Introduction
Seagrass meadows play an important ecological role because they serve as refuge, breeding, and feeding areas for numerous organisms, they are highly productive systems that act as carbon sinks, and they give sediments stability (Fourqurean et al. 2012, Mazarrasa et al. 2015). Together with light and temperature, nitrogen (N) availability is one of the main factors limiting the growth of these phanerogams (Udy et al. 1999). N2 fixation (the process by which N2 is converted into reactive N) can contribute more than 50% of the N required for seagrass growth in tropical zones (Capone 1988, O’Donohue et al. 1991), and in some temperate areas it has been found to contribute between 5% and 30% (McGlathery et al. 1998; Welsh et al. 1996a, 1996b; Cole and McGlathery 2012).
In marine habitats, N2 fixation is a process carried out exclusively by a group of specialized prokaryotes and archaea called diazotrophs, such as some photoautotrophic cyanobacteria (Severin and Stal 2010) and heterotrophic bacteria including sulfate-reducing bacteria (Fulweiler et al. 2013). Heterotrophs are the dominant functional group of N2 fixation in sediments and the phyllosphere of some temperate seagrass meadows (McGlathery et al. 1998, Cole and McGlathery 2012). Fixation rates tend to be coupled to the photosynthetic activity of plants (Capone and Taylor 1977, O’Donohue et al. 1991, McGlathery et al. 1998, Cole and McGlathery 2012), since the exudation of labile organic carbon through the leaves, roots, and rhizomes increases at higher photosynthetic rates and serves as substrate for heterotrophic bacteria (McRoy et al. 1973, Moriarty et al. 1986). The presence of seagrasses also benefits the growth of heterotrophic diazotrophs because the canopy acts as a trap of particulate organic matter that then settles on the meadow sediments (Kennedy et al. 2010).
The bioavailable N in aquatic systems proceeds from the internal regeneration of pre-existing N, from the biological fixation of N 2, and from external inputs, and it is removed primarily by denitrification (Sarmiento and Gruber 2006). Despite the large number of studies that have reported high N2 fixation rates, in benthic systems denitrification tends to be more intense than fixation; however, in a recent study that simultaneously measured denitrification and N fixation rates in Zostera muelleri beds and adjacent sediments, fixation was the dominant process (Russell et al. 2016). Moreoever, in Narragansett Bay (USA), the predominant process switched from denitrification to fixation (Fulweiler et al. 2007). Fulweiler et al. (2013) suggested that the quality and quantity of organic matter can affect the co-occurrence of these two processes and even temporarily uncouple them. In areas where oyster farming occurs the particulate organic matter inputs to sediment tend to increase and denitrification intensifies (Kellogg et al. 2014, Humphries et al. 2016), though N2 fixation may also be stimulated (Newell et al. 2002).
In the case of San Quintín Bay (northwestern Mexico), Camacho-Ibar et al. (2003) suggested that reactive N losses via denitrification exceed inputs from fixation and that N2 fixation may even be negligible; however, they also reported that in the innermost areas of this coastal lagoon N2 fixation and denitrification were almost equal. To date, no direct measurements of these 2 processes have been made in the lagoon, but the isotopic composition reported for Zostera marina and Ulva spp. also suggests that N2 fixation is more intense in the innermost parts of the system (Sandoval-Gil et al. 2015, Carriquiry et al. 2016). As the new N contributed by the adjacent ocean decreases from the mouth to the interior of the lagoon (Camacho-Ibar et al. 2003, Hernández-Ayón et al. 2004) and the continental inputs are null (Aguirre-Muñoz et al. 2001), the macrophytes growing in the innermost areas are likely more dependent on N provided by internal processes such as ammonification and fixation.
In this study we measured N2 fixation rates in the phyllosphere and in sediments collected from within and outside Z. marina meadows in San Quintín Bay, at sites with different oceanic influence and oyster culture activity. We also estimated the possible contribution of fixation to the N demand of Z. marina and the contribution of this process to N balance in the system.
Materials and methods
Study area
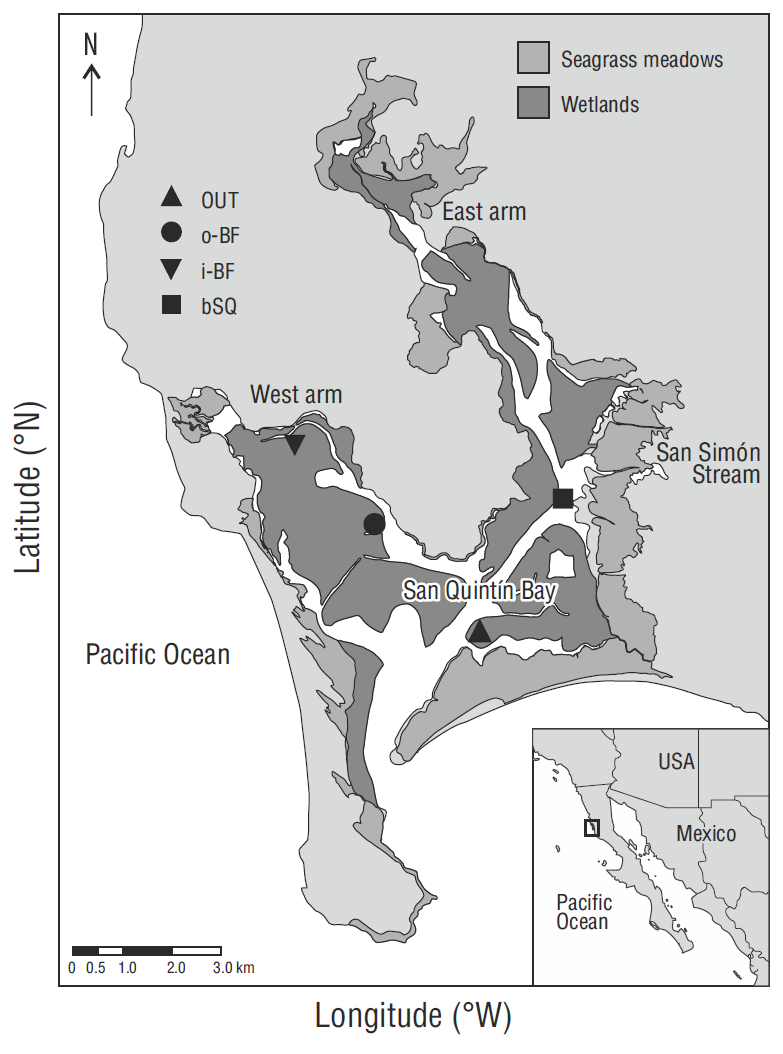
San Quintín Bay (Fig. 1), a hypersaline coastal lagoon on the west coast of the Baja California Peninsula, Mexico (30º27 N, 116º00 W), has an average depth of ~2 m. Fresh-water inflow is practically null as it is restricted to the San Simón stream that only delivers water during winters with higher than average rainfall (150 mm, Aguirre-Muñoz et al. 2001). The dynamics and distribution of the water properties (salinity, temperature, dissolved nutrients, etc.) are controlled by semidiurnal tides (maximum amplitude of ~2.5 m and minimum of ~1 m). The lagoon has 2 arms, the east arm known as Bahía San Quintín and the west arm known as Bahía Falsa, which have a water residence time of 15 and 5 d, respectively (Camacho-Ibar et al. 2003, Melaku Canu et al. 2016). Coastal upwelling intensifies in spring and summer, and nutrient input to the lagoon increases at this