped diatoms such as Ulnaria delicatissima, Fragilaria neotropica or F. crotonensis, but since then, these planktonic diatoms have become increasingly rare in the phytoplankton and sediments of Lake Alberca de Tacámbaro (Caballero et al., 2016). In this way, the discrepancies between the phytoplankton and surface sediment diatom assemblages are an indication of recent changes in the diatom flora of this lake, with a trend towards a reduction in needle shaped planktonic taxa and increasing abundance of centric planktonic taxa such as A. granulata and D. stelligera/pseudostelligera. These changes could be related to a recent increase in nutrient levels in this lake, particularly nitrogen (Montero et al., 2021).
The comparison of the living diatom community with the diatom assemblage in sediments provided valuable information to understand the sedimentary diatom assemblage in Lake Alberca de Tacámbaro, its association with the ecological features of some species and complex processes occurring in the sediments. The surface layer of sediments of this lake is likely to represent a diatom assemblage integrated by diatoms from several zones of the lake and different habits, with an average-time window of about two years. The diatom assemblage in these samples would take a slightly longer time to show the recent changes in the structure of the water column diatom community. The higher silicate fraction in surface sediment samples indicates that horizontal transport and sediment focusing may also have an important influence on the diatom assemblage found in the surface layer of sediments. Even though our results also suggest that in tropical deep lakes some species traditionally considered to have a benthic habit (A. minutissimum and B. vitrea) can be at least temporarily incorporated into the plankton, functioning as tychoplanktonic taxa.











 nueva página del texto (beta)
nueva página del texto (beta)



