Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Hidrobiológica
versión impresa ISSN 0188-8897
Hidrobiológica vol.21 no.3 Ciudad de México sep./dic. 2011
Dynamics of seagrasses and associated algae in coral reef lagoons
Dinámica de los pastos marinos y macroalgas asociadas en lagunas arrecifales coralinas
Brigitta I. van Tussenbroek
Unidad Académica de Sistemas Arrecifales, Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México, Domicilio Conocido, Puerto Morelos, Quintana Roo, 77508. México. e-mail: vantuss@cmarl.unam.mx
Recibido: 17 de junio de 2011.
Aceptado: 14 de noviembre de 2011.
ABSTRACT
Seagrass communities in tropical reef systems are situated in a distinct environmental setting than other seagrass beds around the world: they are exposed to high light intensities and low nutrient concentrations in carbonate sediments. Little is known about the forces which determine the community dynamics in these systems. Here we review studies realized over the last two decades at Puerto Morelos reef lagoon, Mexican Caribbean (Latitude 20°52'N) which highlight the dynamics of seagrasses and rooted macroalgae at time distinct frames. Daily fluctuations in physiology, growth and release of gametes or pollen are driven by light and possibly by herbivore pressure. Growth and sexual reproduction of the seagrasses and algae show seasonal patterns driven by the annual solar cycle. The temporal dynamics of the algae are more intense than those of the seagrasses, possibly due to their respective clonal expansion strategies and rates. Sexual recruitment serves to colonize cleared areas and maintains high genetic variability within populations as was shown for Thalassia testudinum. Hurricanes have a small effect on the seagrass-algal community, selectively removing certain species, but the foundation species T. testudinum and the main producer of calcareous sand Halimeda incrassata are resistant to hurricanes and full community recovery occurs within 2-3 y. Gradual but persistent changes in community structure (relatively more investment in above-ground biomass) and composition (higher relative dominance of faster growing species) reveal increasing input of nutrients in this originally considered pristine and oligotrophic habitat.
Key words: Seagrasses, algae, coral reef lagoon.
RESUMEN
Las condiciones ambientales de las comunidades de pastos marinos en sistemas arrecifales tropicales son distintas a las de otras praderas del mundo. En este ambiente están expuestas a altas intensidades de luz y bajas concentraciones de nutrientes en sedimentos carbonatados. Se sabe poco sobre la dinámica de los pastos marinos y sus macroalgas asociadas en las lagunas arrecifales. En esta revisión bibliográfica, se resumen estudios que abarcaron la dinámica comunitaria de los pastos marinos y macroalgas durante las últimas dos décadas en Puerto Morelos, Caribe mexicano (Latitud 20º52'N). Las fluctuaciones diurnas en el crecimiento, la fisiología y la liberación de gametos o polen están influenciadas por el ciclo diurno de luz, y posiblemente por la presión de los herbívoros. El crecimiento y la reproducción sexual muestran patrones estacionales relacionados con los ciclos solares anuales. La dinámica temporal de las macroalgas fijas al substrato, es más intensa que la de los pastos marinos, lo cual se relacione con sus tasas y estrategias de expansión clonal. El reclutamiento sexual sirve para colonizar áreas perturbadas, y para mantener la variabilidad dentro de las poblaciones, tal y como se demostró para Thalassia testudinum. Los huracanes tienen un efecto pequeño sobre la comunidad pasto-alga, eliminando selectivamente ciertas especies, pero la especie dominante, T. testudinum y la principal productora de arena calcarea Halimeda incrassata, resisten los disturbios causados por los huracanes y la comunidad se recupera en 2-3 años. En las últimas dos décadas se han producido cambios paulatinos en la estructura (relativamente más biomasa de partes de plantas por arriba del sedimento) y composición de la comunidad (con una mayor dominancia relativa de las especies de crecimiento rápido), que son indicadores de incrementos en nutrientes en este sistema arrecifal que originalmente fue considerado como oligotrófico y prístino.
Palabras clave: Pastos marinos, algas, laguna arrecifal.
INTRODUCTION
Many tropical reef systems are composed of a mosaic of the interconnected mangrove forests, seagrass beds and coral reefs extending from the shoreline to the open ocean (Ogden & Gladfelter, 1983; Moberg & Folke, 1999). Coral reefs are the best-known and most widely studied of these three components, because of their beauty, high biodiversity, economic importance, and their high susceptibility to human-induced changes including global climate change (Hughes, 1994; Hoegh Guldberg et al., 2007). Fewer studies exist on the threatened mangrove forests (Valiela et al., 2001), but the extensive seagrass beds covering the sediments of the shallow reef lagoons between the crests of fringing or barrier reefs and the shoreline have received even less attention of the scientific community and general public, which Duarte et al. (2008) attributed to a reduced "charisma", greatly under par to its ecological importance.
Seagrasses are marine flowering plants with global distribution that form extensive meadows and are amongst the most productive ecosystems on Earth (Mateo et al., 2006), sustaining a diverse fauna (Green & Short, 2003) and large fishery industries (Gillanders, 2006). Amongst their ecosystem services are carbon sequestration (Mateo et al., 2006), cycling of nutrients (Romero et al., 2006), nursery habitat (Heck et al., 2003) and protection of coastal areas through stabilization of sediments (Madsen et al., 2001). Coral reef systems obtain their optimal development in the warm oligotrophic coastal waters. In these tropical or subtropical waters, in contrast with temperate ones, seasonal changes are less prominent or absent (Kain, 1989). But on the other hand, many tropical systems are subject to major periodical disturbances by hurricanes (cyclones or typhoons). The transparent waters provide a high light environment in contrast with many other more turbid coastal waters where the seagrasses are found (Dennison & Alberte, 1985; Zimmerman et al., 1991, Carruthers et al., 2002). Nutrient concentrations are generally low in reef systems (Carruthers et al., 2002) and the carbonate sediments of these systems, in contrast with terrigenous ones, tend to have lower availability of nutrients in their pore-water due to distinct biogeochemical interactions (Erftemeijer & Middelburg, 1993; Van Tussenbroek et al., 2006a). In the reef systems, multiple interactions exist between the interconnected mangrove forests, seagrass beds and coral reefs (Ogden & Gladfelter, 1983; Dahlgren & Marr, 2004). The coral reefs function as natural breakwaters, mitigating 75-90% of the wave energy under normal conditions (Brander et al., 2004), favouring the development of mangrove and seagrasses in calmer waters. The geological reefs and sediments can be considered as by-products of calcium carbonate produced by reef organisms of the reefs and the calcareous algae in the reef lagoons (Hubbard et al., 1990). The seagrass and mangrove communities support the existence of the reefs through the export of organic materials and protection of the reefs from direct terrestrial runoffs (Alongi & McKinnon, 2005) and providing nurseries for coral reef fishes and other reef fauna (Nagelkerken et al., 2000, 2001; Verweij et al., 2006; Unsworth et al., 2008). The biodiversity of seagrass beds near the reefs is high, supporting a rich flora (UNESCO, 1998; Van Elven et al., 2004) and fauna (e.g. Monroy-Velasquez, 2000; Eggleston et al., 2004). However, a comprehensive database of fauna associated to seagrass beds in reef systems is lacking, thus absolute comparison with other seagrass communities is not possible. A typical flora associated to seagrasses in reef lagoons is the abundant and diverse community of rooted (rhizophytic) algae (UNESCO 1998; Cruz-Palacios & Van Tussenbroek, 2005). Most of these algae are calcareous and they are important producers of calcareous sand (Van Tussenbroek & Van Dijk, 2007).
The distinct setting of seagrass communities in reef systems, compared to other coastal seagrass beds around the world, most likely has repercussions on the ecosystem dynamics and functioning. The aim of the present work is to highlight the dynamics of the rooted plants which inhabit the shallow reef lagoons at distinct time frames, in order to obtain a better understanding of the forces which determine their community structure. The seagrass community in Puerto Morelos reef system has received relatively more attention than many similar communities, due to the presence of various marine research institutes, resulting in a concentration of research efforts of resident and visiting scientists, and will therefore be used as a case study in this review.
PUERTO MORELOS REEF LAGOON: GENERAL DESCRIPTION
The Puerto Morelos reef system on the NE coast of the Yucatan Peninsula (Fig. 1a-c) is an open-ocean connected highly flushed coastal system with a water residence time of 3 h under normal wave conditions (Coronado et al., 2007). It is situated in the northern part of an extensive barrier-fringing reef complex that extends from Belize to the Strait of Yucatan. An extended fringing reef forms a 2-4 m deep reef lagoon, characterized by calcareous sand that is stabilized by the seagrass meadows. Inland wetlands are separated from the sea by a 2-3 m high and 100-200m broad sand bar (Fig. 1c). Mean surface-water temperatures vary between 25.1 °C in mid winter and 29.9 °C in late summer (monthly averaged temperature from 1993 until 2005 (Rodríguez-Martínez et al., 2010). From November until April cold fronts or "Nortes" produce decreases in air and seawater temperature. The average rainfall is 1060.6 mm y-1 (data from 1993 to 2004), without a clearly defined dry or wet season. During periods of exceptionally heavy rains, overflow of mangrove wetlands bring brackish tannin colored waters into the lagoon. The Yucatan limestone is extremely karstic and rainwater rapidly infiltrates into the aquifer, resulting in the absence of surface drainage or rivers and the water passes through an immense network of underground caves and channels to vent into the marine coastal areas through submarine springs ("Ojos de agua") and fissures (Fig. 1c). Thus, the lagoon environment is principally governed by marine conditions and the salinity varies little throughout the year (between 35.8 and 36.2%). The hurricane season extends from June to November, peaking between August and October. The main hurricanes directly affecting this area over the last 25 y were Gilbert (hurricane force 5, October 1988) and Wilma (hurricane force 4, October 2005) and other five hurricanes passed close to the study area, but did not have a major impact on the biota (Rodríguez-Martínez et al., 2010). The mean tidal range is very small (~17 cm) with a maximal variability in sea level of 32 cm (Coronado et al., 2007). The water in the lagoon is typically oligothrophic with low mean nitrite (0.06 μM), nitrate (13.9 μM) and phosphate (0.46 μM) concentrations (19821983, Merino & Otero (1991). Likewise, porewater nutrient concentrations within the Puerto Morelos Reef Lagoon (NH4+ 1.2-3.42 μM, DIN 2.8-5.1 μM and PO4-3 1.0-2.7 μM (Duarte et al., 1995; Carruthers et al., 2005) are low compared to global mean values for seagrass meadows of 86μM-NH4+ and 12 μM-PO4-3 (Hemminga & Duarte, 2000). Puerto Morelos was a small fishing village until the early 1980's, but since it has developed rapidly as tourism has become the main economic activity. Amongst the major attractions are the crystal clear seas, the white-sanded beaches and the reef ecosystem. The initial population of <1,000 inhabitants in the early 1980s developed into a rapidly growing community of ~15,000 inhabitants in 2008, and the number of hotel rooms experienced a exponential increase from ~400 rooms in 1998 to ~6,500 in 2008 (source INEGI, Mexico). In 1998, Puerto Morelos reef was declared a marine protected area and in average it receives ca. 200,000 visitors per year (http://www.conanp.gob.mx).