time. The concentration of ocean-derived nitrate, which exceeds 10 μM during upwelling events, decreases from the mouth to the innermost parts of the lagoon, where concentrations tend to be <1 μM (Camacho-Ibar et al. 2003, Hernández-Ayón et al. 2004), potentially limiting the N available for uptake by submerged plants. Salinity and temperature increase from the mouth to the inner parts of the system. Salinity ranges from ~33.6 to 39 and temperature from 11 to 27 ºC, though in winter the ranges are smaller, from ~33.6 to 34.1 and from 13.7 to 15.5 ºC, respectively (Camacho-Ibar et al. 2003). Dense Z. marina meadows cover ~46% of the lagoon Surface (Ward et al. 2003). Oyster aquaculture is carried out in the west arm, where concessions cover approximately 30% of the area (Aguirre-Muñoz et al. 2001).
Measurement of N2 fixation rates
In December 2015 sediment cores were collected from areas covered and not covered by Z. marina to measure N2 fixation rates in vegetated and bare sediments. Shoots of the seagrass Z. marina were also collected to measure N2 fixation in the phyllosphere by epiphytic bacteria. N2 fixation rates were measured using the acetylene reduction assay (developed by Hardy et al. 1968 and adapted for the phyllosphere by Capone and Taylor 1977), which measures nitrogenase enzyme activity. This method is based on the low specificity of this enzyme for its natural substrate (N2) and its ability to reduce other molecules of low molecular weight that have triple bonds, such as acetylene (Capone 1988).
Zostera marina meadows, some at oyster farming sites and some not, were chosen along a gradient of oceanic influence (Fig. 1). At the sampling station named OUT, located closest to the lagoon mouth (~4 km) where the oceanic influence is strongest, oyster aquaculture is not carried out. Oyster aquaculture is carried out at the two stations in the west arm, o-BF and i- BF, but the oceanic influence is stronger at the former than at the latter. The fourth station, bSQ, in the east arm, is located at a similar distance from the mouth as station o-BF, but is not affected by oyster farming activities.
At each station, 3 sediment cores were collected from within the seagrass meadow (i.e., vegetated sediments; henceforth referred to as CV cores) and 3 sediment cores were collected in the vegetation-free area adjacent to the meadow (i.e., bare sediments; henceforth referred to as SV cores) . The cores were transported to the laboratory in ice chests and were left to rest for ~12 h. A subcore was taken with a 10-mL syringe and 1-cm sections were obtained from the top 5 cm. Each section was halved and sodium molybdate was added to one of the parts to inhibit the activity of sulfate-reducing bacteria and be able to determine the N2 fixation rate by this group of bacteria. The fixation rates obtained from the analysis of the samples without sodium molybdate were considered total fixation rates. Even though sulfate-reducing bacteria are heterotrophs, throughout the text heterotrophic bacteria will refer to nonsulfate-reducing bacteria. The incubations were carried out in 40-mL glass vials. Sediment was added to these vials and they were closed with a septum cap. After purging with argon, 10 mL of acetylene was injected into the vials to initiate incubation, which was carried out at constant temperature in the dark. Sediments from the first centimeter were also exposed to light. The same procedure was followed but the incubation was performed under an irradiance of 350 mol photons m-2 s-1, the maximum irradiance at the site in winter (Sandoval-Gil et al. 2015). The incubation was ended by transferring the gas to Vacutainer vials previously purged with argon. Two blanks were prepared for each core following the same procedure but without adding sediment; acetylene was not added to one of them and sodium molybdate was not added to either, and the incubations were carried out in the dark.
Zostera marina shoots for the analysis of N 2 fixation in the phyllosphere were collected manually and kept in ice chests with water from the site until processing approximately 6 h after collection. For the incubation, 6 shoots were selected from each site; 3 were used for the treatments carried out under light conditions and 3 for the treatments carried out in darkness. From each shoot we retrieved the first 15 cm (from the apex to the meristem) of leaves 2 and 4. Each leaf was placed in a 140-mL borosilicate flask and 90 mL of water from the collection site was added to the flask. To initiate incubation, 10 mL of acetylene-saturated water was added and the flask was immediately sealed with a septum and aluminium cap. The flasks were shaken for 30 s and kept for 5 h at constant temperature. An irradiance of ~350 mol photons m-2 s-1 was used for the incubations. To end the incubation the flasks were submerged in ice and kept there, for not more than 24 h, until the chromatographic analysis.
Ethylene concentration was measured using a SRI 8610C gas chromatograph equipped with an on-column injector followed by an ethylene trap packed with silica gel, a chromatographic column packed with Porapak N, and a flame ionization detector. Acetylene reduction rates were converted to N2 fixation rates using the 3:1 ratio (McGlathery et al. 1998, Welsh 2000).
