Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de fitopatología
versión On-line ISSN 2007-8080versión impresa ISSN 0185-3309
Rev. mex. fitopatol vol.39 no.1 Texcoco ene. 2021 Epub 07-Mayo-2021
https://doi.org/10.18781/r.mex.fit.2006-8
Scientific articles
Fusarium spp. and inoculum load estimation associated to commercial Agave tequilana offsets at different regional epidemic inductivity levels
1Programa Fitosanidad-Fitopatología; Colegio de Postgraduados, Campus Montecillo, Km 36.5 Carretera México-Texcoco, Montecillo, Texcoco, Estado México, CP 56230, México;
2Laboratorio de Análisis de Riesgo Epidemiológico Fitosanitario (CP-LANREF); Colegio de Postgraduados, Campus Montecillo, Km 36.5 Carretera México-Texcoco, Montecillo, Texcoco, Estado México, CP 56230, México;
3Programa Fruticultura. Colegio de Postgraduados, Campus Montecillo, Km 36.5 Carretera México-Texcoco, Montecillo, Texcoco, Estado México, CP 56230, México;
4Área de Investigación, Casa Sauza, Francisco Javier Sauza #80, Tequila, Jalisco, C.P. 46400., México
The research objective was to identify the Fusarium specie(s) associated with commercial offsets of Agave tequilana and to develop a methodology to quantify the inoculum load in offsets from Jalisco mother plantations with differential epidemic inductivity to wilt and dry bud rot syndrome (SMAP). The purpose was to provide criteria for the certification of mother plantations. The samples were collect between March and May in 2018 and 2019, from 21 commercial plantations of 14 municipalities of Los Altos, South, and Valley of Jalisco. The number of diseased plants (PE) and SMAP severity (S) were estimated in 63 and 200 plants/plantation using App-SIVEA for 2018 and 2019 respectively. The CIFUSAG method was developed and applied in 7055 offsets and 46656 fungal colonies obtained from 2364 basal ‘piña’ wash isolates, internal tissue sections, and offset total maceration. Colony Forming Units (CFU) of Fusarium spp. (Fsp) and total fungi (HT), purification, monosporic, morphological and cultural characterization was made with Komada, water-agar, Sabouraud, SNA, and Sabouraud media, respectively, selecting 557 contrasting isolates. The highest epidemic inductivity in Los Altos was associated to moderate Fusarium Index [(FI) = (∑Fsp) / (∑HT) ] (0.30 and 0.40) and Fsp (20 and 72 CFU) compared to South, which had higher values (0.69, 0.50; 23, 84 CFU, respectively) (Tukey p=0.05). The offset base-size had no influence on FI and Fsp (p=0.183). FI and Fsp of basal ‘piña’ washing were not correlated with S in mother plants (r2 = 0.036 and 0.13), while PE only was correlated with Fsp (r2 = 0.94). The inoculum load obtained by washing was higher than internal tissue with a total of 17,828 CFU (93%) and FI=0.42. Molecular analysis with the EF-1a gene showed an association of 23 isolates with four phylogenetic complexes: Fusarium oxysporum, F. fujikuroi, F. solani and F. incarnatum-equiseti with identity greater than 98%. Offsets of Agave tequilana constitutes a dispersal factor of at least four species of Fusarium spp. in the range of 3.3±3 y 6.83±4.2 CFU/offset.
Key words: Blue Agave; wilt; dry rot; soil; supresive
Esta investigación tuvo como objetivos identificar la especie(s) de Fusarium asociada(s) a hijuelos comerciales de Agave tequilana y desarrollar una metodología para cuantificar la carga de inóculo en hijuelos provenientes de plantaciones madre de Jalisco con inductividad epidémica diferencial al síndrome marchitez y pudrición seca del cogollo (SMAP). La finalidad fue proporcionar criterios para la certificación de plantaciones madre. La fase de campo se realizó entre marzo-mayo 2018 y 2019 en 21 plantaciones comerciales en 14 municipios de Los Altos, Sur y Valles de Jalisco. Se estimó el número de plantas enfermas (PE) y severidad SMAP (S) en 63 y 200 plantas/plantación mediante App-SIVEA para 2018 y 2019 respectivamente. Se desarrolló y aplicó el método CIFUSAG en 7055 hijuelos y 46 656 colonias fungosas obtenidas de 2364 siembras de lavado basal de la ‘piña’, secciones de tejido interno y macerado total de hijuelo. Unidades formadoras de colonia de Fusarium spp. (Fsp) y hongos totales (HT), purificación, monospóricos, caracterización morfológica y cultural se realizó con los medios Komada, agua-agar, Sabouraud, SNA y Sabouraud, respectivamente, diferenciando 557 aislados. La mayor inductividad epidémica en Los Altos significó moderado Índice de Fusarium [(IF) = (∑Fsp) / (∑HT)] (0.30 y 0.40) y Fsp (20 y 72UFC) respecto al Sur que tuvo valores más altos (0.69, 0.50; 23, 84UFC, respectivamente) (Tukey p=0.05). Calibre de hijuelo no tuvo influencia en IF y Fsp (p=0.183). IF y Fsp de lavado basal no estuvieron correlacionadas con S de plantas madres (r2 = 0.036 y 0.13), mientras que PE únicamente se correlacionó con Fsp (r2 = 0.94). La carga de inóculo obtenida por lavado fue superior al de tejido interno con un total de 17,828 UFC (93%) e IF=0.42. Análisis molecular con el gen EF-1a evidenció asociación de 23 cepas con cuatro complejos filogenéticos: Fusarium oxysporum, F. fujikuroi, F. solani y F. incarnatum-equiseti con identidad superior al 98%. El hijuelo comercial de Agave tequilana constituye un factor de dispersión de al menos cuatro especies de Fusarium spp. en el rango de 3.3±3 y 6.83±4.2 UFC/hijuelo.