In this park, three seagrass species (Ruíz-Rentería et al., 1998) and 213 (Dreckmann et al., 1996) or 245 (Collado-Vides et al., 1998) macro-algal species have been found. Collado-Vides et al. (1998) discerned a significantly distinct phycoflora on the reef than in the reef lagoon, the sandy bottom of the reef lagoon being characterized by the dominance of green coenotic (siphonous) algae. The fauna assemblage in the reef system is diverse and in total 669 species of marine invertebrate and vertebrate fauna have been recorded (INE 2000). Specific diversity of various faunal groups in the reef lagoon have been studied, including copepod crustaceans (48 species, Álvarez-Cadena et al., 1998), decapod crustaceans (120 species, Monroy-Velázquez, 2000; Briones-Fourzán et al., 2003), molluscs (48 species, Briones-Fourzán et al., 2003), sea-urchins (11 species, Bravo-Tzompantzi, 1996) and fish (43 species, Álvarez-Guillén et al., 1986). The majority of these studies were not exhaustive and biodiversity of fauna in the reef lagoon most likely is much higher.
The rooted vegetation in the reef lagoon is largely comprised of the foundation species (dominant primary producer in terms of influence and abundance) Thalassia testudinum Bank ex König, accompanied by the seagrass Syringodium filiforme Kützing or Halodule wrightii Ascherson and the rhizophytic green algae. The densities of S. filiforme or H. wrightiiare usually small when growing intermixed with T. testudinum, but these seagrasses can attain high biomass (> 500 g dry m-2) in narrow coastal fringes (Gallegos et al. 1994; Van Tussenbroek 1994a; Ruíz-Rentería et al. 1998). Sexual reproduction is common for all seagrass species (Van Tussenbroek 1994b, unpublished data; Muhlia-Montero, 2011) and the calcified rhizophytic algae (Van Tussenbroek et al., 2006b, Van Tussenbroek & Barba-Santos, 2011).
Plants in the reef lagoon most likely do not suffer from low light stress throughout their principal depth distribution range. The waters are relatively clear (Light extinction coefficient Kd = 0.19-0.47 m-1, Enríquez & Pantoja-Reyes, 2005). On sunny summer days maximal irradiance at the water surface reaches ~2000 pmol quanta m-1 s-1 (Enríquez et al., 2002) and a typical T. testudinum canopy near the reef at a depth between 2.6-3.0 m receives max. ~800 and 1300 pmol quanta m-1 s-1 in the winter and summer, respectively (Van Tussenbroek, unpublished data). Carbonate coral sediments typically have small iron pools (Duarte et al., 1995) and artificial addition of iron to T. testudinum in Puerto Morelos reef lagoon resulted in higher concentrations of chlorophyll a in the leaves and increased leaf growth, suggesting that this element was limiting optimal development of the seagrass (Duarte et al., 1995). In this oligotrophic system the low leaf tissue concentrations of nitrogen (1.8-2.2 % of total dry weight) and phosphorus (0.13-0.19 % of total dry weight, Duarte et al., 1995; Gallegos et al., 1993; Carruthers et al., 2005) indicate deficiency of these elements according to the nutrient limits established by Duarte (1990).
RESULTS AND DISCUSSION
The continuous seagrass-algal vegetation extending along the kilometres-long stretch of coastline of Puerto Morelos reef lagoon may appear stable and rather uniform through time at first glance, but the structure of the community at a certain time and place is the result of dynamic processes. Knowledge concerning the dynamics at distinct time frames aid in understanding the underlying processes and driving forces of this apparently stable community.
Diurnal cycles. Fluctuations on a daily basis in Puerto Morelos reef lagoon are mostly determined by day-night cycles of irradiance because the tidal amplitude is very low. During at least a part of the day, the seagrass canopies are exposed to radiation intensity higher than that required for maximal rates of photosynthesis. Seagrasses have the ability to tolerate high light during solar noon by down-regulating their photosynthetic apparatus through dissipation of excess irradiance as heat (Larkum et al. 2006). In the absence of heat dissipation, the photosynthetic apparatus (particularly Photosystem II) is damaged (photoinhibition). But the light climate within the canopy of T. testudinum decreases through self-shading and the Kd within the canopy varies between 2.1 and 11.5 m-1 (Enríquez et al., 2002; Enríquez & Pantoja-Reyes, 2005). The leaves of T. testudinum have a relatively high elongation rate (~0.25-0.49 cm leaf-1 d-1; annual means 1990-1992, Van Tussenbroek 1995), thus a basal leaf section experiences a 2% daily increase in photonflux density as it elongates (Enríquez & Pantoja-Reyes, 2005). The photosynthetic capacity along the leaves changes accordingly, increasing at first from the base (near the sheath with low chlorophyll concentration) to a certain length (various cms) and then decreasing again towards the apex, where the leaves suffer from photodamage (Enríquez & Pantoja-Reyes, 2005). The apical sections of the leaves, exposed to the high irradiance, show reduced pigment content, leaf absorptance and reductions in the quantum yield of Photosystem II, which are indications of photoinhibition. Photosynthesis by the leaves supports aerobic respiration in the rhizomes and roots of the seagrasses, but the transport of oxygen often exceeds the respiratory requirements of the below-ground tissue and O2 is released from the roots into the sediments (Smith et al., 1984). Thus, apart from the deposition of organic matter, the seagrasses affect biogeoche-mical condition of the sediments through the release of O2 and exudates from the roots, stimulating a diverse bacterial community (Moriarty & Boon, 1989). This influence on biogeochemical processes in the sediments depends on the photosynthetic activity of the seagrasses, which is reflected in the redox potential of the sediments. The redox potential in a well-developed bed of T. testudinum in Puerto Morelos is on average 211 mV higher than adjacent sediments without vegetation and the oxidizing capacity of the seagrasses is highest at the depths with maximal below-round biomass (Enríquez et al., 2001). The redox potential in the rhizosphere declines 45 mV when the incoming irradiance is lowered to 27% of ambient light by experimental shading (Enríquez et al., 2001).
The seagrasses show a clear diurnal pattern in physiology (Smith et al., 1984) and growth (Williams & Dennison, 1990) related to photosynthetic activity, and likewise the calcification of the calcified rhizophytic algae only occurs during light when the algae are photosynthesizing (Van Tussenbroek & Van Dijk, 2007). But the rhizophytic algae may maintain high grow rates during the night, which has been shown for Caulerpa cupressoides by Williams and Dennison (1990). New segments of Halimeda incrassata (Ellis) Lamouroux are formed in the dark and obtain their full-grown size in ~24 h, calcifying during daylight (Hay et al., 1988; Multer, 1988, Van Tussenbroek & Van Dijk, 2007). This has also been observed for other Halimeda spp. (Hay et al., 1988; Drew & Abel, 1990; Larkum et al., 2011) and it is thought to be an adaptation to avoid consumption by juvenile parrotfish that are only active during daylight (Helfman, 1993; Bruggeman et al., 1994). The siphonous green algae also move their chloroplasts away from the periphery during the night when they become considerably paler. Such circadian migration of chloroplasts has been studied in Bryopsis sp. (Menzel & Schliwa, 1986), Caulerpa sp. (whitening of the tips of young growing lamina; Dawes & Barilotii, 1969) and the segments of an opuntoid species of Halimeda spp. (Drew & Abel, 1990). Drew and Abel (1990) hypothesized that the retraction of these vital organels from the plant surface may be a protection from mesograzers such as small green sacoglossan molluscs (Fig. 2) at times when they serve no purpose.
Remarkable are the processes of release of gametes of the rhizophytic calcareous algae or the pollen grains of the seagrass T. testudinum in synchrony with cycles of daylight. Rhizophytic algae of the genera Halimeda, Penicillus, Rhipocephalus and Udotea produce biflagellate gametes by transporting all cellular contents into terminally-located gametangia (Fig. 2). Clifton (1997) and Clifton and Clifton (1999) have reported that the release of gametes occurs early in the morning just after dawn and only lasts for 15-30 min in Panama, and we have observed similar timing of gamete release for the same genera in Puerto Morelos. After release of the gametes, only the white calcareous skeleton with empty gametangia remain (Fig. 2). Dehiscence of the male flowers of the seagrass T. testudinum is also synchronized but occurs at night when the ripe primordia open at dusk and release all pollen within 1-2 h (Fig. 2, Van Tussenbroek et al., 2008a, 2009). Daily synchronized release of gametes (of the algae) or pollen (of T. testudinum ) are typical for coral reef environments and may or may not occur in other habitats. Synchronization may be a mechanism of concentration of gametes (of the algae) or pollen (of T. testudinum), but alternatively may be a response to avoid consumption by nocturnally active zooplankton (Hay, 1997) or daily active parrotfish (Van Tussenbroek et al., 2008a), respectively.