Ancillary measurements
Samples were taken from the first 5 cm of the sediment cores to analyze organic carbon (Corg) content, total N, chlorophyll a, and grain size. A LECO CHNS-932 elemental analyzer was used to determine Corg and N. Grain size was determined using a Horiba LA-910 particle size analyzer. Chlorophyll a concentrations were determined by extraction with 100% acetone over 24 h in darkness (Edmunds et al. 2004). At the end of the extraction, 10 mL of distilled water was added and the aliquots were analyzed using a Varian Cary 50 UV-Vis spectrophotometer. Readings were taken at 664 and 750 nm before and after acidification (0.08 ± 0.01 mL of 0.01 N HCl) to correct for phaeophytin (Lorenzen 1967). To determine the N isotope composition (δ15N), 3 Z. marina leaves from each sampling site were analyzed at the University of California, Davis, Stable Isotope Facility, CA. The isotope ratio was calculated with the equation δ15N (‰) = ((Rsample/R standard) - 1) × 103, where Rsample and R standard are the ratio of the heavy to light isotope (15N/14N) of the sample and standard, respectively.
Data analysis
N2 fixation rates in sediments are expressed in micromoles per square meter per hour (μmol m-2 h-1). Student’s t-tests were used to identify differences in N2 fixation rates in sediments between the treatments under light and dark conditions and between the SV and CV cores. To determine differences between sampling stations, one-way ANOVA was carried out, followed by a Tukey a posteriori test since the data complied with the assumptions of normality and homo-scedasticity. For the N2 fixation rates in the phyllosphere, Student’s t-tests were performed to identify differences between leaves 2 and 4 and between the treatments under light and dark conditions. As the data for N2 fixation in the phyllosphere, grain size, Corg, N, chlorophyll a concentration, and δ15N did not comply with the assumptions of normality and homoscedasticity, the Kruskal-Wallis test was used to determine differences between sampling stations. The results were considered significant when P < 0.05. All statistical analyses were carried out using STATISTICA 8.0.
Results
N2 fixation rates in sediments
N2 fixation rates in surface sediments were in general higher in the treatments carried out in darkness than under light conditions (Fig. 2), though there were no significant differences between treatments (P > 0.05), except for the SV cores from station i-BF (P = 0.006). The fixation rate profiles showed little variation with regard to depth (coefficient of variation <10%) and no clear or consistent pattern. The results for the different depths were therefore integrated for the analysis and discussion, without taking into account the extreme values obtained for one CV core from i-BF. The lowest depth-integrated N2 fixation rates were found at o-BF: 7.0 ± 0.5 and 8.9 ± 3.1 mol m-2 h-1 for the SV and CV cores, respectively. The values for the rest of the stations ranged from 10.3 to 12.0 mol m-2 h-1, but significant differences (P < 0.05) were only observed between the o -BF and OUT SV cores (Fig. 3) . At OUT and bSQ, higher fixation rates were obtained for the SV cores than for the CV cores, and at o -BF and i-BF the rates were higher for the CV cores, but the differences were not significant. N2 fixation rates with and without sodium molybdate were not significantly different (Fig. 3), except at bSQ where ~40% of the fixation was attributed to sulfate-reducing bacteria. The extreme N2 fixation rates found for one of the i-BF CV cores (between centimeters 2 and 5) ranged from 9.6 to 35.0 mol m-2 h-1; these values were not detected in the treatment with sodium molybdate and can therefore be attributed to sulfate-reducing bacteria.
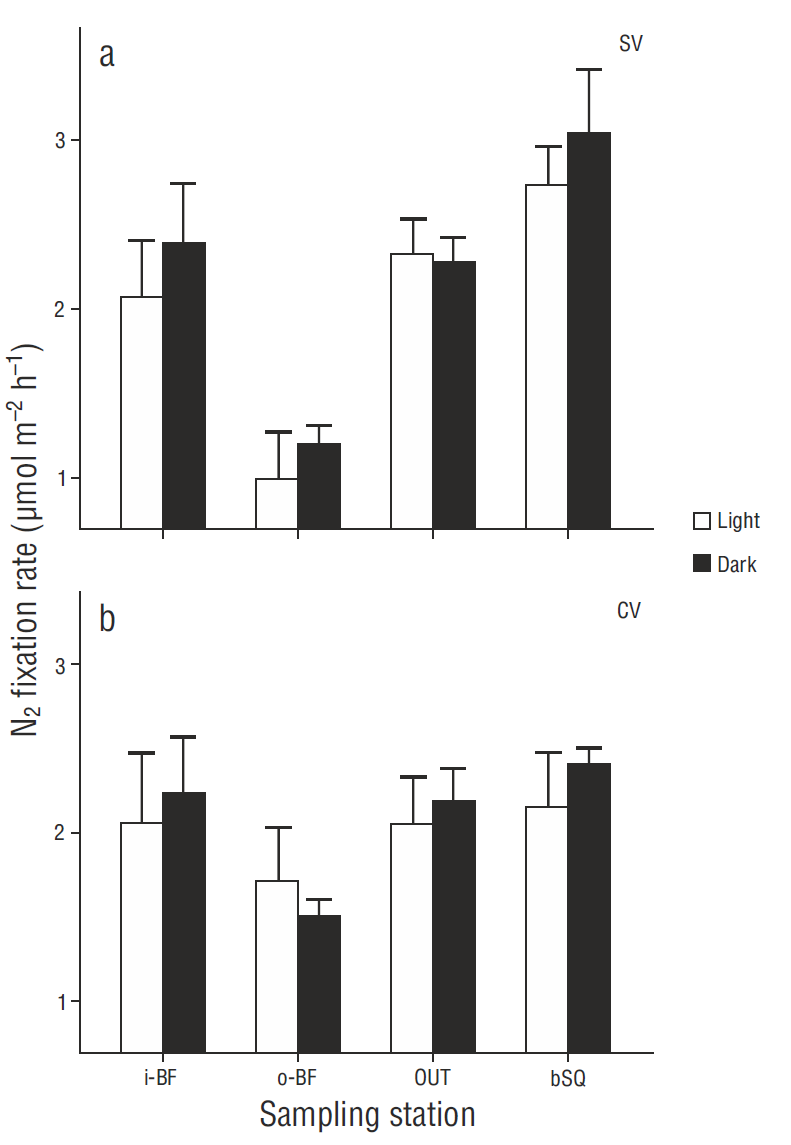
Figure 2 Spatial variation in N2 fixation in (a) bare (SV) and (b) vegetated (CV) surface sediments. N2 fixation rates under light and dark conditions are shown for each sampling station. Values represent the mean ± 1 standard error (n = 3).