Palabras clave: Agave azul; marchitez; pudrición seca; suelo; supresividad
Agave tequilana variety Blue is the raw material used to produce tequila. Mexico is the only tequila producer in the world and holds the Denomination of Origin of Tequila (DOT) since 1977. This beverage is also supported by NOM-006-SCFI-2012. Its DOT condition restricts the growing of Agave tequilana var. Blue for tequila production in five Mexican states, of which Jalisco represents 77% of the total production (SIAP, 2020; López-Bautista et al., 2020; Coria-Contreras et al., 2019). According to records of Mexico’s Consejo Regulador del Tequila (CRT), in 2018 and 2019, the area planted with agave increased approximately 31%, which represents an all-time-record of 96 million of planted offsets (3,200 offsets ha-1), not including offsets to replant harvested hectares (CRT, 2020).
At present, there is an empirical consensus that the agave propagative material contributes to pests dissemination, such as Fusarium, an agent of great productive impact (SENASICA-DGSV, 2017; NOM-083-FITO-2003). However, there are no studies to demonstrate, using A. tequilana offsets, that these are associated with Fusarium spp. nor the inoculum load that they can disseminate and become a risk factor for the establishment of new plantations. Considering the existence of areas with different levels of epidemic inductivity and damage caused by the wilt and dry bud rot syndrome (SMAP) (López-Bautista et al., 2020), and that some plantations are used as mother plantings, with limited or null regulation, for example, the generic sanitary certification is carried out in Guanajuato, but not in any other producing states of DOT, etiological and epidemiological studies at mother plant-offset level are justified to strengthen and justify the establishment of certified plantations where low sanitary risk offsets can be produced and marketed. At commercial plantations of adult plants, which are commonly used as offset mother plants, López-Bautista and collaborators carried out a comprehensive regional study in Jalisco with emphasis on Fusarium species (López-Bautista et al., 2020). In that study, agave wilt and dry bud rot were identified as a syndrome (SMAP) associated with a Fusarium species complex. These researchers reported a total of 16 binomial and phylogenetic species, plus six non-identified species isolated from soil, root and aerial tissue, which belong to four phylogenetic complexes: Fusarium oxysporum [FOSC], F. solani [FSSC], F. fujikuroi [FFSC] and F. incarnatum-equiseti [FIESC]. At least two binomial species had been previously reported: F. oxysporum and F. solani (Vega-Ramos et al., 2013; Ramírez-Ramírez et al., 2017).
The implication of mother plant healthiness and the soil parasitic inductivity in the offset health is important to determine certification strategies and risk management of agave production at regional level. The extensive association of Fusarium species complexes with SMAP in commercial plantations of Agave tequilana (López-Bautista et al., 2020) allows to postulate, as the rational basis of the present work, that the differential quantity and compositional association of Fusarium, both in a non-parasitic external condition and in an internalized form in tissues of blue agave offsets, is dependent upon the offset growth time as well as of the epidemic inductivity level in the region where mother plantations are established. In this context, the purpose of this study was to develop and apply a methodology to demonstrate the parasitic association of Fusarium spp. with A. tequilana offsets, and to estimate the inoculum load based on SMAP inductive epidemic regions in Jalisco and the status of the offsets development by the time they are cut for commercial purposes.
MATERIALS AND METHODS
Establishment of the experimental site in the field. Samples were collected from offsets for commercial use in 2018 and 2019 with nine and 12 commercial plantations of A. tequilana variety Blue between 3-4-years-old, respectively. The samples were collected in 14 municipalities distributed across the agave production regions of Los Altos, Valley, and South Jalisco. The plantations were selected based on the regional epidemic inductivity level represented by diseased plants or showing SMAP symptoms in the 2017 epidemic cycle. Data from SENASICA and Sistema Integral de Protección y Vigilancia Epidemiológica del Agave (SIVEA) campaign against agave regulated pests were used for that purposes (Table 1).
Mother plantation characteristics. Mother plantations were 3-4-year-old and offsets were commercially uprooting during the experiment development. These plantations conformed the regional criteria applied by producers including asymptomatic appearance and good standard agronomic management, which consists in the application of herbicides (June-July), fertilization, “trimming” (removal of side-leaf tips to access the rows) before offsets are cut, hillings in the first two years and removal of flower stalks when found.