Seasonal variability. The growth rates of the seagrass Thalassia testudinum and the alga Halimeda incrassata in Puerto Morelos reef lagoon vary seasonally (Fig. 3). Puerto Morelos, at <210 latitude, experiences a difference of ~2.5 h in daylight and ~5 0C temperature between winter minima and summer maxima (Fig. 3). Most likely light and the solar cycle are the driving forces of seasonal changes in the open-ocean connected reef lagoon and there are only few reports of solar-related seasonal fluctuation this far south (Van Tussenbroek 1995; Van Tussenbroek & Van Dijk, 2007). The magnitude of the seasonal variations in growth rates are reflected in minor fluctuations in the dry weight of the seagrass shoots or thalli (Van Tussenbroek 1994a, 1995; Van Tussenbroek & Van Dijk, 2007). But at the level of community, seasonal changes in standing crop are not obvious as is indicated by the following study.
From June 1990 until October 1991, the above-ground biomass of seagrasses and algae was determined at monthly or bimonthly intervals (and once at an interval of three months) at 11 stations from coast to reef following the protocol described by Van Tus-senbroek & Van Dijk (2007) for H. incrassata. The vegetation was divided into large morphological groups being: the seagrasses Thalassia testudinum and Syringodium filiforme, Halimeda spp., other calcareous algae [Penicillus spp., Rhipocephalus spp., Udotea spp.), Spongy algae (Avrainvillea spp., Cladocephalus spp.) and drift algal groups (mainly Lobophora spp. with less Laurencia spp.). The calcareous algae do not show significant seasonal variations (Table 1), and even though above-ground biomass of the seagrasses varies with time, maximal summer or minimal winter values are not obvious (Fig. 4, Table 1). At this level of community organisation, the effects on population densities of grazing, competition for light with drifting algal masses, storms, biotur-bation, natural recruitment or death may be of more influence to total standing crop than individual growth rates and weights of the plants. The drifting algal group is an exception, and these algae attain their maximal biomass during late Summer-Autumn and minimal abundance in the Winter-Spring (Fig. 4, Table 1). Rodríguez-Almázan (1997) reports that the cover of masses of the dominant drifting brown alga Lobophora variegata (Lamouroux) Womersly (ruffled form) is related to periods of heavy wave-action caused by during periods of northern winds in Winter and Spring or tropical storms (which did not occur in 1990-1991). The drifting algae may play an important role as a habitat to meiofauna, including the juvenile spiny lobster (Panulirus argus Latreille, 1804; Briones Fourzán & Lozano-Álvarez, 2001), thus variations in their abundance affect the diversity and abundance of associated fauna (Estrada-Olivo, 1999).
Both the seagrasses and the rhizophytic algae in the reef lagoon have a well-defined seasonal reproductive period. The reproductive season of the rhizophytic calcareous algae typically occurs from late winter to late summer, with clear peaks at the beginning (February) and the end (September) of the reproductive season (Van Tussenbroek and Van Dijk, 2007; Van Tussenbroek et al., 2006b). Flowering of T. testudinum occurs from March until May with fruit-set from June until September (Van Tussenbroek, 1994b) and H. wrightii and S.filiforme reproduce from February until April or May (Muhlia-Montero, 2011). The onset of the reproductive season of the seagrasses varies by 1 to 3 weeks between years (Van Tussenbroek, unpublished data) and local fluctuations in water temperature and light regime (intensity or photoperiod) are thought to be the main factors influencing the induction of the flowering season of seagrasses in general (McMillan ,1976, 1982; Inglis & Lincoln Smith, 1998; Walker et al., 2001). But in December 2005, 5-6 weeks after the passage of major hurricane Wilma, unusual mass-reproduction rhizophytic algae and fruit-set of S. filiforme were reported (Van Tussenbroek et al., 2006b). The reproductive cycle of T. testudinum also initiated unusually early (January) in the following year (Van Tussenbroek, unpublished data). Oceanographic equipment (Awac Acoustic Doppler Profiler, Nortec AS, Norway) located on the fore reef at 20 m depth registered a drop in seawater temperature from 29 to 19ºC within 12 h during the passage of the hurricane. The reduced temperature event lasted ~18 h, followed by rapid recovery to 25-26ºC once the hurricane had passed (Escalante-Mancera et al., 2009). Temperatures below 25ºC are not registered under usual conditions in this lagoon (Rodríguez-Martínez et al., 2010) and the thermal anomaly registered during Wilma, may have been the trigger for the unusual mass spawning of the green algae and premature flowering of the seagrasses. Reproductive thalli and reproductive shoots of seagrasses S. filiforme and T. testudinum have been found at all times of the year since, until 2010-2011 (Van Tussenbroek & Barba-Santos, 2011; Van Tussenbroek, unpublished data). After the unusual reproductive events following hurricane Wilma, Halime-da incrassata likely produced viable zygotes (Van Tussenbroek & Barba-Santos, 2011) and S. filiforme (Guzmán-Trampe, 2009) and T. testudinum(Troyo-Ballina, 2009) showed viable fruit- and seed-set, indicating that the environmental conditions are favourable for the development of reproductive structures throughout the year in this tropical lagoon. Thus, wether the seasonal patterns of sexual reproduction of the seagrasses and rhizophytic calcareous algae are a collective response to environmental cues or a strategy of reproductive assurance remains to be investigated.
Inter-annual stability. A remarkable trait of the rooted vegetation in the reef lagoon is that all plants are clonal. The vegetative clonal growth occurs through multiplication of the ramets on below-ground runners, which are rhizomes in the case of seagrasses (e.g. Gallegos et al., 1993, 1994; Van Tussenbroek et al., 2006a), the rhizoids of the rhizophytic calcareous algae (Van Tussenbroek & Barba-Santos, 2011). The stalked lamina of the spongy-like algae Avrainvillea and Cladocephalus spp. in contrast, arise from a large amorphous below-ground holdfast (Littler & Littler 1999), and the drift algae tend to multiply through fragmentation (Rodríguez-Almázan, 1997). The clonal growth allows for long-term permanence of the species, and the plant communities in the reef lagoon appear very constant throughout the year and between years when major disturbances are absent.
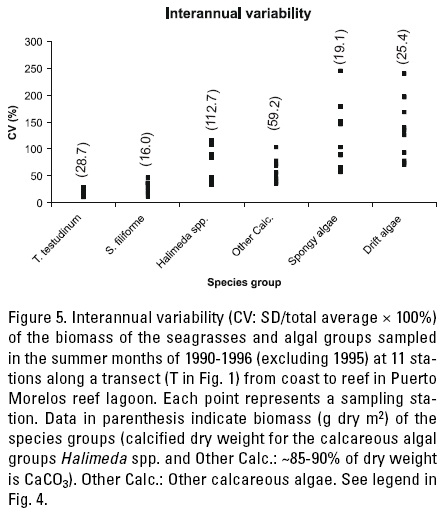
The ramets of the clonal seagrasses and rhizophytic algae are the units of interaction with the neighbours and the environment (Harper, 1985) and the population cycles of these ramets define the dynamics and structure of the community. Interannual stability of the populations of the seagrass and rooted algal species groups and drift algae from 1990 until 1996 (excluding 1995) has been determined along a transect from the coast to the reef, as described above for the seasonal variations in biomass. The interannual coefficients of variation (CV) were smallest for the seagrass species (especially T. testudinum), they were higher for the rhizophytic calcareous algae (Halimeda spp. and other calcareous algae) and they were exceptionally high for the spongy algae (mainly Avrainvillea spp.) and drift algae (Fig. 5). Seagrasses, in contrast to the clonal rhizophytic algae, maintain physical contact with the juvenile shoots and they show physiological integration (Tomasko & Dawes, 1989; Marbà et al., 2002). Such physiological integration results in low mortality of the newly formed shoots (Gallegos et al., 1994, Van Tussenbroek, 2002), which is an indication of support from the older ones (Van Tussenbroek, 2002). Seagrass populations, and especially those of T. testudinum, therefore, tend to be stable through time, in the absence of major disturbances (Fig. 5, Van Tussenbroek, 2002). The thalli (ramets) of H. incrassata, in contrast, have a relative high juvenile mortality (19%), most likely because the juvenile thalli do not maintain physical contact with the older ones (Van Tusssenbroek & Barba-Santos, 2011). The shoots (ramets) of the foundation seagrass T. testudinum attain a high age of 9 y (Gallegos et al., 1993) or > 20 y (Van Tussenbroek, 1994b) depending on the site within the lagoon. The ramets of the seagrasses H. wrightii(max. 1.2 y, Gallegos et al., 1994) and S. filiforme (max. 2.8 y, Gallegos et al., 1994) are much shorter and comparable with the maximal age of the thalli of the calcareous alga H. incrassata (2 y, Van Tussenbroek & Barba-Santos, 2011). Thus, the more intense dynamics of the ramets of the rhizophytic algae (in comparison with S. filiforme, Fig. 5) can thus most likely be attributed to the absence of physiological integration and not to their maximal life-span. Much less is known concerning the population dynamics of the (holdfasts of the) spongy algae, but Avrainvillea longicaulis (Kuetzing) Murray & Boodle can form full proliferations on a holdfast within 3 d through rapid siphon extension (Littler & Littler, 1999). These authors suggest that this rapid proliferation and blade abandonment is a strategy to get rid of epiphytes. In this context, it is interesting to note that few fronds of the drifting algae Lobophora variegata in Puerto Morelos reef lagoon persist after storms as epiphytes on the spongy algae followed by rapid population expansion (Rodríguez-Almázan, 1997).