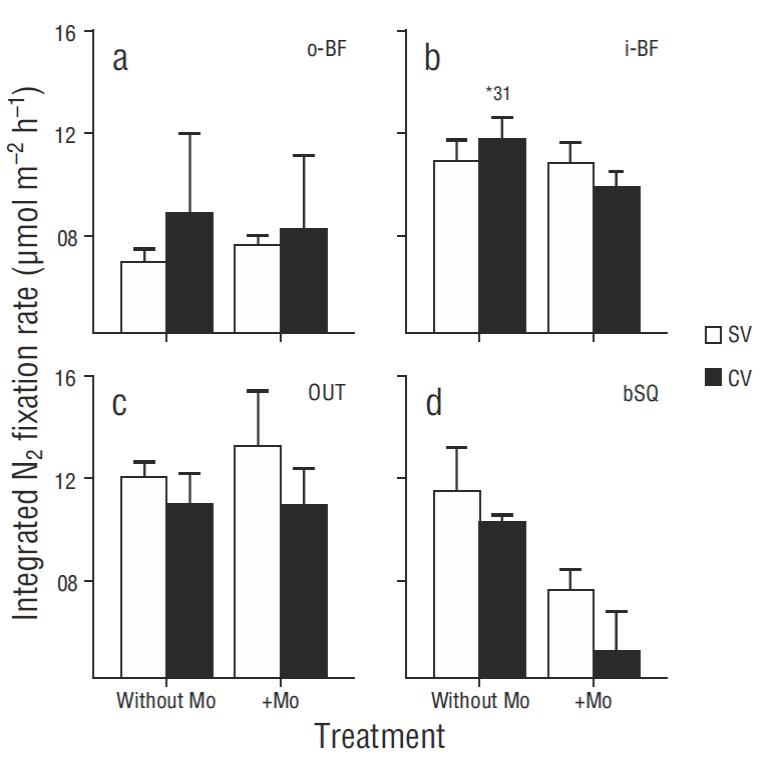
Figure 3 Spatial variation in integrated N2 fixation rates in vegetated (CV) and bare (SV) sediments at the 4 sampling stations: (a) o-BF, (b) i-BF, (c) OUT, and (d) bSQ. The treatments with sodium molybdate (+Mo) and without sodium molybdate are shown. Values represent the mean of the replicates ± 1 standard error (n = 3), except for the i-BF CV cores where n = 2. The asterisk represents the extreme value in one i-BF CV core.
N2 fixation rates in the phyllosphere
N2 fixation rates in the phyllosphere did not show significant differences (P > 0.05) between leaf 2 and leaf 4, so the rates for both leaves were considered as replicates for the subsequent analyses. At most stations (except i-BF), N2 fixation rates were higher in the experiments carried out in darkness than in those carried out under light conditions (Fig. 4a), but the differences were not significant (P > 0.05). Despite there being no significant differences among stations or between the treatments with and without irradiance (H (3, 24) = 6.46, P = 1.00, and H (3, 24) = 3.07, P = 0.385, respectively), the rates tended to increase at the innermost stations.
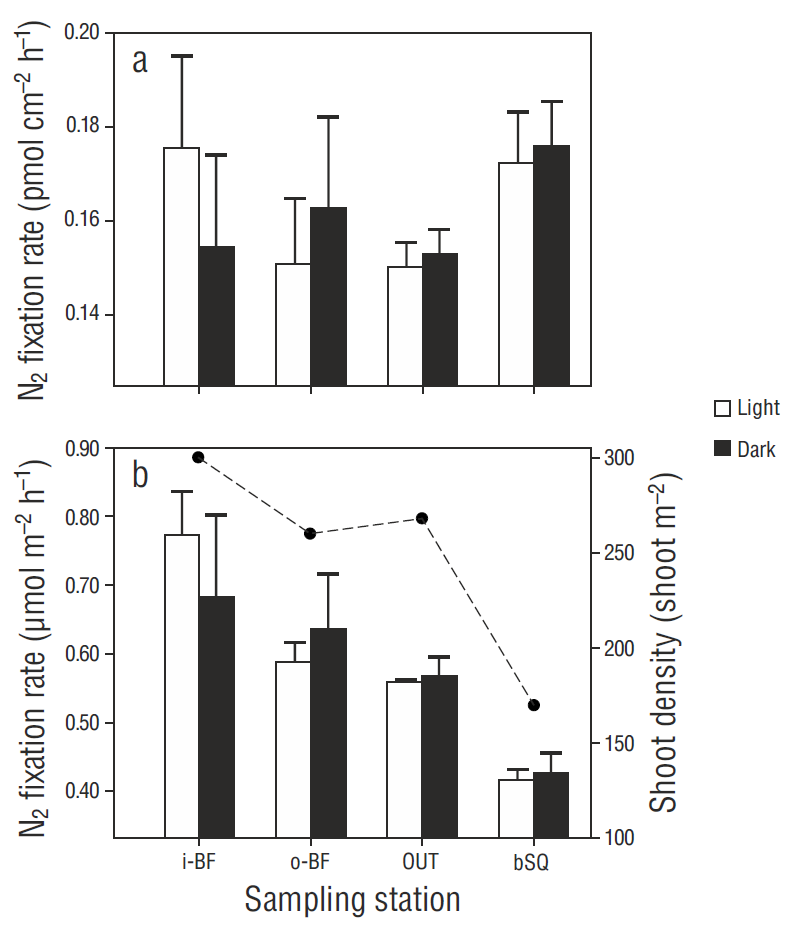
Figure 4 Spatial variation in N2 fixation in the phyllosphere per square centimeter of leaf (a) and per square meter (b). N2 fixation rates under light and dark conditions are shown for each sampling station. Values represent the mean ± 1 standard error (n = 6). The dotted line represents Zostera marina shoot density.
