Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de micología
versión impresa ISSN 0187-3180
Rev. Mex. Mic vol.34 Xalapa dic. 2011
Notas cortas
Antifungal activity of chitosan in Cladosporium cladosporioides isolated from safflower
Actividad antifúngica de quitosano en Cladosporium cladosporioides aislado de cártamo
Eber A. Quintana–Obregón1,2, Maribel Plascencia–Jatomea2, Jaime López–Cervantes1, Luis A. Cira–Chávez1, Dalia I. Sánchez–Machado1, Mario O. Cortez–Rocha 2
1 Departamento de Biotecnología y Ciencias Alimentarias, Instituto Tecnológico de Sonora, Calle Antonio Caso s/n, Col. Villa ITSON, C.P. 85130 Ciudad Obregón, Sonora, México.
2 Departamento de Investigación y Posgrado en Alimentos, Universidad de Sonora, Blvd. Luis Encinas y Rosales s/n. Colonia Centro. C.P. 83000, Hermosillo, Sonora, México.
Autor para correspondencia:
Mario O. Cortez Rocha mcortez@guayacan.uson.mx
Recibido 6 de diciembre 2010;
Aceptado 7 de agosto 2011.
Resumen
El quitosano mostró inhibición sobre la germinación de esporas de Cladosporium cladosporioides a las 24 h (70.99 ± 12.53 y 32.72 ± 10.85%, QB y QA, respectivamente). El quitosano causó cambios morfométricos sobre las esporas, evidenciado por excesivo hinchamiento y disminución de la elongación del tubo germinal. Sin embargo, en comparación con los controles, el quitosano no inhibió las velocidades de extensión radial de los cultivos.
Palabras clave: control biológico, hongos patógenos, crecimiento radial, germinación de esporas.
Abstract
The chitosan showed inhibition of spore germination at 24 h on Cladosporium cladosporioides (70.99 ± 12.53 and 32.72 ± 10.85% for QB and QA, chitosans, respectively). Chitosan caused morphometric changes on spore evidenced by excessive swelling and delay on the germ tube. However, in comparison with the controls, the chitosan not inhibited the colony radial extension rate.
Key words: biological control, pathogenic fungi, radial growth, spore germination.
Recent studies demonstrated that Cladosporium genera are associated to Ramularia and Alternaria in safflower leaves infected with false mildew (unpublished data, Quintana–Obregón et al.). Chitosan has been reported to inhibit and delay the growth of some common pathogens in plants and crops (Badawy and Rabea, 2011; El Hadrami et al., 2010). In studies previews, chitosan (3.4 g L–1) showed inhibition on Ramularia and Alternaria (Quintana–Obregón et al., 2011a; 2011b). The goal of this study was to evaluate the in vitro antifungal activity of chitosan on C. cladosporioides.
Spores of C. cladosporioides grown at 25 °C, 12 h light–dark photoperiod, pH 5.5, and V8 medium. Subsequently, suspensions of spores were prepared after 96 h, using a sterile Tween 20 solution (0.1% v v–1) and stirred for 5 minutes. The number of spores (mL) was determined using a Neubauer chamber. Two types of chitosan were evaluated, chitosan (Aldrich lot: 04924LH) with degree of deacetylation of 84% and molecular weight of 46.31 ± 5.2 kDa (QB), and chitosan (Fluka BioChemika, Lot: 436207/1) with deacetylation degree of 76% and molecular weight of 260.65 ± 10.9 kDa (QA). Flasks with V8 medium and chitosan with acetic acid (CH3COOH) were autoclaved, cooled to 45 °C, 3 and mixed. The concentration of chitosan in the V8 medium was 3.4 g L–1 and acetic acid 0.05M. Subsequently, 20 mL of mixture deposited on Petri dishes (9 cm in diameter) cooled at room temperature. Controls were V8 medium with and without acid.
Petri dishes containing plugs of V8 medium (20x20x5 mm) were by spreading inoculated of a spore suspension containing 104 spores of C. cladosporioides incubated at 25 °C. From each plug, 200 spores per plate (germinated and non–germinated) were randomly counted at different times using an optical microscope. Percentage of inhibition with respect to the acid control was calculated (Plascencia–Jatomea et al., 2003; El Ghaouth et al., 1992). Measurements in diameter and length of spores at 0, 8, and 24 h of incubation were made at 400x with the Image–Pro Plus v. 6.3 (Media Cybernetics, Inc., USA, 1993–2008). In addition, the increase in length and diameter of the spores were calculated using the equation I = (x–x0), where I is the increase in length or diameter, xi is the average length or diameter, and x0 is the average length or diameter in V8 medium (control H2O) before the incubation.
