Potato (Solanum tuberosum) is one of the main global crops, after sugarcane (Saccharum officinarum), corn (Zea mays), rice (Oryza sativa) and wheat (Triticum spp.) (FAO, 2016). Diseases affecting roots, tubers and leaves of the potato plant can prevent proper tuber formation. One of such disease is black scab, caused by the pathogen Rhizoctonia solani (Betancourth et al., 2021). This pathogen infects many wild and cultivated plants in Mexico (DGSV-CNRF, 2020).
To control R. solani, growers often apply synthetic fungicides such as Carbendazim, Copper phosphite and Thiabendazole (Alburqueque and Gusqui, 2018). Chemicals like Azoxystrobin and propiconazole also provide effective disease control (Khan and Bolton, 2010). However, chemical control is not recommended due to the high residue levels (Leadbeater and Gisi, 2010). Therefore, alternative methods to control R. solani have been explored, including biological control.
The antagonistic effect of some Trichoderma species on R. solani was first described in 1932 (Weindling, 1932). Today, Trichoderma is used to protect plants from root pathogens like R. solani, and to stimulate plant defense mechanisms (Korolev et al., 2008). Trichoderma can parasitize and eliminate a wide range of fungal plant pathogens (Romero-Arenas et al., 2017). Its agricultural success and use stems from its diverse mechanisms of action (Infante and Martínez, 2019).
The agricultural utilization of Trichoderma species represents a promising technological approach for producing high-quality biofungicides (Hernández-Mendoza et al., 2012; Companioni et al., 2019). Despite its considerable potential, the application of Trichoderma based solutions for disease management in potatoes remains limited in Mexico, and challenges associated with addressing Rhizoctonia solani infections persist. Thus, the primary aim of this study was to conduct an in vitro assessment of the efficacy of four distinct Trichoderma species as potential biofungicides targeting Rhizoctonia solani.
In this research, isolates of T. viride (Tv), T. koningii (Tk), T. harzianum (Th), and Trichoderma spp. (Tspp.) employed, isolates were obtained from the collection maintained by the Universidad Tecnológica del Sur in Morelos. Concurrently, the R. solani strain was procured from the Zacatepec Experimental Station of the National Institute of Forestry, Agriculture, and Livestock Research (INIFAP). The isolation of R. solani involved obtaining samples from infected potato plants of Fiana variety from Toluca, Mexico state. Subsequent to carefully rinsing the plants to eliminate soil residues through flowing water, symptomatic one squared centimeter sections from various plant segments were disinfected utilizing 3% sodium hypochlorite for 5 min., followed by rinsing with sterile distilled water. These sections were plated on Petri dishes with Sabouraud dextrose agar culture medium (MCD LAB) and subjected to an incubation period of 25 ± 2 °C for 48 hrs. Establishment of pure cultures were achieved by inoculating Petri dishes with hyphal tips possessing characteristics and morphology indicative of R. solani. Both Trichoderma species and R. solani isolates described in this study are currently undergoing molecular identification procedures.
For evaluations, fungi were cultivated on Sabouraud dextrose agar medium at controlled temperature of 25 ± 2 °C for 96 hrs (Trichoderma species) and for 10 days (R. solani).
Competition for resources between Trichoderma species and R. solani were evaluated using the dual confrontation method as outlined by Bell et al. (1982) and detailed by Martínez and Solano (1994). Evaluation were conducted in 90 mm diameter Petri dishes containing Sabouraud dextrose agar medium. Individual 5 mm agar disks harboring mycelium of Tv, Tk, Th, and Tspp. were positioned one centimeter from the edge of each Petri dish (replicate), with a 5 mm disk of R. solani growth positioned at the opposing end. In parallel, a control experiment featuring R. solani growth without of Trichoderma species were conducted employing a completely randomized design, five treatments, with five replicates (Petri dishes), were evaluated. Radial growth measurements during confrontations were taken at 24-hr intervals, up to 120 hrs, within a controlled environment of 25 ± 2 ºC.