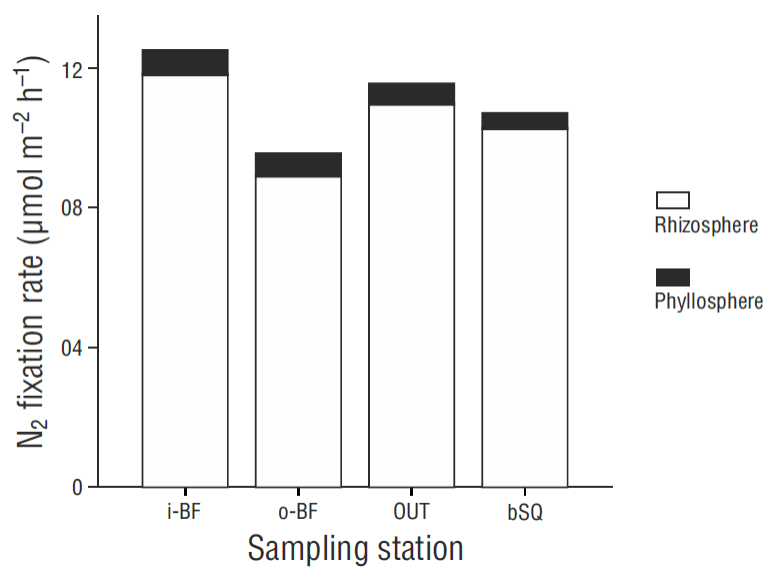
N2 fixation rates in the phyllosphere were prorated by the shoot density obtained in winter 2014 (Sandoval-Gil, unpublished data). The spatial trend of the prorated data was similar to the distribution of the shoot density values (Fig. 4b), increasing from station OUT to the west arm. The values for bSQ were lower than those for OUT. The contribution of epiphytic bacteria within the Z. marina meadows, considering the fixation rates in both the phyllosphere and sediments, was ~7% (0.6 ± 0.1 mol m-2 h-1) (Fig. 5).
Sediment characteristics
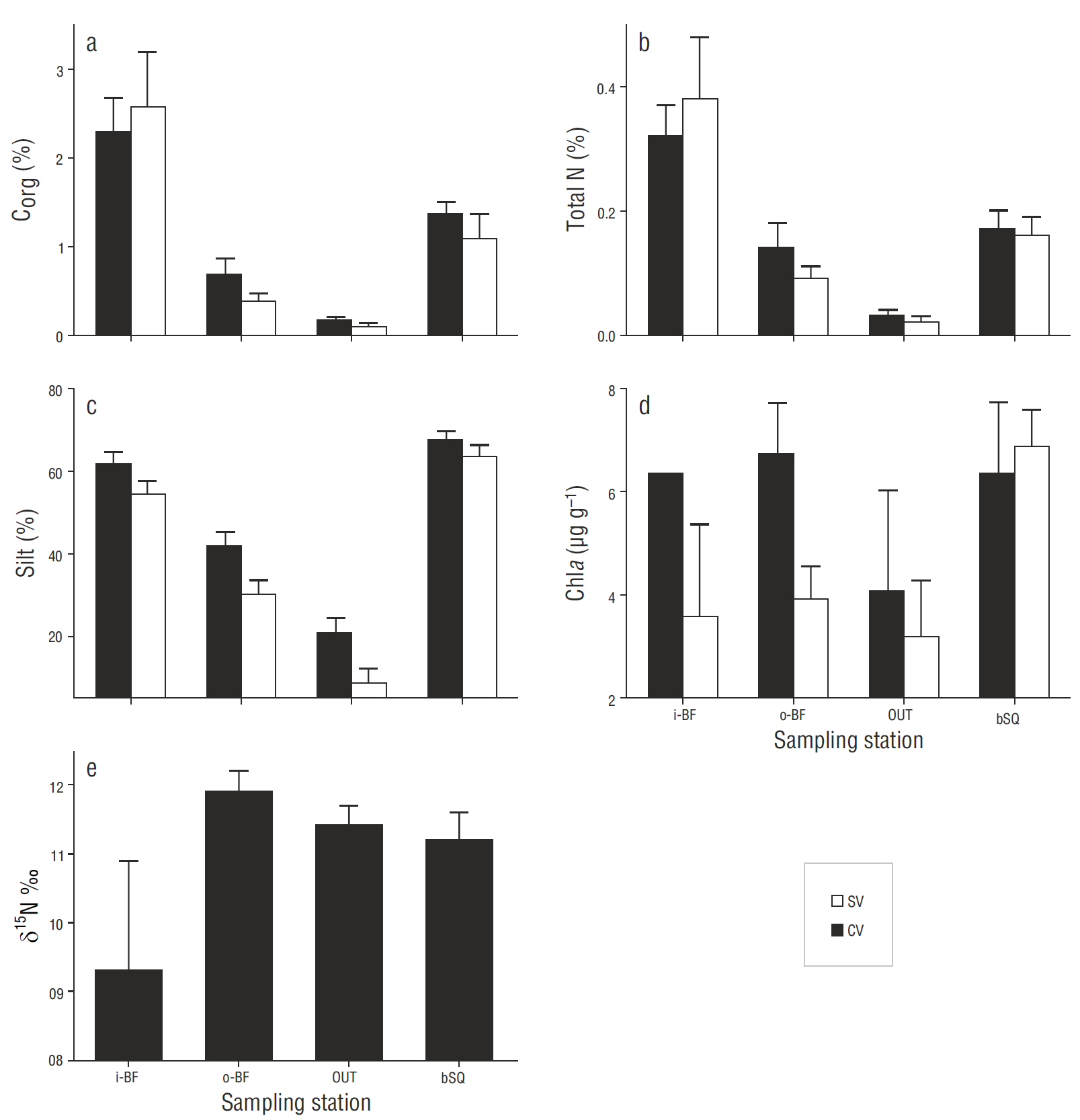
The sediments were mainly composed of sand and silt; clay represented less than 3%. Silt, Corg, and N contents were similar between depths and between the CV and SV cores within each station; however, there were significant differences among stations. Corg and N contents (Fig. 6 a, b) showed a spatial gradient, the highest values occurring at station i-BF, but there were no significant differences (P > 0.05) between the CV and SV cores. The Corg/N ratio was 6.9 ± 0.2 at i-BF, 7.1 ± 0.2 at bSQ, 6.0 ± 1.8 at OUT, and 4.4 ± 0.2 at o-BF. Corg and N showed a significant correlation (P < 0.05) with silt content (r = 0.7). Silt content (Fig. 6c) increased towards the innermost stations and there were significant differences (P < 0.05) between the stations closest to the mouth (OUT and o-BF) and farthest from the mouth (i-BF and bSQ), ranging from 1% to 34% at OUT and from 42% to 72% at the innermost stations. N2 fixation was also significantly correlated with Corg and silt content ( P < 0.05, r = 0.19 and 0.20, respectively). The chlorophyll a content of surface sediments did not show significant differences (P > 0.05) among stations or between the CV and SV cores; however, at i-BF, o-BF, and OUT the concentration of chlorophyll a was higher at the vegetated sites (Fig. 6d). Regarding the correlation between chlorophyll a and the N2 fixation rates and other sediment variables, it was only significantly correlated with silt content (r = 0.44).