Co-existence of the different seagrass species and rooted algae in the tropical seagrass systems, of which the reef systems are an example of excellence, has been an enigma, because in these oligotrophic systems where competition for nutrients is most severe (Williams, 1987, 1990; Davis & Fourqurean, 2001), the seagrass T. testudinum is competitively superior and should thus eventually exclude all other species in due course of succession through facilitation (Williams, 1990). But in oligotrophic reef systems the seagrass and algal species always grow intermixed without forming clear patches with intermediate succession species. Co-existence of early and late-colonizers in stable well-developed seagrass communities has been explained by niche differentiation (e.g. different rooting depths, Williams, 1990; Duarte et al., 1998) or differential resource exploitation during succession (Williams, 1990). But the temporal heterogeneity in clonal spread and resource capture by the rooted plants may also play a role in the maintenance of diversity of the seagrass-algal communities in the Caribbean reef lagoons. The late-colonizing and foundation seagrass T. testudinum has a centrifugal "guerrilla-type" clonal growth form (Marbà & Duarte, 1998; Valdivia- Carrillo, 2011), which is not very typical for a climax species. Lovett-Doust (1981) described the guerrilla growth form as an "opportunistic strategy of rapid spread and sampling of the environment" in contrast with the "phalanx strategy" which is characterized by "consolidation of scarce resources and slow radial spread". Clonal plants that exhibit guerrilla-type growth forms leave unoccupied zones with resources (Harper 1985). T. testudinum produces new shoots in the order of months (Gallegos et al., 1994; Van Tussenbroek, 1998). But rapid growing seagrasses such S. filiforme (Gallegos et al., 1994) or rhizophytic algae such as H. incrassata produce ramets in the order of weeks (Van Tussenbroek & Barba-Santos, 2011) and can thus quickly usurp the unoccupied resource-depletion zones left by T. testudinum, and thus allowing for co-existence of seagrasses and algae.
Clonal growth patterns determine the persistence, proliferation and distribution of the genets (sexual individuals that are originated from seeds or zygotes) in the populations, but the richness of the genets depends on sexual recruitment. T. testu-dinum at a coastal zone in Puerto Morelos reef lagoon consists of many single genotypes with a high clonal richness (R) of 0.79 (proportion of genetically unique individuals, max. = 1, Van Dijk & Van Tussenbroek, 2010). Between 85-90% of the fruits dehisced in situ followed by limited seed dispersal (<1-10 m, Van Dijk et al., 2009). Thus, at least for T. testudinum, sexual reproduction plays an important role in maintaining within population genetic variability. Sexual recruits are also important for long distance dispersal (Van Dijk et al., 2009) and for recolonization of denuded areas after major disturbance within the reef lagoon (see below).
Major disturbances 1: Hurricanes. The seagrass-algal community in Puerto Morelos reef lagoon is surprisingly resilient to the impact of hurricanes and most likely the reef barrier functions as a major protection structure. For example, during hurricane Wilma (October 2005, force 4, winds ~240 km h-1, duration ~63 h) waves attained a mean peak height of ~11 m outside the reef tract (Es-calante-Mancera et al., 2009), which were reduced to ~3 m within the reef lagoon (Hydrological and Meteorological Service, Unidad Academica Puerto Morelos, unpublished data). Major hurricane Gilbert (force 5) passed over Puerto Morelos in September 1988 with winds ~300 km h-1 (duration ~8 h) and its impact on T. testudinum was evaluated by means of retrospective analysis. Most populations of this seagrass suffered from either sediment burial or erosion (Marbà et al., 1994; Van Tussenbroek, 1994c), but depending on environmental settings the population sizes decreased only slightly, and when decreases were more substantial, recovery was initiated after 1-2 y (Van Tussenbroek, 1994c). Passage of hurricane Wilma (October 2005) over Puerto Morelos, Mexican Caribbean, caused significant decrease in population density of the seagrass Syringodium filiforme and rhizophytic calcareous algae, whereas populations of the seagrass Thalassia testudinum and Halimeda spp. were almost unaffected (Van Tussenbroek et al., 2008b). These impacts of the hurricane supported the results of earlier experiments simulating sediment removal and burial by hurricanes (Cruz-Palacios & Van Tussenbroek, 2005). Thus, apart from obvious impacts such as destruction of seagrass beds, hurricanes may also change the community structure of persistent beds through species-specific elimination. In the later case, recovery of the community may be relatively fast. Experimental burial and sediment removal resulted in a distinct community (Cruz-Pa-lacios & Van Tussenbroek, 2005), but the differences in species composition between the experimental and control plots was inexistent after three years (Fig. 6). Hurricane Wilma eliminated the lush vegetation of a coastal fringe of 10-60 m by deposition of a 0.5-1.0 m thick layer of sand. At present, primary succession in this zone follows classical pattern of succession through facilitation (Williams, 1990; Van Tussenbroek et al., 2006a). The early arrivers were seedlings of Halodule wrightii(after ~6 months) and Syringodium filiforme (after ~1 y, Van Tussenbroek, unpublished data) and rhizophytic algae. First recruits of H. incrassata in the denuded coastal area after hurricane Wilma were found 8 months after passage of the hurricane at an average density of 1.3 juvenile or young thalli per 100 m2, and were most likely from sexual origin (Van Tussenbroek & Barba-Santos, 2011).
In addition, a sudden drop in seawater temperature during hurricane Wilma (October 2005) most likely caused loss of synchronization in sexual reproduction of the seagrasses and algae (see section Seasonal variability). This sudden decrease in temperature was due to upwelling during the hurricane which brought sub-superficial waters from deeper zones to the surface in order to restore normal sea levels and hydraulic balance (Escalante et al., 2009). The colder and nutrient-enriched water likely replenished Puerto Morelos reef lagoon with essential trace metals and T. testudinum leaf tissue concentrations of Zn increased from 8.3 to 41.9 pg dry g-1 and concentrations of Fe rose from 24.0 to 78.1 pg dry g-1 (Whelan et al., 2011).
Major disturbances 2: Eutrophication. Over the last three decades, the urban development of Puerto Morelos has increased considerably, with a ten-fold increase in population and establishment of several major hotel complexes. Potential sources of nutrients into the sea are hotels (e.g. from fertilizers for the golf-courses), intensive farming, rubbish disposal and residential sources. The village of Puerto Morelos has no central sewer system and wastes are discharged into septic tanks or directly into the aquifer (see also Fig. 1). The reef system thrives under low natural nutrient concentrations and eutrophication may cause drastic changes in this coastal ecosystem. The seagrass-algal community in the reef lagoon has undergone gradual, almost imperceptible changes over the last decades (Rodríguez-Martínez et al., 2010). Nutrient availability to seagrasses may be derived from C:N:P ratios in the leaf tissues (Duarte, 1992; Fourqurean et al., 1992). Carbonate systems, such as Puerto Morelos reef lagoon, are phosphorus limited (Short et al., 1990; Fourqurean et al., 1992; Carruthers et al., 2005) and the C:P ratio contents in T. testudinum leaves sampled at several sites within the reef lagoon gradually decreased from 1991 to 2005 from average 731:1 to 564:1 (N 4 stations) respectively, indicating increasing availability of phosphorus (Rodríguez-Martínez et al., 2010). The seagrasses T. testudinum and S. filiforme showed a gradual shift to relatively higher biomass invested in above-ground tissues (Rodríguez Martínez et al., 2010), which is consistent with an increasing nutrient load (Zieman & Wetzel, 1980; Erftemeijer & Middelburg, 1993). The total above-ground biomass of the community increased from 1991 to 2005, whereas that of T. testudinum remained constant (Rodríguez-Martínez et al., 2010). Thus, the relative biomass of T. testudinum decreased during that period whereas that of the faster-growing S. filiforme and fleshy and drift macroalgae increased, although these tendencies varied within and between the seagrass and algal groups according to environmental setting and total community biomass (Fig. 7). The calcareous rhizophytic algae, however, showed a distinct tendency with nutrient increase than the other algal groups and their relative abundance decreased when conditions became more eutrophic (Fig. 7). A possible reason may be that at higher densities, light extinction in between the canopy increases (Enríquez & Pantoja-Reyes, 2005) and rhizophytic algae, which are smaller than the seagrass leaves (Cruz-Palacios & Van Tussenbroek, 2005), are likely overshadowed by the seagrasses. Only at the extreme oligotrophic back-reef an increase in the relative abundance of the calcareous increase is observed (Fig. 7). These changes in the community structure also demonstrate that populations of the macroalgae (and fast growing seagrass species) are more dynamic than those of the dominant but slow-growing T. testudinum (see section inter-annual stability). Thus, at a community scale, the algae (and possibly also the faster growing seagrass species) may be better indicators of change than the foundation seagrass T. testudinum. This gradual shift in community structure is consistent with the relative dominance model which predicts that the competitive superiority tends to favor species with faster relative growth rates at increasing nutrient availability (Fourqurean et al., 1995; Rose & Dawes, 1999; Fourqurean & Rutten, 2003). Thus, there are strong indications that Puerto Morelos reef lagoon is slowly changing from a pristine to a more eutrophic system.