Experiment design. The experiment design selected for the analysis of variance consisted in subdivided plots in factorial 3x3x3 that corresponded to Los Altos, Valley and South, the region factor in large plots; high, moderate, and low inductivity, the epidemic intensity factor of wilt and dry rot (SMAP) in medium-size plots; and three offset base-size in small plots. By region, the plantations were selected according to offset uproot scheduled records from cooperative companies and producers and based on their regional distribution to have two sites/inductivity/region available. Each plot was divided into three blocks with three rows each (3 m apart) with an approximated distribution in one hectare. Offsets for each base-size to obtain samples were taken from the site of field “temporary” harvest collection where offsets are stored after uprooting. In each plot, samples of the three base-size were collected: Lime, Orange, and Grapefruit (8-10, 10-12 and 12-15 cm in diameter of base-size or ‘piña’, respectively.) The total sample of 270 offsets plot-1 was made up of 15 offsets per base-size in two replications per block. In 2018, three composite samples per base-size/block were selected, and two samples only in 2019, mainly in Los Altos and South regions, to widen the internalization sampling. Per plot, for external tissue samples from the 2018 production cycle, 27 samples/plot were obtained for a total of 243 total composite samples, and in 2019, 18 samples/plot for a total of 216 samples. For internal tissue samples, in 2018, 30 offsets were collected in a high-inductivity plot in Los Altos, and in 2019, 140 offsets (20 per plot), 120 in Los Altos, and 20 in a low-inductivity plot in the South region.
Table 1 Location and technological characterization of 21 commercial plantations of Agave tequilana with agave wilt and dry bud rot syndrome (SMAP) selected in 2018 and 2019 to study the etiology and inoculum load in commercial offsets.
| Año | Región | Inductividad | Municipio | Latitud | Longitud | Altitud (msnm) | Tecnifi- cación | Manejo | ID Plantación | |
|---|---|---|---|---|---|---|---|---|---|---|
| 2018 | Altos | Alta | Atotonilco | 20.51509 | -102.6127 | 1556 | Medio | Inorgánico | A-ATO-P4 | |
| Moderada | Jesús María | 20.59527 | -102.15688 | 2180 | Alto | Inorgánico | A-JM-M2 | |||
| Baja | Jesús María | 20.76863 | -102.14421 | 2279 | Medio | Inorgánico | A-JM-M1 | |||
| Sur | Baja | Juchitlán | 20.08979 | -104.08275 | 1245 | Alto | Inorgánico | S-JUC-PM6 | ||
| Juchitlán | 20.09765 | -104.07422 | 1199 | Alto | Inorgánico | S-JUC-PM7 | ||||
| Juchitlán | 20.05530 | -104.06809 | 1324 | Alto | Inorgánico | S-JUC-PM8 | ||||
| Valles | Moderada | Amatitán | 20.83527 | -103.66876 | 1224 | Medio | Inorgánico | V-AMA-P9 | ||
| El Arenal | 20.78533 | -103.71006 | 1387 | Medio | Inorgánico | V-ARE-PM4 | ||||
| Baja | Amatitán | 20.87292 | -103.76204 | 1229 | Medio | Inorgánico | V-AMA-PM5 | |||
| 2019 | Altos | Alta | Zapotlanejo | 20.5677961 | -102.891988 | 1856 | Alto | Inorgánico | AZAP-A01 | |
| Arandas | 20.6467737 | -102.273707 | 2078 | Alto | Inorgánico | AARA-A02 | ||||
| Moderada | Tepatitlán | 20.8714019 | -102.685529 | 1979 | Medio | Inorgánico | ATEP-M01 | |||
| Jesús María | 20.5994848 | -102.15713 | 2186 | Alto | Inorgánico | AJM-M02 | ||||
| Baja | Ixtlahuacan del río | 21.0554448 | -103.192341 | 1674 | Alto | Inorgánico | AIXT-B01 | |||
| Cañadas de Obregón | 21.1631611 | -102.675379 | 1829 | Bajo | Orgánico | ACDO-B02 | ||||
| Sur | Alta | Pihuamo | 19.1997579 | -103.426473 | 707 | Medio | Inorgánico | SPIH-A01 | ||
| Pihuamo | 19.2114215 | -103.481563 | 655 | Medio | Inorgánico | SPIH-A02 | ||||
| Moderada | Juchitlan | 20.1064934 | -104.061595 | 1190 | Medio | Inorgánico | SJUC-M01 | |||
| Pihuamo | 19.2475873 | -103.430513 | 681 | Medio | Inorgánico | SPIH-M02 | ||||
| Baja | Tecolotlán | 20.1694819 | -104.042434 | 1177 | Medio | Orgánico | STEC-B01 | |||
| Tuxcacuesco | 19.7303572 | -103.906454 | 927 | Alto | Inorgánico | STUX-B02 | ||||
CIFUSAG method to obtain basal washings. To select offsets based on their base-size, metallic rings with diameters were used: Lime 10 cm, Orange 12 cm and Grapefruit 14 cm. The base (neck) and leaf base per offset of the composite sample were washed. This was done with sterile demineralized water in a 15 L plastic bucket. The offsets were partially submerged and scrubbed with a plastic brush to remove as much adhered soil as possible. Once the wash liquid was obtained, it was agitated with a modified drill to homogenize the sample, and then filtered to another 2 L container with a plastic mesh (2x2 mm opening). The filtrate was left stand for 10-15 min to remove water excess and recover the soil in 50 mL sterile Falcon tubes (Figure 1 A1-A8). The samples were taken to the laboratory in coolers with cooling gels to maintain the temperature and dry them immediately. Figure 1 explains the CIFUSAG methodology: (A1) Offsets distribution in the field. (A2) Composite sample of 15 offsets for Lime, Orange, and Grapefruit base-size. (A3) 1L of sterile demineralized water. (A4) Offset basal washig. (A5) Modified drill. (A6) Sedimentation for 10-15min of washing. (A7) Removal of excess water and collection of solid phase. (A8) Sample of basal external tissue (whasing) in 50 mL sterile Falcon tubes. (B1) Sample of 20 Orange base-size offsets. (B2) Cut of leaves. (B3) Individual washing with soap and tap water to remove residues. (B4) Offset drying. (B5) Removal of external tissue. (B6) Individual cut of offsets. (C1) Scheme of the internal tissue selected per offset. (C2) Selection of tissue for direct in vitro medium-plated. (D1) Offset remaining tissue in disinfestation process. (D2) Tissue maceration at 3000 rpm for 30s. (D3) Tissue of macerated offset. (D4) Bagasse filtering using plastic mesh. (D5) 50 ml homogeneous sample in a sterile Falcon tube. (E1) External tissue in Komada medium. (E2) Internal tissue in culture medium. (E3) Purification of isolates in water-agar. (E4) Monosporic culture (E5) and Culture characterization in Sabouraud medium.