N isotopic composition of Zostera marina leaves
The N isotopic composition (δ15N) of Z. marina tissues was lowest at i-BF, though the variability between the replicates was high (values between 6.9‰ and 12.4‰) so there were no significant differences (P = 0.55) between stations (Fig. 6e). At the other 3 stations the values ranged from 10.8‰ to 11.9‰.
Discussion
Benthic N2 fixation can be an important source of N for seagrasses, an alternative to the direct assimilation of nitrogenous compounds found in the water column and in sediments. Despite its potential importance, few studies have reported fixation rates in sediments and in the phyllosphere of temperate seagrass meadows. The results of the present study conducted in San Quintín Bay suggest that N 2 fixation in winter can be mainly attributed to heterotrophic bacteria, both in the parts of the sediment that light does not reach and at the surface and even in the phyllosphere. Even though the fixation rates showed little variability between sampling stations, the sediments at the innermost stations where ocean-derived N is scarce had the highest rates. The lowest fixation rates were measured at the station where oyster aquaculture activity is greater and where high denitrification rates have been found. The contribution of epiphytic bacteria to benthic N2 fixation (sediments + phyllosphere) in the lagoon was only ~7%.
Contribution of different diazotrophs to N2 fixation
In studies addressing N2 fixation activity, experiments carried out in the light and in the dark are used to discriminate between the activity of cyanobacteria (assuming that they only carry out N2 fixation in the presence of light) and the activity of heterotrophic bacteria (assuming that they carry out N2 fixation in the presence of light and in the dark) in surface sediments exposed to light and in the phyllosphere (see McGlathery et al. 1998). On the other hand, experiments with and without sodium molybdate are used to discriminate between the activity of sulfate-reducing bacteria (inhibited by molybdate) and the activity of the rest of the heterotrophs (see McGlathery et al. 1998, Cole and McGlathery 2012). Based on these assumptions, the similarity between the N2 fixation rates obtained in the experiments with and without light and in the experiments with and without sodium molybdate suggest that heterotrophs are the functional group that contributes the most to N2 fixation rates in San Quintín Bay. This means that, at least during our study period (winter), photosynthetic N2-fixing cyanobacteria in the sections exposed to light (surface sediments and phyllosphere) and sulfate-reducing bacteria showed, in general, little or no N2 fixation activity. Although only a few studies have characterized the functional groups that contribute to N2 fixation in temperate seagrass meadows, some of these studies have also suggested that heterotrophs may be the main N2-fixing bacteria (McGlathery et al. 1998, Cole and McGlathery 2012). Other studies, however, have found (Welsh et al. 1996a) or inferred (Russell et al. 2016) that sulfate-reducing bacteria contribute a high percentage of N 2 fixation in sediments. Despite the importance of N2-fixing heterotrophic bacteria in benthic marine habitats, including seagrass meadows, this functional group, unlike cyanobacteria and sulfate-reducing bacteria, has not been characterized in detail.
There is no clear explanation as to why, as at other temperate sites, the contribution of cyanobacteria to total N2 fixation in surface sediments and the phyllosphere was apparently low. One possibility is that in winter, the light available for the photosynthetic activity of cyanobacteria may have been insufficient. Knapp (2012) suggested that, unlike N2-fixing heterotrophic bacteria, including sulfate-reducing bacteria, the N2-fixing activity of benthic cyanobacteria may be inhibited by exposure for prolonged periods to dissolved inorganic N concentrations commonly observed in San Quintín Bay (>1 M). Like Cole and McGlathery (2012), our conclusion that heterotrophs are the dominant functional group responsible for N 2 fixation in the illuminated substrates (surface sediments and seagrass leaves) is based on the assumption that photoautotrophic N2-fixing cyanobacteria would only contribute to fixation in the treatments with light. However, the contribution of cyanobacteria to N2-fixation in the treatments carried out in the dark cannot be discarded because some unicellular and filamentous cyanobacteria (with and without heterocysts) are capable of fixing N during the night (Bergman et al. 1997, Severin and Stal 2008). Gracia-Escobar et al. (2014) characterized the phytoplankton community at one station in the west arm of the bay and reported that filamentous cyanophytes predominate during ebb tides, which suggests that they are resuspended from inner lagoon sediments. It is important to determine whether these cyanobacteria are benthic diazotrophs and if they fix N during the day or night.
The contribution of sulfate-reducing bacteria to N2 fixation was low at most stations (between 0% and 15%). Sulfate-reducing bacteria have also been found to contribute little (<25%) to total fixation rates at other sites occupied by Z. marina (McGlathery et al. 1998, Cole and McGlathery 2012), and this has been associated with sandy sediments with low concentration of organic matter (<2%) . In general, the San Quintín Bay sediments are characterized by low clay content (<3%). In the more dynamic areas (stations OUT and o-BF) the sediments are sandy and have low C content (<1%), whereas at the inner stations there is a predominance of silty muds (Daesslé et al. 2009) with slightly higher Corg content (Fig. 6a). The hydrodynamics and grain size seem to limit the accumulation of C org in the sediments of the stations closest to the mouth, which in turn prevents the generation of anoxic conditions, thereby limiting sulfate reduction. At station bSQ, however, where the sediments are mainly composed of silt and Corg content was >1%, sulfate-reducing bacteria contributed ~40% to the N 2 fixation rates. Sulfate-reducing bacteria have been reported to contribute >50% to N fixation in sediments both at sites with relatively low organic matter content (<0.4%, Capone 1982) and at sites where organic matter inputs to sediment are high (Welsh et al. 1996b, Fulweiler et al. 2013). In the present study, the highest N2 fixation rates (31 mol m-2 h-1), which were associated with sulfate- reducing bacteria, were measured in one of the 3 replicates of the CV cores from station i-BF that had an aver-age Corg content of 2.1%. Since the other replicates had a similar Corg content but lower N2 fixation rates, these results indicate that the bacterial communities can be distributed het-erogeneously in the sediments, forming microzones of high biomass near the roots and rhizomes with high availability of labile Corg (O’Donohue et al. 1991).