Dynamics of seagrass communities in tropical reef lagoons in general. The present state and dynamics of the seagrass community at Puerto Morelos reef lagoon are in many ways representative of other seagrass systems from similar areas, but several major differences in environmental settings and dynamics are obvious. Other reef systems may receive intermittent nutrient inputs from terrestrial runoff (Gabric & Bell, 1993), or upwelling possibly in combination with tides (Wolanski et al., 1988; Smith et al., 2004). Thus, in many tropical reef systems at similar latitudes or more towards the tropics, other factors than incoming radiation may mask the signal of solar radiation and intra-annual changes in productivity can be forced by upwelling events or increased nutrient input and turbidity during rainy seasons (Day et al., 1982; Flores-Verdugo et al.,1988; Carrurthers et al., 2002). Tidal differences are generally small in the tropical Atlantic, but they are more obvious in many places in the Indo-Pacific and the time of the day when the beds fall dry is of great influence for seagrass biomass and functioning (Erftemeijer & Herman, 1994; Stapel et al., 1997) and the timing of sexual reproduction (Pettitt, 1980, 1984; Cox & Knox, 1989; Cox, 1991). Elsewhere, grazing by sea urchins may play an important role in community structure of tropical beds (Klumpp et al., 1993; Valentine et al., 2000) and excessive grazing by fish may leave areas devoid of seagrasses near the reef (Randall, 1965). These processes are not obvious in Puerto Morelos. Tropical seagrass beds are feeding grounds for marine turtles (Williams, 1988; Moran & Bjorndal, 2005; Murdoch et al., 2007), manatees or dugongs (Preen, 1995; Marshall et al., 2000). But similar to most tropical systems (Jackson, 1997; Valentine & Duffy, 2006), the abundance of these large herbivores are greatly reduced in Puerto Morelos at present. Excessive grazing of sea turtles may cause total collapse of seagrass beds (Fourqurean et al. 2010) and the actual virtual absence of the large herbivores may be unprecedented for the tropical seagrass beds (Jackson, 1997; Valentine & Duffy, 2006). The structure of what we call "pristine" beds at the moment may have been completely different before these animals were hunted down by humans.
On a world-wide scale, seagrass communities suffer from reduction in coverage on a global scale due to human-impacts such as eutrophication, siltation, fisheries (resulting in modification of food webs), introduced invasive algae, dredging, and land reclamation (Short & Wyllie-Echeveria, 1996; Duarte, 2002; Orth et al., 2006; Waycott et al., 2009; Short et al., 2011). But much of the current knowledge on seagrass communities is based on studies in coastal bays (mostly temperate and subtropical), estuaries or other wave-protected areas (e.g. most data used by Waycott et al., 2009 on world-wide loss of seagrass beds are from studies in temperate regions) and less is known concerning the actual state of the seagrasses that occupy the reef lagoons (see also Carruthers et al., 2002). Seagrass communities in reef systems throughout the Greater Caribbean have been monitored as part of the Caribbean Coastal Marine Productivity (CARICOMP) Program, which is a mangrove, seagrass and coral monitoring network initiated in 1993 (UNESCO 1998). Throughout this area, natural disturbances such as hurricanes, earth quakes or excessive rainfalls cause declines in seagrass cover, but the communities usually recover within 6 mo to 3 y (CARICOMP 2004). However, after > decade of observations, there is a general agreement that many coastal systems of this monitoring network have undergone changes for the worse due to increased human pressure. Increased nutrient loading from sewage or sedimentation are amongst the major causes (CARICOMP 2004) and in this respect Puerto Morelos reef lagoon, regrettably, shares the fate of many other Caribbean coastal reef systems. Understanding the dynamics of the foundation and ecologically important species which occupy these diverse coastal reef lagoons may provide accurate indicators of change for monitoring purposes and thereby providing a tool in mitigating the effects of the increasing human presence along the coasts of the greater Caribbean.
ACKNOWLEDGEMENTS
The support and collaboration of M. en C. Guadalupe Barba-Santos and all students which visited our laboratory over the past 20 years are greatly appreciated.
REFERENCES
Alongi, D. M. & A. D. McKinnon. 2005. The cycling and fate of terrestrially-derived sediments and nutrients in the coastal zone of the Great Barrier Reef Shelf. Marine Pollution Bulletin 51: 239-252. [ Links ]
Álvarez-Cadena, J. N., E. Suárez-Morales & R. Gasca. 1998. Copepod assemblages from a reef-related environment in the Mexican Caribbean Sea. Crustaceana 71: 411-433. [ Links ]
Álvarez-Guillén, H., Ma de la C. García-Abad, M. Tapia-García, G. J. Villa-lobos-Zapata & A. Yañez-Arancibia. 1986. Prospección ictioecológica en la zona de pastos marinos de la laguna arrecifal en Puerto Morelos, Quintana Roo, verano 1984. Anales del Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México 13: 317-336. [ Links ]
Bravo-Tzompantzi, D. 1996. Contribución al conocimiento de los equinoideos (Echinodermata: Echinoidea) del Caribe mexicano: Puerto Morelos, Quintana Roo, México. Tesis de Licenciatura, Escuela de Biología, Universidad de Puebla, México, 44 p. [ Links ]
Brander, R. W., P. Kench & D. Hart. 2004. Spatial and temporal variations in wave characteristics across a reef platform, Warraber Island, Torres Strait, Australia. Marine Geology 207: 169-184. [ Links ]
Briones-Fourzán, P. & E. Lozano-Álvarez. 2001. The importante of Lobophora variegata (Phaeophyta: Dictyoales) as a habitat for small juveniles of Panulirus argus (Decapada: Palinuridae) in a tropical reef lagoon. Bulletin of Marine Science 68: 207-219. [ Links ]
Briones-Fourzán, P., V. Castañeda-Fernández de Lara, E. Lozano-Álvarez & J. Estrada-Olivo. 2003. Feeding ecology of the three juvenile phases of the spiny lobster Panulirus argus in a tropical ref. lagoon. Marine Biology 142: 855-865. [ Links ]
Bruggemann, J. H., M. J. H. van Oppen, A. M. Breeman. 1994. Foraging by the stoplight parrotfish Sparisoma viride. II. Intake and assimilation of food, protein and energy. Marine Ecology Progress Series 106: 57-71. [ Links ]
CARICOMP. 2004. Caribbean Coastal Marine Productivity Program: 1993-2003. CARICOMP. 88 p. [ Links ]
Carruthers, T. J. B., B. I. van Tussenbroek & W. C. Dennison. 2005. Influence of submarine springs and wastewater on nutrient dynamics of Caribbean seagrass meadows. Estuarine Coastal and Shelf Science 64: 191-199. [ Links ]
Carruthers, T. J. B., W. C. Dennison, B. J. Longstaff, M. Waycott, E. G. Abal, L. J. McKenzie & W. J. Lee Long. 2002. Seagrass habitats of Northeast Australia: models of key processes and controls. Bulletin of Marine Science 71: 1153-1169. [ Links ]
Clifton, K. E. 1997. Mass spawning by green algae on coral reefs. Science 275:116-118. [ Links ]
Clifton, K. E. & L. M. Clifton. 1999. The phenology of sexual reproduction by green algae (Bryopsidales) on Caribbean coral reefs. Journal of Phycology 35: 24-34. [ Links ]
Collado-Vides, L., I. Ortegón-Aznár, A. Sentíes-Granados, L. Comba-Barre-ra & J. González-González. 1998. Macroalgae of Puerto Morelos reef system, Mexican Caribbean. Hidrobiológica 8: 133-143. [ Links ]
Coronado, C., J. Candela, R. Iglesias-Prieto, J. Sheinbaum, M. López & F. J. Ocampo-Torres. 2007. On the circulation in the Puerto Morelos fringing reef lagoon. Coral Reefs 26: 149-163. [ Links ]
Cox, P. A. 1991. Abiotic pollination: an evolutionary escape for animal-pollinated angiosperms. Philosophical Transactions of the Royal Society London. [ Links ] Biological Sciences 333: 217-224. [ Links ]
Cox, P. A. & R. B. Knox. 1989. Two-dimensional pollination in hydrophylous plants: convergent evolution in the genera Halodule (Cymodoceaceae), Halophila (Hydrocharitaceae), Ruppia (Ruppiaceae), and Lepilaena (Zannichelliaceae). American Journal of Botany 76: 164-175. [ Links ]
Cruz-Palacios, V. & B. I. van Tussenbroek. 2005. Simulation of hurricanelike disturbances on a Caribbean seagrass bed. Journal of Experimental Marine Biology and Ecology 324: 44-60. [ Links ]
Dahlgren, C. & J. Marr. 2004. Back reef systems: important but overlooked components of tropical marine ecosystems. Bulletin of Marine Science 75: 145-152. [ Links ]