Figure 1 CIFUSAG method to collect samples of Agave tequilana mother plants for basal external tissue (A), internal tissue (B and C), macerated tissue (B and D) and laboratory methodology (E). Letters and numerals explanation can be found in CIFUSAG method to obtain basal washings (Pages 7 and 9).
Offset selection for internalization and internal inoculum load studies (IIL). In addition to the external tissue samples, 30 offsets were collected in 2018 from a high-inductivity plot for internalization studies distributed in Lime, Orange, and Grapefruit base-sizes. In 2019, offsets were also selected for individual dissection and collection of internal tissue isolates: Of the 12 total plantations, six from Los Altos were selected, two per inductivity level (high, moderate, and low inductivity) and one from the South region (low inductivity). Per plot, 20 Orange-size offsets were selected and distributed in the three experiment design blocks (Figure 1B1). A total of 140 offsets were collected and processed, and then used to obtain different internal tissue (neck, piña, and leaves and bud base), the rest of the tissue was macerated to evaluate the total internal inoculum load.
Mother plants evaluation. To estimate the commercial plantations phytosanitary status, the phytosanitary mother plants condition at the sampling and collection offsets plot was evaluated using the diagrammatic scale proposed by Jiménez-González et. al. (2017) (Figure 1B-C) and the sampling system (<3 ha) proposed by SIVEA.
The in situ evaluation was performed with App-SIVEA v4.1 (Guzmán-Hernández et al., 2017). The sampling started with the third plant of each row to prevent edge effects. In 2018, 63 plants/plots were evaluated divided in three rows, one per block, with 21 plants each. In 2019, 20 quadrants divided in the three experiment blocks were evaluated using 20 continuous plants for each one, which totaled 200 evaluated plants per plantation for agave wilt and dry bud rot (SMAP) (Figure 1A). The data were sent in real time to the platform www.sivea.org.mx from the corresponding App-SIVEA modules and then the epidemiological matrix data was downloaded for analysis.
Laboratory Processing: Drying of external tissue samples. The samples placed in Falcon tubes arrived to the laboratory after a 8-48 h period because of the distance from the plantations where the samples were taken. During the transportation time, samples were kept in coolers with cooling gels. Once in the laboratory, they were refrigerated at 4 °C for 6-8 h for further precipitation and removal of water excess. Then, the samples were placed on sterile interfolded paper towels (Sanitas®) for 12 h in a laminar flow chamber for dried-out (Figure 1 B2-B4).
External tissue cultivation. Once the samples were dried-out, a mother dilution was prepared in 15 mL Falcon tubes using 1 g in 9 mL (1:9 ratio) of sterile demineralized water. After 15 min left standing, the mixture was manually homogenized for 1 min and diluted at 1x100.5. For that 1 mL of the mother dilution and 1 ml of sterile water were mixed to optimize colony counting. Using a Drigalsky spatula, an aliquot of 0.1 mL was distributed in a Petri dish plate containing Komada medium (Leslie and Summerell, 2006) (Figure 1 E1). The plates were left at room temperature for seven days for counting of colonies of Fusarium spp. and total fungi.
Isolation of internal and macerated tissue for IIL. The 140 Orange base-size offsets were washed in the laboratory to remove as much soil from the outside as possible. Using a machete deep cleaned with sodium hypochlorite (3%) and alcohol (70%), the external parts of the offset was removed until only internal tissue (white ‘piña’) was left (Figure 1 B5). The internal tissue was divided in pieces to take samples of neck, ‘piña’, leave-base and bud-base (Figure 1 B6). Per type of tissue, 25 pieces of 0.5x0.5 cm were cut for direct medium plating. These pieces were disinfested in the laminar flow chamber with hypochlorite (3%), followed by alcohol (70%) for two minutes each, and, finally, rinsed twice with sterile demineralized water (Figure 1 C2). The tissue pieces were placed on interfolded paper towels for 24 h for dry out to reduce bacterial growth. The rest of internal tissue was disinfested in beakers using the previously mentioned procedure to be later macerated. This process was performed independently per each offset.
