INTRODUCTION
Oxidative stress (OS) is the loss of balance between oxidants and antioxidants, caused by accumulation of reactive oxygen species (ROS) within the cell. Cumulative production of ROS causes a loss of balance between these molecules and the cellular antioxidant systems, promoting cellular damage (Kurtoglu, Ugur, Baltaci & Undar, 2003; Rossignol & Frye, 2012; Valko, Rhodes, Moncol, Izakovic & Mazur, 2006). OS and Redox balance can be regulated by dietary components, either with oxidant or antioxidant activity. These components comprise a variety of micronutrients, including iron (Evans & Halliwell, 2001). Damage caused by oxidative stress in cells includes alterations in protein functions due to polypeptide fragmentation, formation of protein-protein networks and modifications of amino acid side chains to form hydroxyl or carbonyl derivatives (Taskiran, Nesil & Alkan, 2007). Nucleic acid damage occurs mainly in mitochondrial deoxyribonucleic acid (mtDNA), as the inner mitochondrial membrane is the main production site of reactive oxygen singlets (O-) (Kirkinezos & Moraes, 2001). In membrane lipids, OS generates an enormous variety of peroxidation products, including aldehydes, hydroxy-alkenals, acrolein, malondialdehyde (MDA), glyoxal, along with more stable products, such as ketones and alkanes, which exhibit a variety of biological properties (Esterbauer, 1993; Negre-Salvayre, Coatrieux, Ingueneau & Salvayre, 2008). Lipid Peroxidation end products can disturb normal cellular processes (Negre-Salvayre et al., 2008).
Iron is considered an essential micronutrient for cellular redox reactions. It is present in different forms in all tissues and interacts with oxygen, generating ROS through the Fenton and Haber-Weiss reactions, which can lead to oxidative stress (Batista-Nascimento, Pimentel, Menezes & Rodrigues-Pousada, 2012). Iron uptake within cells must be regulated to provide an adequate level for cellular functions and prevent its accumulation and possible toxic effects. Both excessive and decreased iron concentrations may lead to OS and cellular damage (Puntarulo, 2005). Oxidative damage due to altered iron homeostasis has been related to mitochondrial dysfunction, since mitochondria use approximately 90% of cellular oxygen, leading to production of a significant amount of superoxide and other RO (Walter et al., 2002). The consequences of iron-dependent OS have been studied in detail, particularly in conditions of iron overload. Various pathological characteristics of neurodegenerative diseases, cancer, atherosclerosis and ageing are attributed to iron-dependent production of ROS and induction of OS (Casanueva & Viteri, 2003). For example, DNA telomere shortening and cellular ageing have been related to increased polyunsaturated lipid oxidation dependent on significant iron overload, as measured by increased ferritin and thiobarbituric reactive species (TBARS) in plasma (Murillo-Ortiz et al., 2016).
Cells from the CNS, particularly neurons, are among the most sensitive to oxidative damage due to their high metabolic rate, limited capacity for regeneration and high iron content (Smith, Park, Krause & Banik, 2013). Furthermore, microglia and astrocytes promote OS mediated by ROS during neuroinflammatory processes (Ortiz et al., 2013). Iron appears to be widely distributed in the CNS and it has been associated with OS and induction of cell death, particularly in pathological conditions related to ageing and neurodegeneration (Farina, Avila, da Rocha & Aschner, 2013; Zorzi, Zibordi, Chiapparini & Nardocci, 2012). Neurodegenerative disorders associated with iron can be a result of both iron accumulation in specific brain regions or defects in iron metabolism and homeostasis (Batista-Nascimento et al., 2012).
Iron deficiency (ID) is a major cause of motor and cognitive dysfunction, particularly in developing organisms, as it decreases dendritic branching, neuronal interconnections and myelinization in different areas of the CNS (Pra, Franke, Henriques & Fenech, 2012). ID anemia is a health problem affecting around one billion people worldwide, according to the World Health Organization (WHO), with children, adolescents, and pregnant women being the most affected groups (WHO, 2001). Normal iron requirements are often not covered by diet, due to insufficient content and low bioavailability (Michaca et al., 2012). Despite its importance for CNS development and functions, the possible relationship between oxidative stress in the CNS and iron deficiency has not been determined. In this study, we sought to determine how chronic ID affects oxidative stress in the CNS, specifically regarding damage to lipids in nervous tissue.
