INTRODUCTION
The heavy metal cadmium (Cd) is a pollutant from the industrial activities (WHO, 2007; Ashraf, 2005), and is known to be harmful to aquatic organisms (Chiarelli & Roccheri, 2012; Liu et al., 2012; Arai et al., 2012; Wei, 2013). Recently, there are many reports of the effects of Cd on various organs from different animals. The studies conducted over the past two decades show that cadmium can damage DNA, producing lipid peroxidation, and protein inactivation (Valko et al., 2005). Sary & Mohammad (2012) reported the accumulation of cadmium in fish Acanthopagrus pilate and Platycephalus indicus caused slow growth and health problems for humans.
Studies about monitoring the exposure to heavy metals at the molecular level in various aquatic animals have been performed to see the effects of heavy metals have been reported by several researchers. The bioassay with embryo-cytotoxicity techniques by Dermeche et al. (2012) has found Paracentrotus lividus is very sensitive to Cd. For this, P. lividus is recommended as bioindicator species of the marine environment. Indicators of stress on freshwater invertebrates were also reported by Molnar & Fong (2012) with the Neutral Red Retention-Lysosomal Destabilization Assay. Liu et al. (2012) reported with a random amplified polymorphic DNA (RAPD) test that Arabidopsis thaliana exposed to Cd presented genetic changes. Besides, Wei (2013) research with FTIR spectra techniques applied on gonads of sea urchins Strongylocentrotus nudus exposed to Cd, found the occurrence of stress at the molecular level with damage to lipids and proteins. Sea urchin embryos are very sensitive to environmental stress, and Cd constitutes an inductor from modulator factors, like protein kinases and phosphatases, caspases, heat shock proteins, metallothioneins, transcription factors, reactive oxygen species, apoptosis and autophagy (Chiarelli & Roccheri, 2012; Chatterjee et al., 2014).
Related with the utilization of various protein molecules to see the responses elicited by heavy metals, Jung & Lee (2012) reported that Heat Shock Proteins (HSPs) plays a role in the process of homeostasis under stress conditions. HSPs act as molecular chaperones in living organisms, where the expression of HSP27, HSP70, and HSP90A-1 have been recommended as a molecular marker to monitor the toxicity of heavy metals in the aquatic environment. Protein molecules that have been used as a stress response markers for Cd (P41087, P4825, P2746, P2811) have shown strong correlation with Cd located in the locus in maintaining the integrity of the Ref-1 and hOGG1 genomes or responses as a result of cellular activation due to oxidative stress (Liu et al., 2012). Additionally, Rumahlatu et al. (2012) used methalotionine protein (MT) a molecular marker to see the damage to the spines, shells, gonads, and intestines accumulated with Cd. Previously, Shimoda et al. (2003) found that MT protein expression was related to resistance to apoptosis in cardiomyocytes so that MT protein was used as a marker of failure to treat tumor cell apoptosis. The use of Tumor Necrosis Factor-alpha (TNF-α) protein to see the repair mechanisms in the health sector has also been reported. TNF-α plays a role in the pathogenesis of diabetes mellitus type 2 and can reduce obesity with the increased levels of cytokines (Swaroop et al., 2012). Interestingly, TNF-α is a pro-inflammatory cytokine which has a biological effect, and expressions of TNF-α can mediate the effects of stress from the hypotension of arteries, coagulation of blood vessels, and hypoglycemia (López-Bojórquez et al., 2004; Cavalcanti et al., 2012).
Previous research reviews (Jung & Lee, 2012; Liu et al., 2012; Shimoda et al., 2003; Rumahlatu et al., 2012) have investigated more on the response of biological molecules of HSP and MT proteins as molecular markers caused by exposure to heavy metals. However, there have not been many types of research on the molecular response of Diadema setosum as a result of the exposure to Cd using TNF-α. Therefore, the purpose of this study was to investigate the molecular response in the form of activation, expression, and concentrations of TNF-α D. setosum caused by the exposure to Cd in basin treatment.