For maceration, an industrial blender was used (Blendtec®, model TB-621-26) at 3000 rpm for 30 s. The macerated was poured in a beaker using a plastic mesh (0.5 x 0.5 cm opening) to remove bagasse, and a 50 mL sample was taken in sterile Falcon tubes. The samples were left to sediment for approximately 2-3 h to obtain supernatant (Figure 1 D1-D5). This was selected over the solid phase due to the abundant colonies growth in preliminary tests.
Five pieces of each type of tissue (neck, ‘piña’, and leave-base and bud-base) were placed independently in Petri dishes containing Komada medium (Leslie and Summerell, 2006). Similarly, 100 µL of the supernatant obtained from maceration were distributed in Petri dishes with Komada medium. A total of five Petri dishes per offset were obtained (four of tissues and one of supernatant) (Halfeld-Vieria and Nechet, 2005). The Petri dishes were incubated at room temperature for seven days and then Colony Forming Units (CFU) were counted and the Fusarium index was estimated (López-Bautista et al., 2020)..
DNA extraction, PCR, and phylogenetic analysis. The EF-1a gene was used for DNA extraction; this gene has been previously identified because of its consistency with Fusarium species in blue agave and other systems (O´Donnell et al., 2015; López-Bautista et al., 2020). Extraction was carried out following the AP protocol with modifications (SDS 1%) (Green and Sambrook, 2012). The DNA quality was quantified and determined using a Nano Drop 2000 (Thermo Fisher Scientific, USA), which was previously adjusted to 40 ng µL-1. For PCR, the final volume of the reaction mixture was of 25 µL [1X of PCR buffer (10X), 1.6 mM of MgCl2, 0.16 mM of deoxynucleotide triphosphates (dNTPs), 200 nM of each primer, EF1* (5’-ATG GGT AAG GAR GAV AAG AC) / EF2* (5’-GGA DGT ACC AGT RAT CAT G), 0.5 U of Platinum® Taq DNA polimerase (Invitrogen), and 2.5 µl of DNA (40 ng µL-1). A T-100 thermocycler from BioRad was used. The thermocycler program consisted in an initial denaturation at 94 °C for 5 min and 30 denaturation cycles at 94 °C for 30 s, alignments at 58 °C for 40 s, extension at 72 °C for 55 s and a final extension at 72 °C for 7 min. The amplified fragments were analyzed in 1.5% agarose gel and TBE 1X. Each gel deposit was loaded with 4 µL of the PCR product and 4 µL of load buffer (PROMEGA). The molecular weight marker (MMP) 1KB plus from Invitrogen was used with 1.5 µL+4 µL buffer. Bands of 760 bp were visualized with ethidium bromide and UV light. The corresponding PC product was sequenced by Macrogen Inc. from Korea.
The amplicon sequences were edited with the SeqAssem program (v07/2008) and blasted at the GenBank for preliminary identification and to obtain reference sequences for phylogenetic analyses. For alignments, the MEGA X program (v10.1.8) was used with the ClustalW algorithm. The phylogenetic relation was established using the maximum likelihood model of the General Time Reversible Model (GTR) + Gama Distributed With Invariant (G+I) with 1000 Bootstrap replications.
RESULTS
Validation of the sampling method. The CIFUSAG method was validated with different tools and methods in 2018 to standardize the process described in Figure 2. A thorough review suggests that, up until now, there are no reports of studies of agave or similar crops to quantify Fusarium spp. in propagating material, such as offsets, corms or bulbs. For external washing in the field, the use of brushes helped remove most of the soil and tissue adhered to the offset base, which includes the neck and leaves base; the modified drill added with a paint-mixture tool was also a useful method in sites where sandy soils prevail and the obtained sample was limited to 5-15 g of soil, unlike plantations with clay soils where up to 30 g of soil were collected. The CFU per plate reached an average of 129 total fungi colonies (Min=3, Máx=422) and six Fusarium colonies (Min=0, Máx=269).
Washing and removal the external green tissue of ‘piñas’ for the internal tissue and macerated processing in the laboratory prevent contamination and ensure that only internalized organisms are grown. In addition, maceration and mother dilution used for plating to obtain IIL ensured a greater number of CFU of total fungi (TF) (4 617), of which 22.5% (1 038) belonged to Fusarium spp. Colonies (Fsp). Thus CIFUSAG allowed CFU of TF and Fsp, enabling their analysis for management and control of this and other pathogenic organisms present in the crop.
Colony forming units by region in external tissue. The analysis of subdivided plots did not show effects on the large-intermediate and small plot interaction (Tukey p>0.05) and, therefore, they were individually analyzed. Overall, the Fusarium index [ (FI) = (∑Fsp) / (∑HT) ] showed differences by region and year. At regional level, in 2018 and 2019, the South region had lower epidemic inductivity level compared to the other regions, but its IF was statistically higher (0.5-0.7) compared to that of Los Altos (0.3-0.4) and Valley regions (0.2) (Tukey p<0.05) (Figure 3A).