Classification of Tv, Tk, Th, and Tspp. isolates as antagonists against R. solani was executed using the five class scale introduced by Bell et al. (1982). Percentage Inhibition of Radial Growth Rate (PIRGR) was calculated using the formula PIRGR = (R1 - R2) / R1 x 100, where R1 represents the radial growth of the phytopathogen colony within the control treatment, and R2 signifies the radial growth of the pathogenic isolate when confronted by the antagonist (Rahman et al., 2009). Statistical analysis of PIRGR data were performed employing IBM SPSS® Statistics for Windows version 25 (IBM Corp, Armonk, New York, USA). Prior to analysis, normality and homogeneity of variance were assessed using the Levene and Kolmogorov-Smirnov tests. Subsequently, an analysis of variance and Tukey’s mean comparison test (p ≤ 0.01) was executed.
Mycoparasitism was observed in the contact zone between the two fungi (antagonist pathogen), and the array of hyphal interactions; rolling, penetration, vacuolization, and lysis were assessed in accordance with the methodology outlined by Chet et al. (1981). To conduct this analysis, adhesive tape was utilized to obtain samples from the interaction zone, which were subsequently mounted on slides with a drop of lactophenol blue. Examination of samples were carried out under an optical microscope (LABOMED, model CXL TRINOCULAR 9135007) at 40X magnification. Each replicate (Petri dish) encompassed three distinct preparations.
The four Trichoderma isolates subjected to evaluation were classified within antagonism class 2 of the scale established by Bell et al. (1982) (Figure 1). Their growth encompassed more than 60% of the surface area occupied by R. solani. By applying the same scale, in vitro assessments conducted by Pérez et al. (2020) demonstrated that T. atroviride, T. konigiopsis, and T. harzianum exhibited an overgrowth exceeding 85% over R. solani, warranting their classification within antagonism class 1. Likewise, when assayed against Botrytis sp., strains of T. koningii, T. atroviride, T. inhamatum, and T. harzianum were classified as belonging to class 1 (Acosta et al., 2021), a classification shared by T. asperellum and Trichoderma spp. in relation to B. cinerea (Pincay et al., 2021).

Figure 1 Confrontation of Trichoderma isolates with R. solani were carried out on Sabouraud agar, followed by the assessment of R. solani’s radial growth inhibition at 24 hr intervals. The used abbreviations were as follows: Tspp. (Trichoderma spp.), Th (T. harzianum), Tv (T. viride), Tk (T. koningii), with the control group represented by R. solani.
The antagonistic interaction between fungi is mediated through an intricate network of attack and counter-response mechanisms. Within the arsenal of attack strategies employed by Trichoderma species, a diverse array is noteworthy, including parasitism, antibiosis, competition for resources and space, enzymatic disruption of other microorganisms metabolisms, elicitation of defense responses, and facilitation of germination and growth (Morales-Mora et al., 2020).
All treatments exhibited an antagonistic effect exceeding 50% after 96 hrs, as indicated in Table 1. Growth of R. solani colony displayed noticeable changes, as illustrated in Figure 1. At 120 hrs, Tk, Th, and Tspp., treatments showed the most substantial inhibition percentages. Notably, all three treatments exhibited statistically significant differences in their mean values (p ≤ 0.01). Furthermore, data revealed that after 72 hrs, treatments demonstrated an increased growth rate. This phenomenon could be attributed to potential metabolites released by R. solani, as detailed in Table 1.
Table 1 Radial Growth Inhibition (RIGI) of Trichoderma on R. solani.
| Treatments | 24 h | 48 h | 72 h | 96 h | 120 h |
|---|---|---|---|---|---|
| Tspp. | 17.8 c | 26.2 c | 32.7 c | 55.1 c | 65.5 c |
| Th | 22.2 b | 27.9 b | 36.3 b | 57.3 b | 66.7 b |
| Tv | 11.1 d | 20.5 d | 29.8 d | 50.4 d | 57.0 d |
| Tk | 24.4 a | 31.2 a | 43.3 a | 61.2 a | 67.5 a |
| Es χ | 1.31* | 1.0* | 1.3* | 1.0* | 1.08* |
| CV (%) | 27.76 | 15.12 | 14.66 | 7.17 | 6.74 |
Tspp. = Trichoderma spp; Th = T. harzianum; Tv = T. viride; Tk = T. koningii; Es χ = standard error of the mean; CV = coefficient of variation. Different letters in the same column indicate significant differences according to Tukey’s multiple range test (p ≤ 0.01).