MATERIALS AND METHODS
One-year-old Diadema setosum specimens were treated on 6 basins in the laboratory of Research Center for Deep Sea, The Indonesian Institute of Sciences Ambon. A total of 42 individuals of D. setosum with the same characteristics body weight and diameter of 90 g and 15 cm, respectively were used for this study. The exposure experiments were conducted in 7 basin aquaria (100 x 60 x 70 cm3) with 1 control basin and 5 basins for the experimental concentrations of CdCl2.
The experimental concentrations were 0.0, 1.0, 3.0, 6.0, 9.0, and 12.0 mg/L of Cd. Each basin treatment was filled with 200 L seawater, which was changed once a week with electric-aerator air circulation. Each basin was applied one level of Cd concentration treatment, and each basin was inhabited by seven specimens of D. setosum as individual replicates. The sea urchin was fed with seagrass every morning by binding the seagrass on the boulder and placed in the treatment basin and spreading the seagrass on the surface of the water of the treatment basin.
After 4 weeks of the treatment, surgery on the 42 individuals was performed to take the liver which was transferred into a sample pot for further examination activation of TNF-α protein, the concentration measurements of TNF-α protein in the Laboratory of Medical Physiology, Brawijaya University, Malang.
Examination of TNF-α protein Activation. The Examination of TNF-α protein activation in the liver of D. setosum was performed by using immunohistochemical methods. Livers were prepared through the stages of tissue fixation, embedding, and sample sectioned. Tissue slides were activated by soaking with xylene twice, 15 minutes each. Then, the slide was incubated in a series of ethanol series 100% I, 100% II, 95%, 90%, 80%, and 70%, each solution for 5 minutes. Then it was incubated again in the water for 5 minutes. The provided slides were soaked into the H2O2 0.3% for 30 minutes at room temperature.
The incubation time was 10-30 minutes for frozen sections, and 5-10 minutes for frozen tissue arrays. Then the slides were rinsed with water followed by 1 x PBS (Sigma Aldrich) (pH 7.4) once, then tissue sections were circled with Pap Pen. The samples were incubated with 1% of PBS serum [Mix 1x 3.5 ml of PBS, pH 7.4 as much as 1 drop (ca. 35 µl/drop) normal serum in the tube for 30 minutes at room temperature. Those slides were incubated with PBS which was diluted with the antibody in a humid chamber for 1 hour at room temperature, then rinsed with 1xPBS 3 times in 5 minutes. After that, slides were incubated with a Biotin-labeled secondary antibody for 30 minutes at room temperature. The slides were rinsed with 1xPBS 3 times for 5 minutes. Then, the detection solution was added to the tissue sections and it was incubated at room temperature for 30 minutes. The slides were rinsed with 1XPBS for 3 times, each for 5 minutes. The slides were dripped with DAB liquid (Diamino-benzidine tetrahydrochloride). After that, the cells were dripped with counterstains with hematoxylin for 10 minutes. Cells were washed with flowing water and then with distilled water for 10 minutes. Cells were left at room temperature. The tissue was placed on an object glass and dripped with Entellan®. After that, the liver cells undergoing activation of TNF-α protein were observed using a photonic microscope (Olympus) with slide blot shooting at a 400x magnification field of view.
Measurement of TNF-α Protein Concentration. The measurement of TNF-α protein concentration used an ELISA method (Enzyme-Linked Immunosorbent Assay) (Lequin, 2005). Sample preparation was carried out by softening the liver organ of D. setosum through the thawing step. The ELISA reader was performed by making an ELISA plate plan and coating buffer based on the sample code and location of the sample. After that, Coating Antigen was performed with the levels 1:40 diluted with coating buffer and incubated at a temperature of 40C overnight. The next day the plate was washed with a solution of 0.2% PBS Tween as much as100 µl and repeated 6 times. After that, 100 µl of primary antibody anti-TNF-α (1: 400) was added into assay buffer. Then, the ELISA plate was incubated at room temperature for 2 hours while being shaken with ELISA plate shaker.