MATERIALS AND METHODS
All experiments were performed in the Neurochemistry Laboratory at the Faculty of Medicine, Universidad Autónoma del Estado de México. All animal experiments were carried out in accordance with local regulations. All experiments were approved by the Research Ethics Committee of the Faculty of Medicine, Universidad Autónoma del Estado de México.
Animals
Male BALB/c mice were kept under controlled standard conditions (a temperature of 22 °C ± 2 °C; a 12/12 hour light cycle and access to purified water ad libitum). Mice were divided into two groups (N = 6 each), which were fed commercial diets with known concentrations of iron: control (control diet: Lab Diets AIN76A/100, 100 parts per million (ppm) of iron, added as ferrous sulfate) and iron deficient (ID, iron-deficient diet: Lab Diets AIN-76A/10; 10 ppm iron added as ferrous sulfate). Iron deficiency was generated in utero by feeding the mothers an iron-deficient diet for 14 days prior to mating and during pregnancy and lactation. Mice born to these females were weaned on day 21. The groups continued with the aforementioned diet in the post-weaning period until sacrifice on postnatal day 60.
Dissection of the central nervous system and spleen
At 60 days of age, mice from each group were sacrificed by lethal dose (1.5 µL/g weight) of 6.3% sodium pentobarbital intraperitoneally. Cardiac perfusion with 30 mL of sterile phosphate buffer solution (PBS) at 4 °C was performed. The brain, spinal cord (CNS) and spleen were removed. Tissue samples were frozen at -20 °C for posterior analysis.
Determination of lipid peroxidation products
Frozen CNS (brain and spinal cord, together) and spleen were analyzed according to the procedure of the TBARS Assay Kit (catalog #0801192, ZeptoMetrix, USA). Briefly, samples were weighed and homogenized in a 0.9%, isotonic saline solution, adjusting to a concentration of 50 mg/mL. Reagents and controls were prepared at the time of analysis and added to the tissue suspension and controls, incubated at 95 °C for 60 min, then cooled at 0 °C for 10 min and centrifuged at 3000 rpm for 15 min, using supernatant for analysis. The absorbance of each sample was determined at a wavelength of 532 nm using a Bio-Rad UV-Vis SmartSpecTM Plus spectrophotometer. The results were analyzed using GraphPad Prism v5.0.3 and compared by unpaired t test, considering a significant value of p < 0.05.
RESULTS
Determination of oxidative damage to lipids in the spleen and CNS
Analysis of thiobarbituric acid-reactive species (TBARS) is one of the most commonly applied tests for oxidative damage, due to its simplicity and low cost. The sample is heated with thiobarbituric acid at low pH, forming a pink chromogen ([TBA] 2-malondialdehyde) that is measured by its absorbance at or near 532 nm. The TBARS test is used to measure formation of malondialdehyde (MDA) as a result of lipid peroxidation.
To determine the effect of iron deficiency on the oxidative damage to lipids in the CNS and spleen, TBARS production in duplicate samples from the ID and control groups were assessed. Mean values, standard deviation and error for the analysis were obtained. The TBARS production range in the tissues under analysis was between 105 and 197 nmol/mL (Table 1).
Table 1 TBARS concentration in CNS and spleen.
| Average (nmol TBARS/mg protein) | Std deviation | Std error | Min. (nmol TBARS/mg protein) | Max. (nmol TBARS/ mg protein) | Confidence Interval p = 0.05 (nmol TBARS/mg protein | |
| Co CNS | 185.66 | 10.81 | 6.24 | 177.80 | 197.00 | 170.43-197.89 |
| ID CNS | 122.46 | 14.29 | 8.25 | 106.80 | 134.80 | 106.29-138.63 |
| Co Spleen | 112.46 | 7.63 | 4.40 | 105.80 | 120.80 | 103.83-121.08 |
| ID Spleen | 135.46 | 13.05 | 7.53 | 121.80 | 147.80 | 120.70-150.21 |
Control (Co), Iron Deficiency (ID), thiobarbituric acid reactive substances (TBARS).