N2 fixation rates in sediments
The N2 fixation rates measured in the lagoon sediments in this study (167 to 288 mol m-2 d-1) are within the ranges reported for other temperate seagrass meadows (Capone 1982, Welsh et al. 1996a, McGlathery et al. 1998, Cole and McGlathery 2012, Cook et al. 2015); however, when compared to measurements carried out only during the winter, our results are relatively high. For example, McGlathery et al. (1998) reported rates ranging from 86 to 193 mol m-2 d-1 for sediments from a Z. marina meadow in Limfjord, Denmark, and Welsh et al. (1996a) reported rates ranging from 7.1 to 14.3 mol m-2 d-1 for Zostera noltii meadows in Bassin d’Archachon, France.
In general, N2 fixation rates exhibited reduced spatial variability in the different spatial levels examined here (different depths in the sediment column, within and outside the seagrass meadows at each sampling station, and between sampling stations). The little spatial variation in N2 fixation rates could be related to low biomass and scanty growth of Z. marina during the winter months. At San Quintín Bay, seagrass biomass is maximum in spring and summer (~200 g dry weight m-2) and minimum in winter (<100 g dry weight m-2) in accordance with the seasonal cycle of temperature and light intensity (Cabello-Pasini et al. 2003, Sandoval-Gil et al. 2015). N2 fixation rates in seagrass meadows are closely related to the variability in seagrass metabolism, with higher rates during the growing season and lower rates in winter (McGlathery et al. 1998, Welsh 2000) . An explanation for this relationship is that during the most productive periods, seagrasses exude greater amount of labile Corg through the roots and rhizomes, favoring the activity of heterotrophic bacteria, including sulfate-reducing bacteria (Moriarty et al. 1986, Welsh 2000).
Low seagrass productivity in winter may also explain why there were no significant differences in N2 fixation rates between vegetated and bare sediments, as has been observed in other studies (Russell et al. 2016). Cole and McGlathery (2012) found similar fixation rates within and outside young Z. marina meadows (2 to 3 yr old, density <100 shoots m-2), whereas in the case of older meadows (7 to 8 yr old, density >400 shoots m-2), fixation rates were higher within the meadows. These authors suggest that shoot density could play an important role in controlling the differences in fixation rates at vegetated and bare sites. At San Quintín Bay, shoot density at the sampling stations in winter ranged from 170 to 300 shoots m-2 (Fig. 4), which is probably a low density to induce differences in the fixation rates within and outside the meadows. Slightly higher fixation rates were measured in the bare sediments from stations OUT and bSQ (Fig. 3) . The bSQ sediments also had a slightly higher chlorophyll a content (Fig. 6d). Since chlorophyll a content is considered an indicator of microalgal biomass (Nagel 2004), the fact that N2 fixation is higher in bare sediments than in vegetated sediments suggests that N2-fixing cyanobacteria are contributing to fixation in bare sediments (Nagel 2004, Russell et al. 2016).
Fixation rates were significantly lower at station o-BF than at the other 3 stations; the highest δ15N values (~12‰) determined for seagrass leaves were also recorded at this station (Fig. 6e). In other studies (Sandoval-Gil et al. 2015 and unpublished data), maximum δ15N values have also been found for Z. marina at this same site, suggesting that denitrification is an intense process that dominates over N2 fixation. The sediments at this oyster farming site were the most affected by the feces and pseudofeces produced by oysters (Sandoval-Gil et al. 2016), as reflected by the low Corg/N ratios (4.4 ± 0.2). Several studies have shown that oyster cultures input a large amount of labile organic matter (as feces or pseudofeces) to sediments (Newell et al. 2005) and that these inputs intensify the denitrification process (Kellogg et al. 2014). Other studies have shown that inputs of labile organic matter to sediments can induce a predominance of denitrification over N 2 fixation (Fulweiler et al. 2013). It can thus be assumed that denitrification dominates in sediments at station o-BF, possibly limiting the activity of diazotrophs. Further studies are necessary to understand the interaction between denitrifying bacteria and diazotrophs and to identify the possible causes limiting or intensifying the activity of both groups.
N2 fixation rates in the phyllosphere
Only a small fraction (~7%) of the benthic N2 fixation rates (sediments + phyllosphere) in San Quintín Bay can be attributed to fixation in the phyllosphere (by epiphytic bacteria), as has been reported for other temperate sites occupied by Zostera spp. in winter (McRoy et al. 1973, Welsh 2000); however, N2 fixation rates in the phyllosphere were low (<13 mol m-2 d-1) compared with rates measured in Z. marina meadows in summer (>100 mol m-2 d-1, Cole and McGlathery 2012). As in the case of sediments, one of the factors stimulating fixation rates in the phyllosphere is labile C exuded by seagrasses, in this case through the leaves, since this C serves as substrate for diazotrophs (Capone et al. 1979). The amount of N2-fixing epiphytes in the leaves can also determine the variation in fixation rates in the phyllosphere (Reynolds et al. 2015). In this study we did not measure epiphyte biomass, but visually there were no differences in coverage between leaves 2 and 4 of the same shoot, nor between the leaves from different meadows. This apparent homogeneity in epiphyte biomass between leaves and stations probably contributed to the low variation in N2 fixation rates in the phyllosphere. It would be recommendable for future studies to measure epiphyte biomass and characterize the community of N2-fixing bacteria.