In the next stage, the plate was washed with a solution of PBS Tween 0.2% as much as 200 µl for 6 times, and then 100 µl secondary antibody IGg biotin anti-rabbit (1:800) was added into assay buffer and incubated at room temperature for 1 hour while being shaken. After that, the plate was washed again with PBS Tween 0.2% 6 times. Next, 100 µl SAHRP solutions (1:800) were added to the assay buffer and incubated at room temperature for 1 hour while being shaken. Then, the solution was washed with PBS Tween 0.2% 200 µl for 6 times. After that, each was added 100 µl of good substrate sure blue TMB microwell, incubated for 20-30 minutes in a dark room. At this stage, if their reaction occurs between the antigen and the antibody, the solution would turn blue. The solution that was previously blue would turn yellow. The sample was analyze by using an ELISA reader at a wavelength of 450 nm., and the levels of TNF-α protein of each sample would be observed eventually.
Examination of the Expression of TNF-α Protein. The examination of the expression of TNF-α protein used the western blotting method (Young & Hongbao, 2010). The examination of TNF-α protein expression was preceded by the examination of SDS-PAGE, namely by performing electrophoresis on the samples of protein standard broad range (Biolab). The gel, the results of SDS PAGE, was soaked in 100 mL deionized water (pH 7.0) for 5 minutes. Then the gel, NC membrane, and sponge were soaked in transfer buffer for 5 minutes. Furthermore, it was arranged sequentially in each well: black able, sponge, two sheets of filter paper, gel, NC membrane, 3 pieces of filter paper, sponge, and white able. Then it was put into the chamber, electrified from the negative to the positive pole (100 volts, 120 minutes). Furthermore, the NC membrane was rinsed with deionizing 3 times, and immersed in blocking buffer (5% BSA), and incubated at 40C overnight. NC membrane was washed with TBS 0.2% Tween 3 times for 5 minutes and added antibodies in TBS BSA 1%. It was incubated for 2 hours and shaken. After that, the gel was washed with TBS-Tween 0.2% 3 times for 5 minutes. Then IgG biotin anti-rabbit was added in TBS, incubated for 1 hour and shaken. It was washed again with TBS-Tween 0.2% for 3 times for 5 minutes, and SAHRP was added in TBS, incubated for 1 hour and shaken. After that, it was washed with TBS-Tween 0.2% 3 times for 5 minutes. After that, the TMB substrate was added to the membrane for 15-30 minutes until the ribbon on the membrane emerged. Stop the reaction with distilled water.
Data analysis. The data were analyzed descriptively to describe the activation, concentration, and expression of TNF-α protein and to determine the effect of the concentration of heavy metals Cd on the concentrations of TNF-α protein. Inferential statistical analysis one-way ANOVA followed by Duncan test (p <0.05) was performed to determine whether there were statistical differences between the mean concentrations of TNF-α protein in D. setosum.
RESULTS
Activation of TNF-α Protein Diadema setosum due to heavy metal exposure. The results of the immunohistochemical assay on the liver tissue of D. setosum using rabbit anti-TNF-α antibodies (Fig. 1) show that TNF-α activated cells were brown, while the cells that are not activated TNF-α were blue. The cells undergoing the activation of TNF-α protein seem to spread and form clusters of cells. This brown cell morphology reflects the activation of TNF-α protein that occupies the cytoplasm and the nucleus of D. setosum liver cells. It can be seen that the higher the concentration of Cd exposure can affect liver cells so that the cell is brown. This brown color shows the activation of TNF-α protein. This means that the concentration of heavy metal Cd tends to increase the activation of TNF-α protein.