Davis B. C. & J. W. Fourqurean. 2001. Competition between the tropical alga, Halimeda incrassata, and the seagrass, Thalassia testudinum. Aquatic Botany 71: 217-232. [ Links ]
Dawes, C. J. & D. C. Barilotti. 1969. Cystoplasmic organisation and rhythmic streaming in growing blades of Caulerpa prolifera. American Journal of Botany 56: 8-15. [ Links ]
Day, J. W., R. H. Day, M. T. Barreiro, F. Ley-Lou & C. J. Madden. 1982. Primary production in the Laguna de Terminos, a tropical estuary in the southern Gulf of Mexico. Oeanological Acta Proceedings International Symposium on coastal lagoons, SCOR/IABO/UNESCO, Bordeaux, France, 8-14 September, 1981: 269-276. [ Links ]
Dennison, W. C. & R. S. Alberte. 1985. Role of daily light period in the depth distribution of Zostera marina (eelgrass). Marine Ecology Progress Series 25: 51-61. [ Links ]
Dreckmann, K. M., I. Stout & A. Sentíes Granados. 1996. Lista actualizada de las algas marinas bentónicas de Puerto Morelos, Quintana Roo, Caribe mexicano. Polibotánica 3: 1-17. [ Links ]
Drew, E. A. & K. M. Abel. 1990. Studies on Halimeda III. A daily cycle of chloroplast migration within segments. Botánica Marina 33: 31-45. [ Links ]
Duarte, C. M. 1990. Seagrass nutrient content. Marine Ecology Progress Series 67: 201-207. [ Links ]
Duarte, C. M. 1992. Nutrient concentrations of aquatic plants: patterns across species. Limnology and Oceanography 37: 882-889. [ Links ]
Duarte, C. M. 2002. The future of seagrass meadows. Environmental Conservation 29: 192-206. [ Links ]
Duarte, C. M., M. Merino & M. Gallegos. 1995. Evidence of iron deficiency in seagrasses growing above carbonate sediments. Limnology and Oceanography 40: 1153-1158. [ Links ]
Duarte, C. M., M. Merino, N. S. R. Agawin, J. Uri, M. D. Fortes, M. E. Gallegos, N. Marbá & M. A. Hemminga. 1998. Root production and below-ground seagrass biomass. Marine Ecology Progress Series 171: 97-108. [ Links ]
Duarte, C. M., W. C. Dennison & R. J. W. Orth. 2008. The charisma of coastal ecosystems: Addressing the imbalance. Estuaries and Coasts 31: 233-238. [ Links ]
Eggleston, D. B., C. P. Dahlgren & E. G. Johnson. 2004. Fish density, diversity and size-structure within multiple back reef habitats of Key West National Wildlife refuge. Bulletin of Marine Science 75: 175-204. [ Links ]
Enríquez S., N. Marbá, C. M. Duarte, B. I. van Tussenbroek & G. Reyes-Zavala. 2001. Effects of seagrass Thalassia testudinum on sediment redox. Marine Ecology Progress Series 219: 149-158. [ Links ]
Enríquez, S., M. Merino & R. Iglesias Prieto. 2002. Variations in the photosynthetic performance along the leaves of the tropical seagrass Thalassia testudinum. Marine Biology 140: 891-900. [ Links ]
Enríquez, S. & N. I. Pantoja-Reyes. 2005. Form-function analysis of the effect of canopy morphology on leaf self-shading in the seagrass Thalassia testudinum. Oecologia 145: 235-243. [ Links ]
Erftemeijer, P. L. A. & P. M. J. Herman. 1994. Seasonal changes in environmental variables, biomass, production and nutrient contents in two contrasting tropical intertidal seagrass beds in South Sulawesi, Indonesia. Oecologia 99: 45-59. [ Links ]
Erftemeijer, P. L. A. & J. J. Middelburg. 1993. Sediment-nutrient interactions in tropical seagrass beds: a comparison between a terrigenous and carbonate sedimentary environment in South Sulawesi (Indonesia). Marine Ecology Progress Series 102: 187-198. [ Links ]
Escalante-Mancera, E., R. Silva-Casarín, E. Mendoza-Baldwin, I. Mariño-Tapia & F. Ruíz-Rentería. 2009. Análisis de la variación del nivel del mar y de las corrientes marinas inducidas por el huracán Wilma frente a Puerto Morelos, Quintana Roo, México. Ingeniería hidráulica en México 24: 111-126. [ Links ]
Estrada-Olivo, J. J. 1999. Riqueza específica y abundancia de la macro-fauna bentica asociada a pastizales marinos en la laguna arrecifal de Puerto Morelos, Quintana Roo, México. Tesis de Licenciatura, Facultad de Ciencias, Universidad Nacional Autónoma de México, 67 p. [ Links ]
Flores-Verdugo, F., L. Mee & R. Briceño-Dueñas. 1988. Phytoplankton production and seasonal biomass variation of seagrass Ruppia maritima L., in a tropical Mexican lagoon with an ephemeral inlet. Estuaries 11: 51-56. [ Links ]
Fourqurean, J. W. & L. M. Rutten. 2003. Competing goals of spatial and temporal resolution: monitoring seagrass ecosystem on a regional scale. In: Busch D.E. & J.C. Trexler (Eds.). Monitoring ecosystems. Island Press, Washington D.C. pp. 257-288. [ Links ]
Fourqurean, J. W., J. C. Zieman & G. V. N. Powell. 1992. Phosphorus limitation of primary production in Florida Bay: Evidence from C:P:N ratios of the dominant seagrass Thalassia testudinum. Limnology and Oceanography 37: 162-171. [ Links ]
Fourqurean, J. W., G. V. N. Powell, W. J Kenworthy & J. C. Zieman. 1995. The effects of long-term manipulation of nutrient supply on competition between seagrasses Thalassia testudinum and Halodule wrightii in Florida Bay. Oikos 72: 349-358. [ Links ]
Fourqurean, J. W., S. Manuel, K. A. Coates, W. J. Kenworthy & S. R. Smith. 2010. Effects of excluding sea turtle herbivores from a seagrass bed: Overgrazing may have led to loss of seagrass meadows in Bermuda. Marine Ecology Progress Series 419: 223-232. [ Links ]
Gabric, A. J. & P. R. F. Bell. 1993. Review of the effects of non-point nutrient loading on coastal ecosystems. Australian Journal of Marine and Freshwater Research 44: 261-283. [ Links ]
Gallegos, M. E., M. Merino, N. Marbá & C. M. Duarte. 1993. Biomass and dynamics of Thalassia testudinum in the Mexican Caribbean: elucidating rhizome growth. Marine Ecology Progress Series 95: 185-192. [ Links ]
Gallegos, M. E., M. Merino, A. Rodríguez, N. Marbá & C. M. Duarte. 1994. Growth patterns and demography of pioneer Caribbean seagrasses Halodule wrightiiand Syringodium filiforme. Marine Ecology Progress Series 109: 99-104. [ Links ]
Gillanders, B. M. 2006. Seagrasses, Fish, and fisheries. In: Larkum, A. W. D., R. J. Orth & C. M. Duarte (Eds.). Seagrasses: Biology, Ecology and Conservation. Springer, The Netherlands. pp. 503-536 [ Links ]
Green, E. P. & F. T. Short. 2003. World Atlas of seagrasses. Prepared by the UNEP World Conservation Monitoring Centre. University of California Press, Berkeley, USA, 298 p. [ Links ]
Guzmán-Trampe, S. 2009. Desarrollo de fruto y semilla, banco de semillas y germinación de Syringodium filiforme. Tesis de Licenciatura, Facultad de Ciencias, Universidad Nacional Autónoma de México, 54p. [ Links ]
Harper, J. L. 1985. Modules, branches and capture of resources. In: Jackson J. B. C., L. W. Buss L. W., & R. E. Cook (Eds). Population biology and evolution of clonal organisms, Yale University Press, New Haven and London, pp. 1-34. [ Links ]
Hay, M. 1997. Synchronous spawning-When timing is everything. Science 21:1080-1081. [ Links ]
Hay, M. E., V. J. Paul, S. M. Lewis, K. Gustafson, J. Tucker & R. N. Trindell. 1988. Can tropical seaweeds reduce herbivory by growing at night? Diel patterns of growth, nitrogen content, herbivory, and chemical versus morphological defense. Oecologia 75: 233-245. [ Links ]
Heck, K. L., Jr., G. Hays & R. J. Orth. 2003. Critical evaluation of the nursery role hypothesis for seagrass meadows. Marine Ecology Progress Series 253: 123-136. [ Links ]
Helfman, G. S. 1993. Fish behaviour by day, night and twilight, In: Pitcher, T. J. (Ed.). Behaviour of teleost fishes, 2nd Edition, London. pp. 479-512. [ Links ]
Hemminga, M. A. & C. M. Duarte. 2000. Seagrass Ecology. Chapter 4: Light, carbon and nutrients. Cambridge University Press, Cambridge. pp. 99-145. [ Links ]
Hoegh-Guldberg, 0., P. J. Mumby, A. J. Hooten, R. S. Steneck, P. Greenfield, E. Gomez, C. D. Harvell, P. F. Sale, A. J. Edwards, K. Caldeira, N. Knowlton, C. M. Eakin, R. Iglesias-Prieto, N. Muthiga, R. H. Bradbury, A. Dubi & M. E. Hatziolos. 2007. Coral reefs under rapid climate change and ocean acidification. Science 318: 1737-1742. [ Links ]
Hubbard, D. K., A. I. Miller & D. Scaturo. 1990. Production and cycling of calcium carbonate in a shelf-edge reef system (St. Croix, U.S. Virgin islands): Applications to the nature of reef systems in the fossil record. Journal of Sedimentary Petrology 60: 335-360. [ Links ]
Hughes, T. P. 1994. Catastrophes, phase shifts and large-scale degradation of a Caribbean coral reef. Science 265: 1547-151. [ Links ]
Instituto Nacional de Ecología (INE). 2000. Comunidad de Puerto Morelos, Quintana Roo. Programa de Manejo del Parque Nacional Arrecife de Puerto Morelos. Instituto Nacional de Ecología, México, DF 222 p. [ Links ]