Contribution of N2 fixation to seagrass N demand
Based on the specific N demand values ( mol g-1 dry weight h-1) for the growth and foliar biomass of Z. marina in winter reported by Sandoval-Gil et al. (2015), N demand values of 114 to 238 mol m-2 h-1 were estimated for Z. marina (Table 1). The benthic N2 fixation rates obtained in this study (phyllosphere + sediment) were 9.5 to 13.0 mol m-2 h-1, so this process would potentially supply between 5% and 10% of the N required by Z. marina in San Quintín Bay, similar to that reported for other systems (5-12%) (Welsh et al. 1996a, McGlathery et al. 1998); however, the values are low compared with the direct uptake of dissolved inorganic N by foliar and subterranean tissues (>300 mol m-2 h-1, Sandoval-Gil et al. 2015). Given that shoot density is higher at i-BF, N2 fixation rates per square meter are higher at this station (Fig. 4b), which is coherent with the higher percent contribution of N2 fixation to the N demand of plants growing at that station. The latter could also be related to lower δ15N values (<11‰) obtained for Z. marina leaves at station i-BF in the present and previous studies (Sandoval -Gil et al. 2015; Hernández-López et al., unpublished data), since the composition of the source, atmospheric N2, is close to 0‰ (Sharp, 2007); hence, this process can cause a decrease in δ15N values.
Table 1 Values of N2 fixation (phyllosphere + rhizosphere), N demand of Zostera marina, and percent contribution of N2 fixation to Z. marina N demand. DW = dry weight.
| Sampling station | Biomass* (gDW m-2) | N demand* (µmol gDW-1 h-1) | N demand mol m-2 h-1) | N fixation mol m-2 h-1) | Percent contribution |
| i-BF | 178 | 0.7 | 125 | 12.5 | 10.8 |
| o-BF | 229 | 0.5 | 114 | 9.5 | 8.4 |
| OUT | 199 | 1.2 | 238 | 11.5 | 4.8 |
| bSQ | 242 | 0.8 | 194 | 10.7 | 5.5 |
Contribution of N2 fixation to the N budget of San Quintín Bay
Finally, we estimated the contribution of N2 fixation to the N budget of San Quintín Bay to test the assumption that this process in not very important in the lagoon (Camacho-Ibar et al. 2003). According to Ward et al. (2003), seagrass meadows, mudflats, and channels cover an area of 20.7, 8.9, and 8.5 km2, respectively, in San Quintín Bay. The mean N2 fixation rate (sediments + phyllosphere) for the vegetated sediments from all 4 sites was 266 ± 34 mol m-2 d-1, and for the bare sediments, 266 ± 98 mol m-2 d-1. Prorating these rates by the area of each habitat produces N inputs to the system by N2 fixation of 5.5 ± 0.7 × 103 mol d-1 in the area occupied by Z. marina, 2.4 ± 0.8 × 103 mol d-1 in the mudflats, and 2.3 ± 0.8 × 103 mol d-1 in the channels, resulting in a total input of 10.1 ± 2.4 × 103 mol d-1 (0.26 ± 0.06 mmol m-2 d-1).
Using a mass balance model (the LOICZ model), Camacho-Ibar et al. (2003) calculated an oceanic input of dissolved inorganic N (nitrate + ammonium) by advection during winter of 2.2 × 103 mol N d-1 and an input by tidal mixing of 86 × 103 mol N d-1, for a total oceanic input of 88 × 103 mol N d-1. Our results show that N2 fixation is equivalent to ~12% of the oceanic dissolved inorganic N inputs (Fig. 7). With the same model, Camacho-Ibar et al. (2003) calculated that the difference between fixation and denitrification in winter was -0.7 mmol m-2 d-1. Therefore, with our fixation estimate we can calculate a denitrification rate of 1.0 mmol m-2 d-1, which corresponds to 38 × 103 mol N d-1.
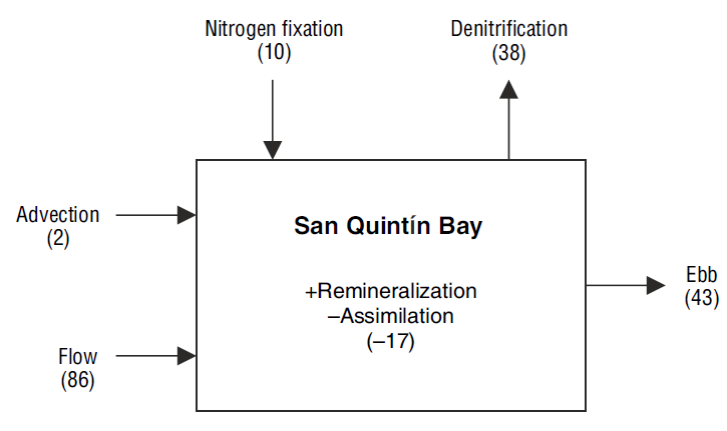
Figure 7 Fluxes of dissolved inorganic N (×103 mol N d-1) between San Quintín Bay, the adjacent ocean, and the atmosphere. The arrows indicate the direction of the flux. Inside the box, the plus sign represents input and the minus sign represents removal of dissolved inorganic N at the bay.
In summary, our estimates indicate that in winter, N2 fixation may be equivalent to a third of the N lost via denitrification. This result is relevant because it indicates that the input of new N by fixation cannot be considered a negligible flux in the N budget of San Quintín Bay. Moreover, our study contributes to the growing evidence indicating that N2 fixation rate measurements should not be restricted to oligotrophic systems, since N2 fixation by heterotrophic bacteria, including sulfate-reducing bacteria, in temperate environments can contribute to reduce the uncertainty in the global oceanic N budget (Knapp 2012). In San Quintín Bay, N2 fixation in the sediments and in the phyllosphere will likely be greater during the growing season of Z. marina (spring and summer) than in winter, as has been reported for other sites (McGlathery et al. 1998, Welsh 2000). We therefore think it is important to measure N2 fixation rates throughout the year, as well as to characterize the N2-fixing organisms using molecular methods (see, e.g., Bentzon-Tilia et al. 2015, Newell et al. 2016).











 nueva página del texto (beta)
nueva página del texto (beta)