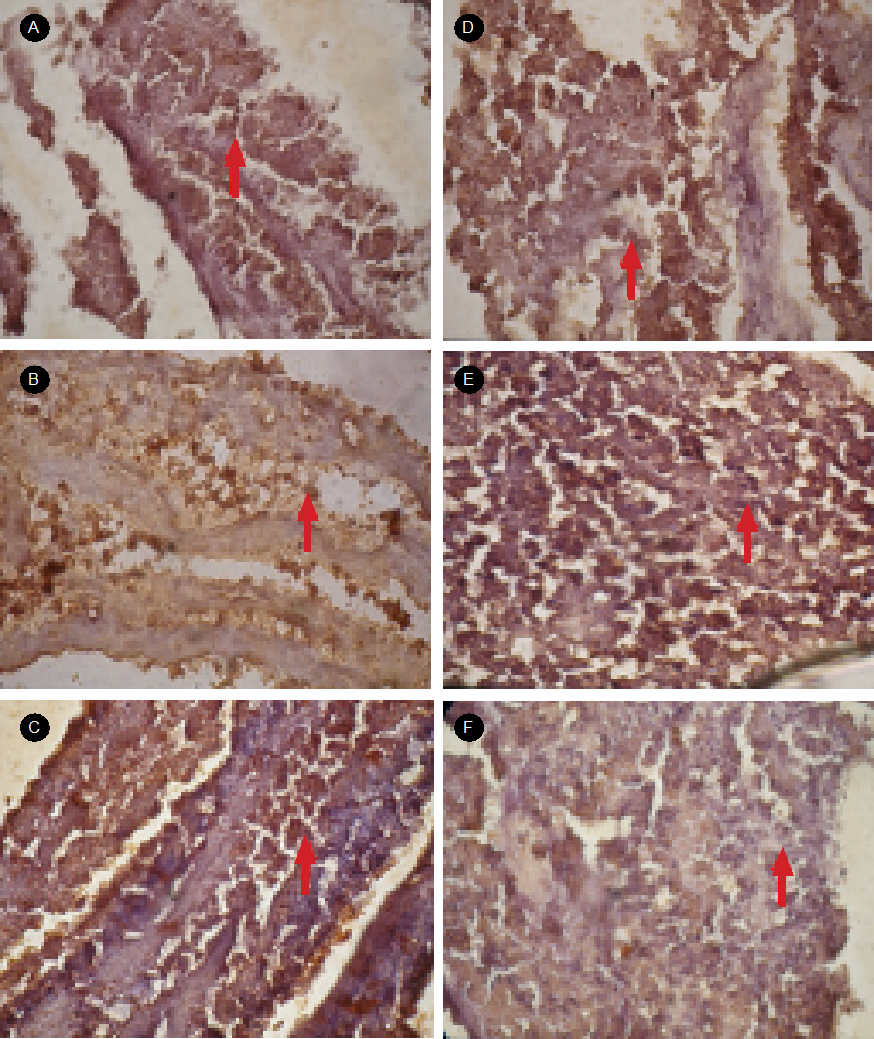
Figure 1 The results of immunohistochemical smearing using rabbit anti-TNF-α antibody in liver tissues of Diadema setosum. Observations with Olympus microscope slides for shooting four dots with 400x magnification zoom. Image with Notation: A) control; B) concentration of 1.0 µg/L Cd; C) the concentration of 3.0 µg/L Cd; D) concentration 6.0 µg/L Cd; E) concentration of 9.0 µg/L Cd; and F) concentration of 12.0 µg/L Cd. The arrows show the liver cells where the TNF-α protein is activated the brown signal.
Concentration and Expression of TNF-α Protein Diadema setosum due to heavy metal exposure. The concentration of TNF-α protein by ELISA reader (Table 1) measurement showed an increase in concentration with the increasing exposure to heavy metals Cd. It can be seen that the levels of TNF-α protein concentrations increase from low to high, that is, in basin 1 <2 <3 <4 <5 <6. On the other hand, the results of the analysis of variance (Table 2) indicate that the concentrations of Cd show a significant influence on the concentration of TNF-α protein in the liver of D. setosum, with the value of F = 7.961 and p = 0.000. In addition, the results of Duncan test with a=0:01 (Table 3) show that there is a significant difference in the concentrations of heavy metals Cd of 0.0 (control), 1 .0, 3, 0, 6.0, 9.0, and 12.0 µg/L Cd on the concentration of TNF-α protein in the liver of D. setosum.
Table 1 The mean concentrations of TNF-α protein in 6 treatment basins of the concentration of Heavy Metal Cd
| Heavy metals Cd concentration at 6 treatment basins (µg/L) | The mean concentrations of TNF-α protein (ng/ml) | |
| Mean | Standard deviation | |
| 0.00 (Control) | 152.60 | 24.50 |
| 1.0 | 167.83 | 19.73 |
| 3.0 | 212.36 | 65.45 |
| 6.0 | 227.60 | 72.51 |
| 9.0 | 239.74 | 55.06 |
| 12.0 | 297.71 | 37.83 |
Table 2 The results of ANOVA, effect of heavy metal Cd concentration on the TNF-α protein concentration in the liver of D. setosum
| Source variance | Sum of squares | df | Mean square | Value F | Sig. (P-value) | |
| Concentration TNF-α protein | Between Groups | 96096.826 | 5 | 19219.365 | 7,691 | .000 |
| Within groups | 89964.584 | 36 | 2499.016 | |||
| Total | 186061.410 | 41 |
Table 3 Duncan test results of the ANOVA there is a significant effect of the concentration of heavy metals Cd on the concentration of TNF-α protein in the liver of Diadema setosum.