Inglis, G. J. & M. P. Lincoln Smith. 1998. Synchronous flowering of estuarine seagrass meadows. Aquatic Botany 60: 37-48. [ Links ]
Jackson, J. B. C. 1997. Reefs since Columbus. Proceedings of the 8th International Coral Reef Symposium 1: 97-106 [ Links ]
Kain J. M. 1989. The seasons in the subtidal. British Phycological Journal 24: 203-215. [ Links ]
Klumpp, D. W., Salita-Espinosa, J. T. & M. D. Fortes. 1993. Feeding ecology and trophic role of sea urchins in a tropical seagrass community. Aquatic Botany 45: 205-230. [ Links ]
Larkum, A. W. D., E. A. Drew & P. J. Ralph. 2006. Photosynthesis and metabolism in seagrass at the cellular level. In: Larkum, A. W. D., R. J. Orth & C. M. Duarte (Eds.). Seagrasses: Biology, Ecology and Conservation. Springer, The Netherlands, pp. 323-345. [ Links ]
Larkum A. W. D., Salih A. & M. Kuhl. 2011. Rapid Mass Movement of Chloroplasts during Segment Formation of the Calcifying Siphonalean Green Alga, Halimeda macroloba. PLoS ONE6 (7): e20841. [ Links ]
Littler, M. M. & D. S. Littler. 1999. Blade abandonment/proliferation: A novel mechanism for rapid control in marine macrophytes. Ecology 80: 1736-1746. [ Links ]
Lovett-Doust, L. 1981. Population dynamics and local specialization in a clonal perennial (Ranunculus repens): I. The dynamics of ramets in contrasting habitats. Journal of Ecology 69: 743-755. [ Links ]
Madsen, J. D., P. A. Chambers, W. F. James, E. W. Koch & D. F. Westlake. 2001. The interaction between water movement, sediment dynamics and submersed macrophytes. Hydrobiologia 444: 71-84. [ Links ]
Marbá N & C. M. Duarte. 1998. Rhizome elongation and seagrass clonal growth. Marine Ecology Progress Series 174: 269-280. [ Links ]
Marbá, N., M. E. Gallegos, M. Merino & C. M. Duarte. 1994. Vertical growth of Thalassia testudinum: seasonal and interannual variability. Aquatic Botany 47: 1-11. [ Links ]
Marbá, N., M. A. Hemminga, M. A. Mateo, C. M. Duarte, Y. E. M. Mass, J. Terrados & E. Gacia. 2002. Carbon and nitrogen translocation between seagrass ramets.Marine Ecology Progress Series 226: 287-300. [ Links ]
Marshall, C. D., P. S. Kubilis, G. D. Huth, V. M. Edmonds, D. L. Halin & R. L. Reep. 2000. Food-handling ability and feeding-cycle length of manatees feeding on several species of aquatic plants. Journal of Mammalogy 81: 649-658. [ Links ]
Mateo, M. A., J. Cebrián, K. Dunton & T. Mutchler. 2006. Carbon flux in seagrass ecosystems. In: (Larkum, A. W. D., R. J. Orth, C. M. Duarte (eds.), Seagrasses: Biology, Ecology and Conservation. Springer, The Netherlands, pp. 159-192. [ Links ]
McMillan, C. 1976.Experimental studies on flowering and reproduction in seagrasses. Aquatic Botany 2: 87-92. [ Links ]
McMillan, C. 1982. Reproductive physiology of tropical seagrasses. Aquatic Botany 14: 245-258. [ Links ]
Menzel, D. & M. Scliwa. 1986. Motility in the siphonous green alga Bryopsis I. Spatial organisation of the cytoskeleton and organelle movements. European Journal of Cell Biology 40: 275-285. [ Links ]
Merino, M. & L. Otero. 1991. Atlas ambiental costero, Puerto Morelos-Quintana Roo. Ferrandiz SA, Mexico, DF, 80 p. [ Links ]
Moberg, F. E. & C. Folke. 1999. Ecological goods and services of coral reef ecosystems. Ecological Economics 29: 215-233. [ Links ]
Monroy-Velázquez, L. V. 2000. Variaciones en la composición y abundancia en la fauna de decapados asociados a pastizales marinos en el Caribe Mexicano. Tesis de Maestría, Programa de Posgrado en Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México, 80 p. [ Links ]
Moriarty, D. J. W. & P. I. Boon. 1989. Interactions of seagrasses with sediment and water. In: Larkum, A. W. D., A. J. McComb & S. A. Shepherd (Eds.). Biology of seagrasses. A treatise on the biology of seagrasses with special reference to the Australian region. Elsevier, The Netherlands. pp. 500-535. [ Links ]
Moran, K. L. & K. A. Bjorndal. 2005. Simulated green turtle grazing affects structure and productivity of seagrass pastures. Marine Ecology Progress Series 305: 235-247. [ Links ]
Muhlia-Montero, M. 2011. La herbivoría de las flores masculinas de 3 especies de pastos marinos en la laguna arecifal de Puerto Morelos. Tesis de Maestría, Programa de Posgrado en Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México, México, 139 p. [ Links ]
Multer. 1988. Growth rate, utrastructure and sediment contribution of Halimeda incrassata and Halimeda monile, Nonsuch and Falmouth Bays, Antigua, W.I. Coral Reefs 6: 179-186. [ Links ]
Murdoch, T. J. T, A. F. Glasspool, M. Outerbridge, J. Ward, S. Manuel, J. Gray, A. Nash, K. A. Coates, J. Pitt, J. W Fourqurean, P. A. Barnes, M. Vierros, K. Holzer & S. R. Smith. 2007. Large-scale decline in offshore seagrass meadows in Bermuda. Marine Ecology Progress Series 339: 123-130. [ Links ]
Nagelkerken, I., G. van der Velde, M. W. Gorissen, G. J. Meijer, T. van't Hof & C. den Hartog. 2000. Importance of mangroves, seagrass beds and the shallow coral ref. as a nursery for important coral reef fishes, using a visual census technique. Estuaries, Coastal and Shelf Science 51: 31-44. [ Links ]
Nagelkerken, I., S. Kleijnen, T. Klop, R. A. C. J. van den Brand, E. Cocheret de la Moriniere & G. van der Velde. 2001. Dependence of Caribbean reef fishes on mangroves and seagrass beds as nursery habitats: a comparison of fish faunas between bays with and without mangroves/seagrass beds. Marine Ecology Progress Series 214: 225-235. [ Links ]
Ogden J. C. & E. H. Glaffelter. 1983. Coral reefs, seagrass beds and mangroves: Their interaction in the coastal zones of the Caribbean. UNESCO Reports in Marine Science 23: 1-450. [ Links ]
Orth, R. J., T. J. B. Carruthers, W. C. Dennison, C. M. Duarte, J. W. Fouquerean, K. L. Heck J. R., A. R. Hughes & G. A. Kendrick. 2006. A global crisis for seagrass ecosystems. Bioscience 56: 987-996. [ Links ]
Pettitt, J. M. 1980. Reproduction in seagrasses: Nature of the pollen and receptive surface of the stigma in Hydrocharitaceae. Annals of Botany 45: 257-271. [ Links ]
Pettitt, J. M. 1984. Aspects of flowering and pollination in marine an-giosperms. Oceanography and Marine Biology Annual Reviews 22: 315-342. [ Links ]
Preen, A. 1995. Impacts of dugong foraging on seagrass habitats: observational and experimental evidence for cultivation grazing. Marine Ecology Progress Series 124: 201-213. [ Links ]
Randall, J. E. 1965.Grazing effects on seagrasses by herbivorous reef fishes in West Indies.Ecology 46: 255-260. [ Links ]
Rodríguez-Almazán, C. 1997. Evaluación de la dinámica de los manchones de Lobophora variegata (Dictyotales, Phaeophyta) en la laguna arrecifal de Puerto Morelos, Quintana Roo. Tesis de Licenciatura, Facultad de Ciencias, Universidad Nacional Autónoma de México, México, 59 p. [ Links ]
Rodríguez-Martínez, R. E., F. Ruíz-Rentería, B. van Tussenbroek, G. Barba-Santos, E. Escalante-Mancera, G. Jordán-Garza & E. Jordán-Dahlgren. 2010. State and environmental tendencies of the Puerto Morelos CARICOMP site, Mexico. Revista Biología Tropical 58: 23-43. [ Links ]
Romero, J., K-S Lee, M. Pérez, M. A. Mateo & T. Alcoverro. 2006. Nutrient dynamics in seagrass ecosystems. In: Larkum, A. W. D., R. J. Orth & C. M. Duarte (Eds.). Seagrasses: Biology, Ecology and Conservation. Springer, The Netherlands. pp. 227-254. [ Links ]
Rose, C. D. & C. J. Dawes. 1999. Effects of community structure on the seagrass Thalassia testudinum. Marine Ecology Progress Series 184: 83-95. [ Links ]
Ruíz-Rentería, F., B. I. van Tussenbroek & E. Jordán-Dahlgren. 1998. Puerto Morelos, Quintana Roo, México. Pp. 57-66. In Kjerfve, B. J. (Ed.) CARICOMP-Caribbean Coral Reef, Seagrass and Mangrove Sites. UNESCO, Paris. 345 p. [ Links ]
Short, F. T., W. C. Dennison & D. G. Capone. 1990. Phosphorus limited growth of the tropical seagrass Syringodium filiforme in carbonate sediments. Marine Ecology Progress Series 62: 169-174. [ Links ]
Short, F. T. & S. Wyllie-Echeverria. 1996. Natural and human-induced disturbance of seagrasses. Environmental Conservation 23: 17-27. [ Links ]
Short, F. T., B. Polidoro, S. R. Livingstone, K. E. Carpenter, S. Bandeira, J. S. Bujang, H. P. Calumpong, T. J. B. Carruthers, R. G. Coles, W. C. Dennison, P. L. A. Erftemeijer, M. D. Fortes, A. S. Freeman, T. G. Jagtap, A. H. M. Kamal, G. A. Kendrick, W. J. Kenworthy, Y. A. La Nafie, I. M. Nasution, R. J. Orth, A. Prathep, J. C. Sanciangco, B. van Tussenbroek, S. G. Vergara, M. Waycott & J. C. Zieman. 2011. Extinction Risk Assessment of the World's Seagrass Species. Biological Conservation 144: 1961-1971. [ Links ]