Radial growth. A 6 mm well in the center of the culture medium in each Petri dish (6 cm in diameter) was with a sterile Pasteur pipette done, deposited inside a 105 spores solution. The diameters of the mycelia were manually at different times measured until the colony in the control reached the border of the plate. To identify the growth phases of the fungal colony an arithmetic and logarithmic growth (LOG10) kinetics were plotted (Trinci, 1969). In addition, the rate of radial expansion of the mycelia U (µm h–1) in the exponential phase (log) and stationary phase, were calculated from the slope by plotting the experimental colony radius against time, using the start and end time of each phase through the logarithmic plot of colony radius previously obtained.
A completely randomized design was used and the JMP 2004 program for the analysis of variance and Tukey multiple range test (P <0.05) (JMP 5 vs. 5.0, SAS Institute Inc., USA) to rank the means of various treatments.
Inhibition of spore germination by QA and QB was 32.72 ± 10.85 and 70.99 ± 12.53% after 24 h, respectively. The dimensions of the C. cladosporioides spores grown in V8 medium were 6.91 ± 1.50 µm of length and 3.43 ± 0.70 µm in diameter. There were no significant difference in the length and diameter of the spores at the first 8 h of incubation (P<0.05); however, after 24 h, an excessive increase (I) was observed in spores treated with chitosan, whereas in controls 80% was polarized and/or germinated (Figure 1, Table 1). During polarization and conidial development, spores from chitosan treatments showed an excessive swelling and delay in the elongation of the germ tube (24 h). After 30 h of incubation, the spores exhibited multiple polar initiation points (not quantified) and many germ tubes on the spore surface (not quantified) treated with chitosan QB and QA (before the germination, only 1–2 poles were in the control conditions observed). Before 8 h of incubation, the chitosan not affected the spores (Table 1). However, the presence of multiple polar in spore suggest a high level of nuclear division (polarization) and elongation of the germ tubes. The spore polarization is related to the formation of septa, division nuclear, mitosis and development of the cell wall (Bartiniki–Garcia and Lippman, 1977; Bartnicki–Garcia et al., 1968; Osherov and May, 2001) and chitosan affects the polarization and elongation of the germ tube (Plascencia–Jatomea et al., 2003). These changes may be due to interaction of the charges in the cell wall with chitosan amino groups to form a composite polyelectrolyte (Hirano and Nagao, 1989). In our study, both evaluated chitosans have a high deacetylation degree (76%), which indicates high density of positively charged amino groups in the molecule.
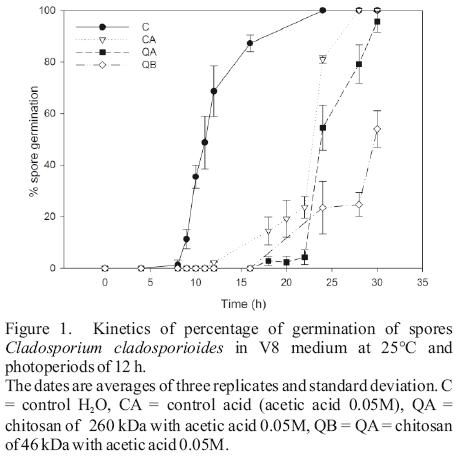
In radial growth, the results determined significant differences between water and the acid controls before 126 h of incubation (Figure 2). From the logarithmic kinetics obtained, in the controls were identified four stages of fungal growth (see captions of Figure 3). The radial expansion rates in the growth phase after 162 h in the QB was high (Table 2), and no significant differences were observed between their rates of the two growth phases, Phase II and Phase after 162 h (Table 2). After spore germination (Phase I in Figure 3), the tube hyphae developed germs which initiates the mycelial growth of the colony (Phase II in Figure 3). Radial expansion rates of the colony showed that chitosans was induced at higher rates after 162 h of incubation (Table 2). In addition, QB maintained the expansion rate, while that QA decreased (Table 2). However, fungi growth in the controls changed from the log phase to steady growth phase (Figure 3), that means a decrease in the radial expansion rates.