| Concentration of heavy metals Cd | The mean concentrations of TNF-α protein in liver of D. setosum | Notation Duncan |
| 0.00 (control) | 1.52595E2 | a |
| 1.0 | 1.67833E2 | a b |
| 3.0 | 2.12357E2 | a b |
| 6.0 | 2.27595E2 | a b c |
| 9.0 | 2.39738E2 | b c |
| 12.0 | 2.97714E2 | c |
The observed differences in the group of Cd concentration levels showed the effect of the exposure of Cd concentration levels on the concentrations of TNF-α protein in the liver of D. setosum. The concentration of Cd significantly increases the concentrations of TNF-α protein. This shows that the higher the concentration of Cd exposed can increase the concentration of TNF-α protein of the D. setosum. On the other hand, the expression of TNF-α protein based on the results of western blotting test (Figure 2) revealed that the TNF-α protein was colored by the rabbit anti-TNF-α antibody with the molecular weight of 33.57 kDa if the protein tape is the TNF-α protein. Figure 2 also showed increased expression with higher exposure to the concentration of heavy metals Cd.
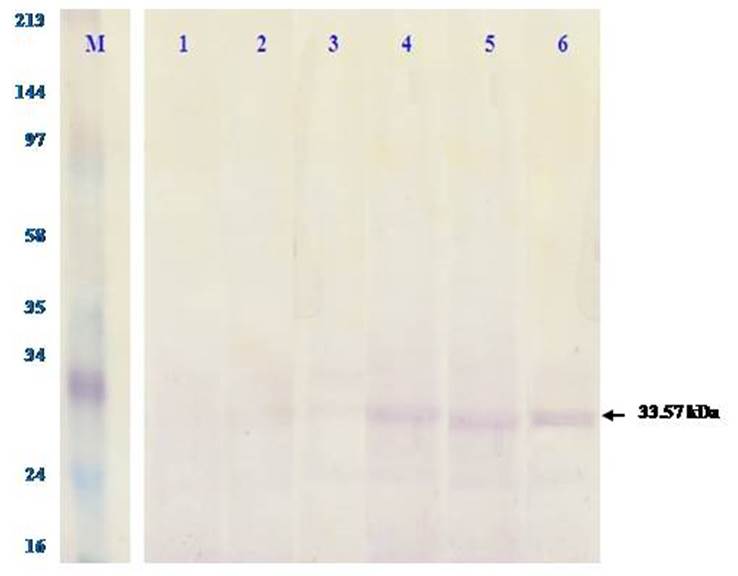
Figure 2 TNF-α protein expression by western blotting test on the liver of Diadema setosum at 6 treatment basins of heavy metal Cd concentration. The gel results of electrophoresis SDS-PAGE were performed western blotting test, that is, buy incubating it with monoclonal antibody rabbit anti-TNF-α as a primary antibody and secondary rabbit anti IgG biotin. Shown in the top row of the concentration levels at 6 treatment basins, the purplish-brown tape is TNF-α protein marked with arrows. M: marker, 1 to 6: Cd concentration at 6 treatment basins.
DISCUSSION
The concentration of Cd which became higher caused changes in cell tissue, and it was related to the protective strategy of D. setosum to combat stress due to the accumulation of Cd. This is evidenced by the growing activation of TNF-α protein of D. setosum with the higher concentrations of heavy metals Cd. It has been shown that the induction of Cd can trigger histopathological changes and cause disturbances in the lipid composition so that the macrophages will release TNF and increases oxidative stress in the liver organ (Faix et al., 2005; Smiri et al., 2010). Besides, cadmium can lead to osteoporosis, and it is generally deposited in the liver, kidney and damage the functioning of the brain, lungs (Ohta et al., 2000; Johri et al., 2010; Jaishankar et al., 2014).