Smith, R. D., W. C. Dennison & R. S. Alberte. 1984. Role of seagrass photosynthesis in root aerobic processes. Plant Physiology 74:1055-1058. [ Links ]
Smith, J. E., C. M. Smith, P. S. Vroom, K. L. Beach & S. Miller. 2004. Nutrient and growth dynamics of Halimeda tuna on Conch reef, Florida Keys: Possible influence of internal tides on nutrient status and physiology. Limnology and Oceanography 49: 1923-1936. [ Links ]
Stapel, J., R. Manuntun & M. A. Hemminga. 1997.Biomass loss and nutrient redistribution in an Indonesian Thalassia hemprichii seagrass bed following seasonal low tide exposure during daylight. Marine Ecology Progress Series 148: 251-262. [ Links ]
Tomasko, D. A. & C. J. Dawes. 1989. Evidence for physiological integration between shaded and unshaded short shoots of Thalassia testudinum. Marine Ecology Progress Series 54: 299-305. [ Links ]
Troyo-Ballina, A. 2009. Taninos en los frutos de Thalassia testudinum y proteccion de peces herbfvoros. Tesis de Licenciatura, Facultad de Ciencias, Universidad Nacional Autónoma de México, Mexico, 44 p. [ Links ]
UNESCO. 1998. CARICOMP-Caribbean coral reef, seagrass and mangrove sites. Coastal Region and small island papers 3, UNESCO, Paris XIV + 347 p. [ Links ]
Unsworth, R. K. F., P. Salinas de León, S. L. Garrard, J. Jompa, D. J. Smith & J. J. Bell. 2008. High connectivity of Indo-Pacific seagrass fish assemblages with mangrove and coral reef habitats. Marine Ecology Progress Series 353: 213-224. [ Links ]
Valdivia-Carrillo, T. 2011. Genética poblacional en pastizales de Thalassia testudinum (Bank ex König) en la laguna arrecifal de Puerto Morelos, Quintana Roo. Tesis de Licenciatura, Facultad de Ciencias, Universidad Nacional Autónoma de México, México, 99 p. [ Links ]
Valentine, J. F & J. E. Duffy. 2006. The central role of grazing in seagrass ecology. . In: Larkum, A. W. D., R. J. Orth & C. M. Duarte (Eds.). Seagrasses: Biology, Ecology and Conservation. Springer, The Netherlands. pp. 463-501. [ Links ]
Valentine J. F., K. L. Heck Jr., K. D. Kirsch & D. Webb. 2000. Role of sea urchin Lytechinus variegatus grazing in regulating subtropical turtlegrass Thalassia testudinum meadows in the Florida Keys (USA). Marine Ecology Progress Series 200: 213-228. [ Links ]
Valiela, I., J. L. Bowen & J. K. York. 2001. Mangrove forests: One of the world's threatened major tropical environments. Bioscience 51: 807-815. [ Links ]
Van Dijk, J. K., B. I. van Tussenbroek, K. Jiménez Durán, G. J. Márquez Guzmán & J. Ouborg. 2009. High levels of gene flow and low population genetic structure related to high dispersal potential of a tropical marine angiosperm. Marine Ecology Progress Series 390: 67-77. [ Links ]
Van Dijk, J.K. & B.I. van Tussenbroek. 2010. Clonal diversity and structure related to habitat of the marine angiosperm Thalassia testudinum along the Atlantic coast of Mexico. Aquatic Botany 92: 63-69. [ Links ]
Van Elven, B. R., P. S. Lavery & G. A. Kendrick. 2004. Reefs as contributers to biodiversity of epiphytic macroalgae assambleges in seagrass meadows. Marine Ecology Progress Series 276: 71-83. [ Links ]
Van Tussenbroek, B. I. 1994a. Spatial and seasonal variability in biomass and leaf morphology of the manatee grass Syringodium filiforme in a tropical coral reef lagoon, Mexico. Anales del Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México 21: 15-22. [ Links ]
Van Tussenbroek, B. I. 1994b. Aspects of the reproductive ecology of Thalassia testudinum in Puerto Morelos reef lagoon, Mexico. Botánica Marina 37: 413-419. [ Links ]
Van Tussenbroek, B. I. 1994c. The impact of Hurricane Gilbert on the vegetative development of Thalassia testudinum in Puerto Morelos reef lagoon, Mexico: a retrospective study. Botánica Marina 37: 421-428. [ Links ]
Van Tussenbroek, B. I. 1995. Thalassia testudinum leaf dynamics in a Mexican Caribbean reef lagoon. Maine Biology 122: 33-40. [ Links ]
Van Tussenbroek, B. I. 1998. Above- and below-ground biomass and production of Thalassia testudinum in a tropical reef lagoon. Aquatic Botany 61: 69-82. [ Links ]
Van Tussenbroek, B. I. 2002. Static life-table analysis and demography of the foliar shoots of the tropical seagrass Thalassia testudinum. Bulletin of Marine Science 71: 1247-1256. [ Links ]
Van Tussenbroek, B. I. & M. G. Barba-Santos. 2011. Demography of Halimeda incrassata (Bryopsidales, Chlorophyta) in a Caribbean reef lagoon. Marine Biology 158: 1461-1471. [ Links ]
Van Tussenbroek B. I. & J. K. Van Dijk. 2007. Spatial and temporal variability in biomass and production of psammophytic Halimeda incrassata (Bryopsidales, Chlorophyta) in a Caribbean reef lagoon. Journal of Phycology 43:69-77 [ Links ]
Van Tussenbroek, B. I., J. A. Vonk, J. Stapel, P. L. A. Erftemijer, J. J. Middelburg & J. C. Zieman. 2006a. The biology of Thalassia: Paradigms and recent advances in research. In: Larkum, A. W. D., R. J. Orth & C. M. Duarte (Eds.). Seagrasses: Biology, Ecology and Conservation. Springer, The Netherlands. pp. 409-439. [ Links ]
Van Tussenbroek, B. I., M. G. Barba-Santos & J. K. van Dijk. 2006b. Unusual synchronous spawning by different species of green algae (Bryopsidales), after the passage of hurricane Wilma (2005). Botánica Marina 49: 270-271. [ Links ]
Van Tussenbroek, B. I., J. G. R. Wong & J. Márquez-Guzmán. 2008a. Synchronized anthesis and predation on pollen in the marine angiosperm Thalassia testudinum (Hydrocharitaceae). Marine Ecology Progress Series 354: 119-124. [ Links ]
Van Tussenbroek, B. I., M. G. Barba-Santos, J. K. van Dijk, S. N. M. Sanabria-Alcaraz & M. L. Téllez-Calderón. 2008b. Selective elimination of rooted plants from a tropical seagrass bed in a back-reef lagoon: A hypothesis tested by hurricane Wilma (2005). Journal of Coastal Research 24: 278-281. [ Links ]
Van Tussenbroek, B. I., J. Márquez-Guzmán & R. Wong. 2009. Phenology of marine angiosperms [seagrasses]: reproductive synchrony in the sea. In: Pandalai, S.G. (Ed). Functional Approach to Sexual Plant Reproduction. Research Signpost, India. pp. 17-46. [ Links ]
Verweij, M. C., I. Nagelkerken, D. de Graaff, M. Peeters, E. J. Bakker & G. van der Velde. 2006. Structure, food and shade attract juvenile coral reef fish to mangrove and seagrass habitats: a field experiment. Marine Ecology Progress Series 306: 257-268. [ Links ]
Walker, D. I. , B. Olesen & R. C. Phillips. 2001. Reproduction and phenology in seagrasses. In: Short, F.T. & R.G. Coles (Eds.). Global seagrass research methods. Elsevier Science B.V. The Netherlands. pp. 59-78. [ Links ]
Waycott, M. C. M. Duarte, T. J. B. Carruthers, R. J. Orth, W. C. Dennison, S. Olyarnik, A. Calladine, J. W. Fourqurean, K. L. Heck, Jr, A. R. Hughes, G. A. Kendrick, W. J. Kenworthy, Short F. T. & S. L. Williams. 2009. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proceedings of the National Academy of Sciences USA 106:12377-12381. [ Links ]
Whelan III, T., B. I. van Tussenbroek & M. G. Barba Santos. 2011. Changes in trace metals in Thalassia testudinum after hurricane impacts. Marine Pollution Bulletin 62: 2797-2802. [ Links ]
Williams, S. L. 1987. Competition between the seagrasses Thalassia testudinum and Syringodium filiforme in a Caribbean lagoon. Marine Ecology Progress Series 35: 91-98. [ Links ]
Williams, S. L. 1988. Thalassia testudinum productivity and grazing by green turtles in a highly disturbed seagrass bed. Marine Biology 98: 447-455. [ Links ]
Williams, S. L. 1990. Experimental studies of Caribbean seagrass bed development. Ecological Monographs 60:449-469. [ Links ]
Williams, S. L., W. C. Dennison. 1990. Light availability and diurnal growth of a green macroalga (Caulerpa cupressoides) and a seagrass (Halophila decipiens). Marine Biology 106: 437-443. [ Links ]
Wolanski, E., E. Drew, K. M. Abel & J. O'Brien. 1988. Tidal jets, nutrient upwelling and their influence on the productivity of the alga Halimeda in the Ribbon reefs, Great Barrier reef. Estuarine, Coastal and Shelf Science 26: 169-201. [ Links ]
Zieman, J. C. & R. G. Wetzel. 1980. Productivity in seagrasses: methods and rates. In: Phillips R. C. & C. P. McRoy (Eds). Handbook of seagrass biology: An ecosystem perspective. Garland STPM Press, New York. pp. 87-116. [ Links ]
Zimmerman, R. C., J. L. Reguzzoni, S. Wyllie-Echverria, M. Josselyn & R. S. Alberte. 1991. Assessment of environmental suitability for growth of Zostera marina L. (eelgrass) in San Fransisco Bay. Aquatic Botany 39: 353-366. [ Links ]