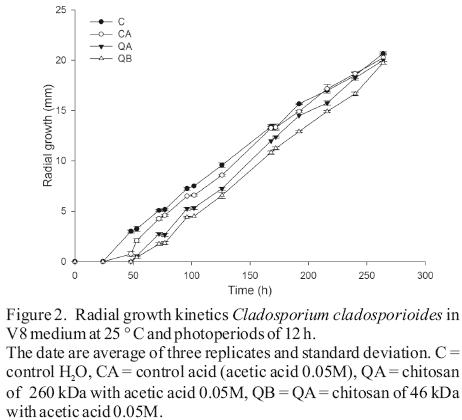
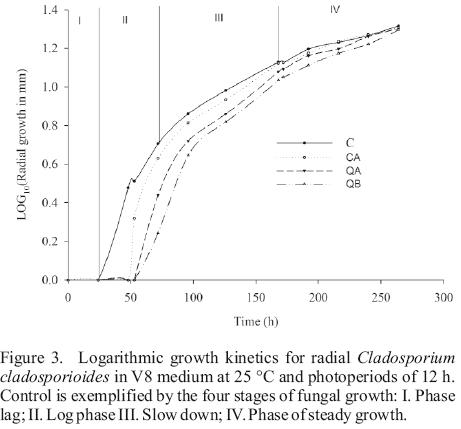
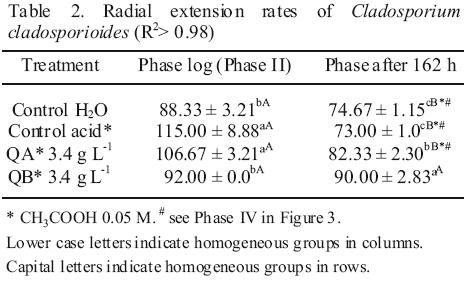
These morphometric changes in the radial growth of the colony might be due to defense mechanisms that fungus uses for adaptation to the culture medium, especially in treatment with low molecular weight chitosan. It is possible to maintain the growth rate in the log phase, which permits the colony to release a greater amount of enzymes (chitinases, deacetylases, and deaminase) which partially hydrolyze chitosan (Palma–Guerrero et al., 2010). This allows an optimal growth and movement to the steady growth phase.
References
El Hadrami, A., L.R. Adam, I. El Hadrami, F. Daayf, 2010. Chitosan in plant protection. Marine Drugs 8(4):968–987. [ Links ]
Badawy, M.E.I., E.I. Rabea, 2011. A biopolymer chitosan and its derivatives as promising antimicrobial agents against plant pathogens and their applications in crop protection. International Journal of Carbohydrate Chemistry Article ID 460381, 29 pages, doi: 10.1155/2011/460381. [ Links ]
Bartnicki–Garcia, S., E. Lippman, 1977. Polarization of cell wall synthesis during spore germination of Mucor rouxii. Experimental Mycology 1(3): 230–240. [ Links ]
Bartnicki–Garcia, S., N. Nelson, E. Cota–Robles, 1968. Electron microscopy of spore germination and cell wall formation in Mucor rouxii. Archives of Microbiology 63(3): 242–255. [ Links ]
El Ghaouth A., G. Arul, J. Grenier., A. Asselin, 1992. Antifungal activity of chitosan on two postharvest pathogens of strawberry fruits. Phytopathology 82(4): 398–402. [ Links ]
Hirano, S., N. Nagao, 1989. Effects of chitosan, pectic acid, lysozyme and chitinase on the growth of several phytopathogens. Agricultural Biology and Chemistry 53(11): 3065–3066. [ Links ]
Osherov, N., G.S. May, 2001. The molecular mechanisms of conidial germination. FEMS Microbiology Letters 199(2): 153–160. [ Links ]
Palma–Guerrero, J., S. Gómez–Vidal, V.E. Tikhonov, J. Salinas, H.B. Jansson, L.V. Lopez–Llorca, 2010. Comparative analysis of extracellular proteins from Pochonia chlamydosporia grown with chitosan or chitin as main carbon and nitrogen sources. Enzyme and Microbial Technology 46(7): 568–574. [ Links ]
Plascencia–Jatomea, M., G. Viniegra, R. Olayo, M.M. Castillo–Ortega, K. Shirai, 2003. Effect of chitosan and temperature on spore germination of Aspergillus niger. Macromolecules Bioscience 3(10): 582–586. [ Links ]
Quintana–Obregón, E.A., J. López–Cervantes, L.A. Cira–Chávez, D.I. Sánchez–Machado, M. Plascencia–Jatomea, M.O. Cortez–Rocha, 2011a. Actividad antifúngica del quitosano contra Alternaria tenuissima in vitro y en semilla de cártamo (Carthamus tinctorius L.). Revista Mexicana de Fitopatología vol. 29 (en prensa). [ Links ]
Quintana–Obregón, E.A., M. Plascencia–Jatomea, R.I. Sánchez–Mariñez, A. Burgos–Hernandez, G.A. González–Aguilar, J. Lizardi–Mendoza, M.O. Cortez–Rocha, 2011b. Effects of middle–viscosity chitosan on Ramularia cercosporelloides. Crop Protection 30(1): 88–90. [ Links ]
Trinci, A.P.J., 1969. A kinetic study of the growth of Aspergillus nidulans and other fungi. Journal of General Microbiology 57: 11–24. [ Links ]