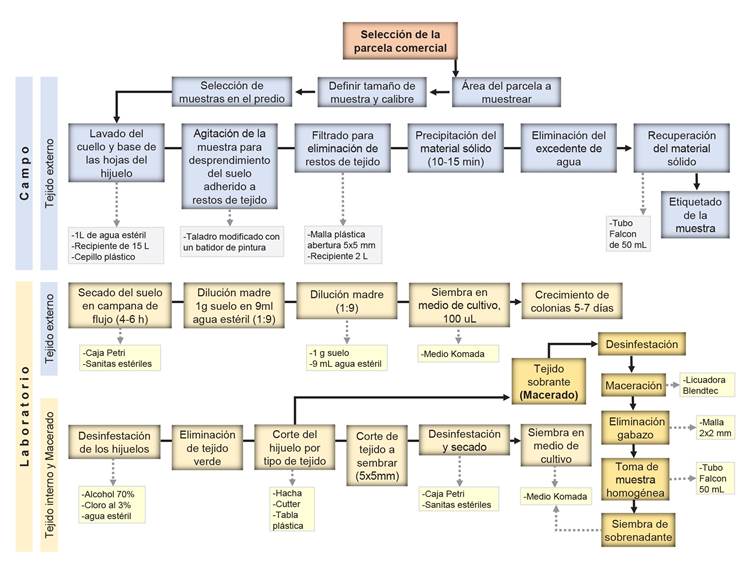
Figure 2 Scheme of the CIFUSAG method developed to estimate the Fusarium spp. inoculum load using A. tequilana offsets with field and laboratory phases. Samples included basal washing, internal tissue sections and total maceration of the offset.
In external tissue, the Fusarium CFU was statistically different (p<0.05) inter-regions for 2018, but not in 2019, despite the double of CFU obtained in the two regions (Figure 3B). The diseased plants in 2018 were less than five, in average, per assessed plot whereas in 2019, 15-20 plants showed SMAP with predominance of wilt symptoms (Figure 3C). The range percent of plant disease severity was 4.0-19.32% in 2018 being statistically different in Los Altos compared to that of the South and Valley regions (p<0.05), while in 2019 the disease severity ranged 29-31% in Los Altos and South regions with not statistical diference (Figure 3D).
Overall, the analyses of variance did not show significant interaction effects of base-size offset with region in 2018 and 2019 for CFU and Fusarium index (FI) (p>0.05) (Figure 4B, C and D), but there were differences in CFU of other fungi (p<0.05), mainly in offset samples collected in 2019, where Grapefruit base-size had the greatest average of CFU (107.1) (Figure 4A).
These results suggest that there is a significant diversity in the amount of fungi per region and base-size offsets. Although the pathogenic capacity per regional epidemic inductivity was not known in Fusarium, through the analysis conducted it can be inferred that there is a higher risk when offsets are mobilized.
Colony forming units per region in internal and macerated tissue. Initial preliminary tests were done for macerated tissue. The results showed that the supernatant, from a homogeneous sample, had the greatest amount of CFU of total fungi and Fusarium, especially in selective culture medium, with 62% of total colonies. The CFUs were greater in plantation with moderate inductivity in Tepatitlán, with a total counting of 378 (min=0, máx= 42, std=59.5 / plate), followed by Zapotlanejo with 206 (min=0, máx= 32, std=31.2 / plate). The plantation with lower inductivity in the South region, corresponding to Tuxcacuesco, was ranked third regarding Fusarium CFU with 188 total counting (min=0, máx= 37, std=61.8 / plate). The samples of Cañadas de Obregón plantation, with lower inductivity, in did not produced Fusarium colonies, a fact that could be attributed to the seasonal climatic conditions, where frosts were registered during the experiment. The offset weight, which was of 158 g in average, the lowest of all the plantations in Los Altos region, could also support the effect of the cold stress.
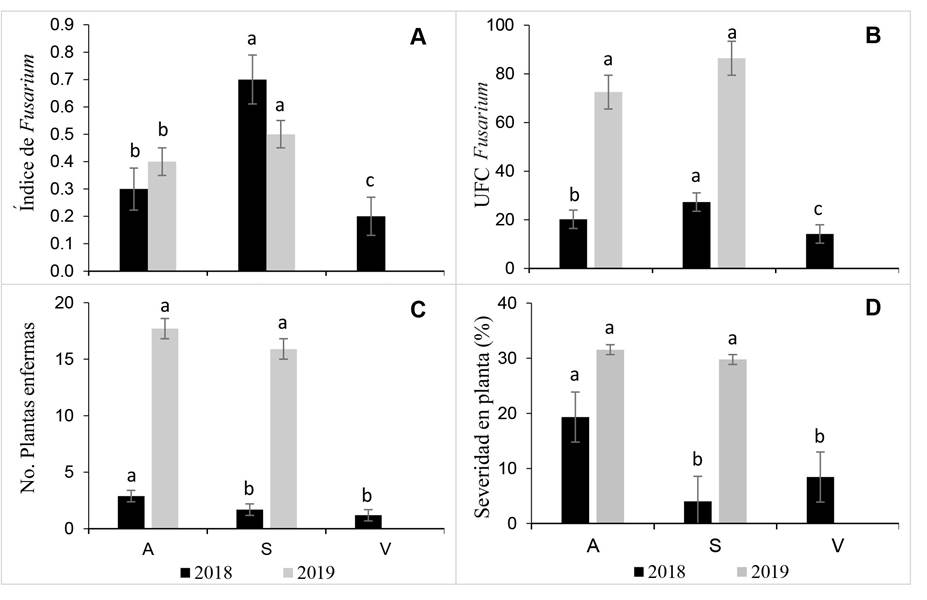
Figure 3 Fusarium spp. inoculum load in offsets external tissue (washing), number of infected plants and severity in mother plants with wilt and dry bud rot syndrome in Los Altos (A), South (S) and Vally regions (V) of Jalisco in 2018 and 2019. A) Fusarium spp. Index; B) Fusarium spp. colony forming units; C) Infected plants; D) Percent of disease severity in plants. At least one letter in common per year is statistically equal (Tukey p<0.05).