Other in vitro assessments, Trichoderma spp. exhibited inhibition rates exceeding 50% against Trichothecium sp., Cladosporium sp., and Fusarium sp.; meanwhile, it demonstrated a remarkable 75% inhibition against Didymella bryoniae (Martínez et al., 2013). Using the native strain TC05 of Trichoderma spp., both under in vitro conditions and within a mesh house, revealed a substantial antagonistic potential against Fusarium spp. (Rodríguez-Pinto et al., 2021). Elucidation of underlying mechanisms of action exhibited by these antagonist isolates in vitro forms the foundation for their selection. This selection process pertains not only to subsequent assessments under semi-controlled and field conditions but also guides the formulation of production strategies aimed at achieving enhanced stability and efficacy in field results.
Figure 2, shows the confrontation zone between Th and R. solani, where similar to Tspp., four distinct types of hyphal interactions were observed, encompassing coiling, penetration, vacuolization, and lysis of hyphae. Meanwhile, Tv and Tk exhibited two and three types of interactions, respectively. On the other hand, coiling and hyphal penetration emerged as the predominant mycoparasitic strategies employed by all evaluated Trichoderma isolates (Table 2). Literature has documented that the amalgamation of diverse mycoparasitic tactics contributes to a more effective biocontrol action against phytopathogens (Companioni et al., 2019).
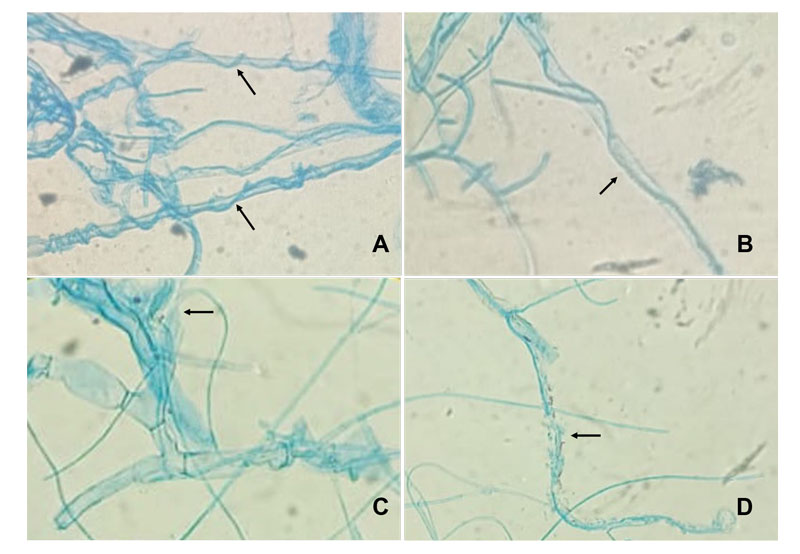
Figure 2 Mechanisms of antagonistic action of T. harzianum on R. solani: coiling (A), penetration (B), vacuolization (C) and lysis (D).
Table 2 Types of hyphal interaction between Trichoderma isolates and R. solani.
| Treatments | Hyphal interaction type |
|---|---|
| Trichoderma spp. (Tspp.) | E-P-L-V |
| T. harzanium (Th) | E-P-L-V |
| T. viridie (Tv) | E-P |
| T. koningii (Tk) | E-P-L |
Coiling (E), Penetration (P), vacuolization (V) and Lysis (L).
Trichoderma species produce enzymes, including ß-1,3-glucanases, ß-1,6-glucanases, chitinases, and proteases, capable of breaking down cell walls of various fungi (Ait-Lahsen et al., 2001). In this process, Trichoderma species hyphae exhibit chemotropic growth towards the host. When in close proximity to the pathogen, their attachment and coiling initiate, ultimately leading to lytic activity.
Results from dual confrontation of T. harzianum and T. koningii with R. solani revealed significant radial growth inhibition rates. Correspondingly, both T. harzianum and Trichoderma spp. displayed four distinct types of hyphal interactions with the pathogen. Furthermore, all assessed species were categorized under class 2 on the Bell et al. scale (1982).
While all Trichoderma isolates exhibited comparable levels of antagonism against R. solani, exploring in vitro interactions with diverse R. solani strains, varying phytopathogenic fungi, and the original crop environments could aid in selecting Trichoderma species showcasing optimal parasitic and control characteristics.











 texto en
texto en 