Source: Author´s own elaboration
The CNS contains a high iron and polyunsaturated fatty acid content (Diniz et al., 2015; Shichiri, 2014), which make it highly sensitive to oxidative stress. Free radical production has a very important role in the brain regulationon of biological functions, as well as in the pathogenesis of neurodegenerative disorders (Aguiar et al., 2012). In the CNS, the concentration of lipid peroxidation products was lower in the ID group (122.46 nmol/mL ± 14.29 nmol/mL), presenting a significant difference (p < 0.0001) with the control group (185.66 nmol/mL ± 10.81 nmol/mL) (Figure 1). Thus, decreased peroxidation of lipids in the CNS might be reflective of diminished ROS production within nervous tissue, which may be related to the neuronal dysfunction observed under iron-deficient conditions.
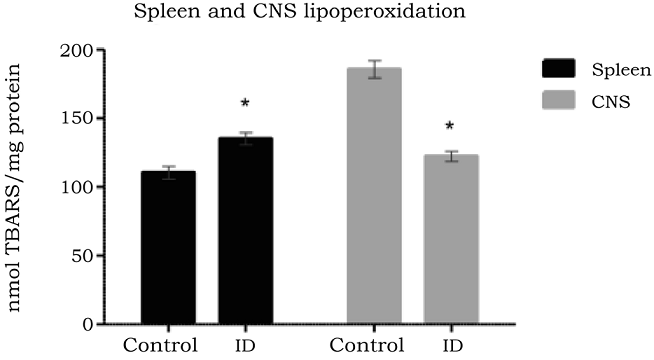
Source: Author´s own elaboration.
Figure 1 TBARS concentrations in the CNS and spleen of control and ID in 2-month-old mice. Tissue was collected after sacrifice and TBARS were measured by TBARS assay kit n = 6 in duplicate. * indicates p < 0.05.
In contrast to the CNS, the spleen combines the innate and adaptive immune system in a uniquely organized way. The structure of the spleen enables it to remove older erythrocytes from the circulation, leading to efficient recycling of hemoglobin-bound iron (Knutson & Wessling-Resnick, 2003) Leukocyte activation during immune reactivity to blood borne antigens also increases production of ROS, which are relevant for immune protection of the host (Mebius & Kraal, 2005). In the spleen, the ID group presented a statistically significant (p < 0.0033) increase of lipid peroxidation products compared to the control group (135.46 nmol/mL ± 13.05 nmol/mL and 112.46 nmol/mL ± 7.63 nmol/mL, respectively) (Figure 1). Increased peroxidation in the spleen may be relevant for the enhanced susceptibility to infections under iron deficient conditions.
DISCUSSION
It has been established that the nervous system is vulnerable to injury mediated by oxidative stress given its high metabolic activity, limited antioxidant capacity, increased production of ROS (Diniz, et al., 2015), high polyunsaturated fatty acid (PUFA) content (Shichiri, 2014), high iron content, particularly in the substantia nigra and dopaminergic neurons (Jellen et al., 2012), and its high surface to membrane ratio (Tewari, Mahendru, Sinha & Bilotta, 2014). ROS are highly reactive and can induce lipid peroxidation and accumulation of its products when generated near cell membranes, promoting cellular damage (Rahman & Adcock, 2006).
As noted previously, significant iron concentrations are widely distributed in the CNS (Zorzi et al., 2012). Since iron is known to participate in intracellular redox reactions and its concentration has been related to the induction of oxidative stress, our initial assumption was that iron deficiency might increase oxidative damage in the central nervous system, as has been observed in other organs such as liver, kidney and the intestinal mucosa (Knutson, Walter, Ames & Viteri, 2000; Walter et al., 2002), were oxidative damage to mitochondrial DNA and lipid membranes has been related to a decrease in the mitochondrial respiratory efficiency caused by iron deficiency (Kirkinezos & Moraes, 2001; Walter et al., 2002). However, the results contradict the initial assumption, since under conditions of iron deficiency in the CNS, a significant decrease in the formation of lipid peroxidation products is observed, compared to basal values in the control group, indicating as well that iron deficiency may impair the production of ROS in nervous tissue in the absence of antigenic stimulation.