The Fusarium inoculum load in internal tissue compared to the inoculum load in external tissue per offset was lower (3.52 CFU) in Orange base-size. The inoculum load in external tissue was similar among base-size offsets. Orange base-size had a 5.37 CFU average. These results suggest a higher dispersion of Fusarium through external tissue. However, the CFU found internally are not to different so it may require both a systemic and contact product that help reduce the offset inoculum load (Figure 4D).
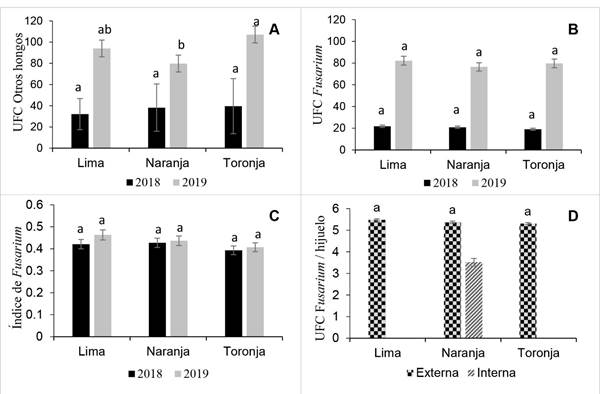
Figure 4 Inoculum load per Lime, Orange and Grapefruit base-size of agave offsets in experiments conducted in 2018 and 2019. A) Colony forming units (CFU) of other fungi; B) Fusarium spp. CFU; C) Fusarium spp. index; and D) Fusarium/offset per type of external or internal tissue and offsets base-size. Lime and Grapefruit base-size without data. At least one letter in common per year (A-C) or among externalt issue (D) are statistically equal (Tukey p<0.05).
In internal tissue, Fusarium colonies had a significantly different behavior with 75% of total isolates found in the leaf base, with a minimum of 18, and a maximum of 51 per plantation, followed by bud base (15%), ‘piña’ (8%) and neck (2%). This suggests a possible Fusarium species tissue specialization at offset level, as reported by López-Bautista et al. (2020), where different Fusarium isolates were reported in aerial tissue, roots and soil. These data also suggest that there is not ascendant fungus movement from the neck to exposed aerial tissue as leaves but rather there are multiple entrance ways.
Molecular identification and phylogeny. From 30 offsets used to obtain Fusarium isolates in internal and external tissue, 16 and seven isolates were collected respectively. Basal leaves represented 56% of the total, followed by neck (19%), bud (12.5%) and ‘piña’ (12.5%). The phylogenetic analysis of the amplicon sequences with the EF-1a gene determined the presence of three Fusarium binomial species belonging to different complexes; F. oxysporum (FOSC), F. verticillioides (FFSC), F. solani (FSSC), two phylogenetic species previously associated with adult plants (FIESC 9, FIESC 12) and one that was not identified also associated with adult plants (Fusarium 2-Agave) (López-Bautista et al., 2020) (Table 2).
The Fusarium FOSC and FFSC complexes were found in both tissues (internal and external), while FSSC was isolated only from internal tissue and FIESC# on external tissue. The leaf base had the greatest diversity and number of species: F. verticillioides (five), F. oxysporum (two) and F. solani (two). In bud, only F. oxysporum (one) and F. solani (one) were found, whereas in neck, F. oxysporum was consistent (three). However, in ‘piña’ tissue, the two sequences were aligned with a maximum of 97% identity with a Fusarium sp. species of the GenBank but had 99.7-100% identity with the non-identified species Fusarium 2-agave from A. tequilana adult plants. F. verticillioides and F. oxysporum were found in external tissue. The phylogenetic analysis using the EF-1a gene showed groups by complex with a statistical support higher than 96% and of 80% by species. Variability was also observed on clades, especially in the FIESC and FSSC complexes, where Fusarium spp. phylogenetic and non-identified species are placed (Figure 5).
DISCUSSION
The wilt and dry rot bud syndrome (SMAP) in A. tequilana represents one of the greatest challenges in blue agave production in DOT areas. The investigative work with this disease, which is caused by a complex of Fusarium species, is limited and mainly focused on commercial plantations with adult plants exhibiting the syndrome due to productive stress predisposition (Vega-Ramos et al., 2013; Ramírez-Ramírez et al., 2017; López-Bautista et al., 2020). Usually, this type of plantations, between 3-4 years-old, can be used as a source of offsets to establish new plantations, a fact that rise key phytosanitary questions because of the close relation among mother plant-offset-soil. Under this rational framework, this study provides three lines of knowledge: 1). Development and validation of a field and laboratory methodology, named as CIFUSAG (acronym of Carga de Inóculo Fusarium en Agave), to estimate the Fusarium spp. inoculum load, external and internalized in offsets; 2). Demonstrates the existence of differential inoculum load with strong regional effect; 3). Provides etiological basis by showing associative evidence of a complex of Fusarium species with offsets, both at external and internal tissue level.