In the present study, we found a remarkable increase in the TNF-α levels in the D. setosum tissue of the liver after Cd exposure. Quantitatively, the ELISA test (Table 1) shows that the concentrations of TNF-α protein increase along with the increasing exposure to the concentration of Cd. Semi quantitatively, the western blotting test (Fig. 2) shows that the expression of TNF-α protein increases along with the increasing exposure of the concentration of Cd. This means that the higher the concentration of TNF-α protein effected the thicker the tape showing the expression of the protein. The concentration of the TNF-α protein at the concentration (of 12 µg/L Cd) treatment was the highest, which was almost 5 times compared to the controls, with the highest level of expression. The description of the expression of TNF-α protein based on western blotting is following each concentration of the TNF-α protein recorded, and consistent with these findings is that the metals may affect the expression of TNF-α and, hence, disturb the cell metabolism of organisms (Marth et al., 2001). On the other hand, Cd exposure also significantly elevated the level of TNF-α in the intestine of microbiota (Liu et al., 2014). This means that Cd addition has an anti-proliferative and anti-inflammatory effect when associated with TNF-α stimulation. According to Goetz et al. (2004) and Min et al. (1998), TNF-α is involved in a variety of cellular activities as cell proliferation, differentiation, and cell death. In addition, the results of the analysis of variance (Table 2) showed that the concentrations of heavy metals Cd had a very significant effect (p <0.05) on the concentration of TNF-α protein in the liver organ of D. setosum, which is consistent with this finding is that lead metal causes a significant increase in the levels of TNF-α and caspase-3 in the liver when compared to controls (p <0.05) (Ponce-Canchihuamán et al., 2010). On the other hand, Cd exposure significantly increased plasma TNF-α and IL-6 levels in mice (p < 0.001) compared to normal mice (Alghasham et al., 2013). Besides, the increased oxidative stress causes interference with the permeability of the mitochondrial membrane, causing the cytochrome-c release from mitochondria to the cytoplasm so that it binds to Apaf-1, and activates the cascade, which causes cell death (Chu, 2013; Gulbins et al., 2003; Reed, 2000).
The concentration and expression of TNF-α protein as a molecular response was caused by the accumulation of heavy metal Cd. Kersshaw & Flier (2004) explained that TNF-α is a cytokine produced by macrophage cells and if the levels within the cell increase, they can be associated with the mechanism to suppress the oxidation process in the liver.
The exposure to Cd increases the production of TNF-α, and it is associated with the molecular response in the form of activation, expression, and concentrations of TNF-α D. setosum which caused by the exposure to Cd. Abbas et al., (2000) described that when the cells undergoing severe infection, TNF-α is produced in large quantities and can cause a pathological state. The results in the form of concentration and expression of TNF-α protein are a biomolecular response due to the exposure to heavy metals Cd. The response is a defensive mechanism against oxidative stress caused by exposure to heavy metals Cd.
The results of the study revealed that the higher the concentration of heavy metals Cd exposed can increase the macrophage cells produce TNF-α so that the concentration and expression of TNF-α protein in the cell. The increased TNF-α triggers the formation of NO (nitric oxide) by activating iNOS to convert L-arginine into 2 NO molecules and causes oxidative stress (Gulbins et al., 2000; Machida et al., 2006). This proves that the concentration and expression of TNF-α protein can be molecular markers of oxidative stress caused by the exposure to heavy metals Cd on D. setosum can be used as biomonitoring species of heavy metals Cd in the waters.
CONCLUSION
Biomolecular response in the form of activation, concentration, and the expression of TNF-α protein in the liver of D. setosum due to the exposure to heavy metals Cd showed that concentration of TNF-α protein increased when the concentration of heavy metals Cd exposed increased. The occurrence of the increased expression with higher exposure to the concentrations of heavy metals Cd was characterized by the ticker tape of TNF-α protein based on the results of western blotting test. The research results on the concentration and expression of TNF-α protein could serve as a biomonitoring model of the exposure to heavy metals Cd at the molecular level using D. setosum as biomonitoring species.











 nueva página del texto (beta)
nueva página del texto (beta)