Low levels of ROS have physiologically relevant functions as signaling molecules, and in innate and adaptive immune cells and are essential for pathways initiated by Toll-Like Receptors (TLRs), including TLR1, TLR2, and TLR4, and for optimal bactericidal activity of macrophages. Low ROS concentrations activate proliferative pathways, serving as signals to support stem-cell proliferation. By contrast, high levels of ROS impair stem-cell activate signaling pathways that limit self-renewal but do not necessarily cause cellular damage (Ray, Huang & Tsuji, 2012). Redox signaling is required for numerous cellular processes, as indicated by the role of ROS in proper cellular differentiation, tissue regeneration, and prevention of aging. It is also crucial in regulating signaling pathways that control various disease states, including tumor genesis, autoimmunity, and loss of tissue regeneration with age (Ray et al., 2012; Schieber & Chandel, 2014). Thus, decreased ROS production in the nervous tissue due to iron deficiency might be related to the observed decreases in dendritic branching, neuronal interconnections and myelinization that have been associated to motor and cognitive dysfunction in ferropenic iron deficiency anemia (Pra et al., 2012).
The spleen, like the CNS, has a high metabolic rate, due to leukocyte activity and its iron recycling functions, making it susceptible to oxidative stress damage (Javed et al., 2010). While the CNS contains microglia as immune effector cells (Kreutzberg, 1996), macrophages in the spleen respond to a variety of activation stimuli with the production of high concentrations of ROS (Cathcart, 2004; Hume et al., 2002).
In the spleen the red pulp is anatomically well suited for its blood-filtering function, by the combination of an open and sinusoidal venous system, and also contains macrophages that have special properties for fighting bacteria and facilitating iron metabolism. An important task of splenic macrophages is iron recycling, regulated by the requirements of the bone marrow (Knutson & Wessling-Resnick, 2003). Macrophages in the spleen express NRAMP1 (natural-resistance-associated macrophage protein 1), a transporter for divalent metals across the phagosomal membrane, useful for shuttling iron outside the phagosomes, reducing iron availability for ingested microorganisms and limiting their growth (Mebius & Kraal, 2005). Although the spleen has an elevated amount of immune effector cells, including macrophages that are involved in the production of large quantities of ROS upon activation, our results show that oxidative damage in the spleen appears to be lower than in the CNS, which contains a lower number of macrophage-like cells (microglia).
Nonetheless, in contrast to the effect of iron deficiency in CNS, we observed a significant increase in MDA production in the ID group, compared to the controls. These data could suggest an impaired function of spleen cells derived from an increased requirement for iron recycling from a limited stores source, leading to possible tissue damage or impaired stem-cell generation in this tissue, due to oxidative stress by enhanced ROS production in splenocytes, activating signaling pathways that limit self-renewal (Mittler et al., 2011).
CONCLUSIONS
In conclusion, this results show altered profiles of the lipid peroxidation end product MDA in the CNS and spleen of ID mice. Reduced oxidative damage in the CNS might be related to a decreased production of ROS in nervous tissue, a possibility that should be addressed in future studies, since even in the absence of lipid damage in the tissue, decreased production of these molecules may be related to known alterations in neuronal signaling and functions that become manifest in the long term as cognitive and motor deficits observed in anemic individuals. Increased MDA production in the spleen may be relevant for diminished leukocyte functions and an impaired immune response under iron-deficient conditions, promoting enhanced susceptibility to infections. Our identification of changes in lipid peroxidation products under ID identifies additional adverse effects of this pathology on the organism and emphasizes the need for adequate prevention and correcting measures for it, given the high incidence and prevalence of iron deficiency worldwide.











 nueva página del texto (beta)
nueva página del texto (beta)