Table 2 PCR molecular identification of 23 Fusarium isolates of 30 offsets in 2018 through source isolation using the EF-1a gene.
| Fuente de aislamiento | Especie/Complejo | ID aislado | %Identidad | Secuencia Referencia | |
|---|---|---|---|---|---|
| Base de la hoja | F. verticillioides | [FOSC] | NF2bh | 100 | MH582325 |
| NF3bh | 99.70 | MH582331 | |||
| NF4bh | 99.85 | MH582331 | |||
| PF1bh | 99.41 | JF740737 | |||
| PF8bh | 100 | MH582325 | |||
| F. oxysporum | [FOSC] | LF6bh | 99.23 | AF008497 | |
| PF7bh | 99.39 | AF008497 | |||
| F. solani | [FSSC] | LF5bh | 99.85 | JF740774 | |
| NF21bh | 99.86 | JF740846 | |||
| Cuello | F. oxysporum | [FOSC] | LF9b | 99.23 | AF008497 |
| NF10b | 100 | AF246837 | |||
| PF11b | 99.85 | JF740855 | |||
| Cogollo | F. oxysporum | [FOSC] | NF12cb | 99.85 | AF008497 |
| F. solani | [FSSC] | NF22ca | 99.86 | JF740846 | |
| Piña | Fusarium | sp. [FSSC] | PF23d | - | - |
| Fusarium | sp. [FSSC] | PF24d | - | - | |
| Tejido externo | F. verticillioides | [FOSC] | PF17s | 99.85 | MH582325 |
| PF19s | 100 | MH582325 | |||
| F. oxysporum | [FOSC] | NF13s | 99.54 | AF008497 | |
| PF20s | 99.85 | AF008497 | |||
| Fusarium | sp. [FIESC] | LF14s | 97.92 | MH582434 | |
| NF16s | - | - | |||
| NF18s | 97.73 | MH582433 | |||
The regional coverage of 14 municipalities in Jalisco with two years field data, a total of 7055 offsets tested and 46 656 fungal colonies counted, support the inoculum load results and proof the CIFUSAG methodology applicability. Conversely, the molecular characterization was limited to Fusarium isolates obtained from 30 offsets collected at a high inductivity plantations in Los Altos. Interestingly, the composition and diversity of the species complex found was analogous to the one found by López-Bautista and associates (López-Bautista et al., 2020). However, it is necessary to expand the studies of molecular characterization, with greater regional coverage, including other genes in addition to the elongation factor used in this study. Of particular interest are the Fusarium species with internalization capacity because they can be more efficiently disseminated and may represent a greater control challenge. Productive systems similar to agave, regarding the use of propagative material associated with Fusarium spp. have been reported in crops such as Musa paradisiaca (Bermúdez, 2014), Tulipa sp. (Bergman and Bekker, 1978) and Aloe vera (Avasthi et al., 2018). However, in general, a qualitative association with Fusarium is reported without any estimation of inoculum loads to establish actionability criteria with preventive purposes. Similarly, the studies of taxonomic diversity are usually limited to identify the specie or race of interest.
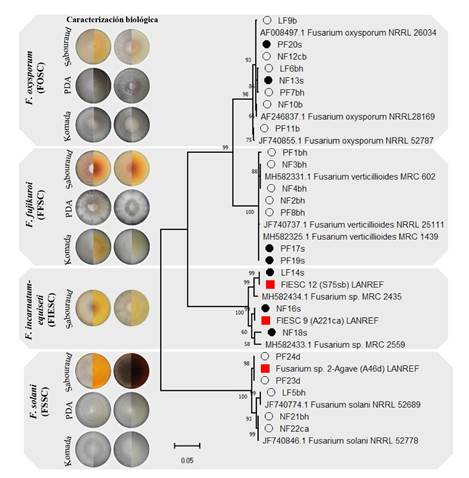
Figure 5 Phylogeny of 23 Fusarium spp. isolates associated with external (washing) and internal tissue of Agave tequilana offsets based on the EF-1a gene, maximum likelihood, and general time reversible model (GTR) + gama distributed with invariant (G+I), with 1000 bootstrap replications. The black bullet indicates isolates from external tissue; the white indicates isolates from internal tissue; the red are the phylogenetic species and the non-identified species of A. tequilana adult plants (López-Bautista et al., 2020). The rest, non-marked, are reference sequences from the GenBank.
With more than 96 million A. tequilana offsets planted between 2018 and 2019, this is the main propagation approach and therefore represents a potential pathway for Fusarium spp mobilization, and possibly of other pests, due to operational, agronomic and/or phytosanitary restrictions of other multiplication strategies such as botanical seed or in vitro culture. In this context, the results of this research address the importance to improve our understanding of the offset role in the productive sustainability of a region with designation of origin and limited margin for land expansion. It has been widely documented that Fusarium exhibits several adaptation strategies to ensure its establishment and parasitic fitness once settled in a new agroecological niche. A recent example of this is F. oxysporum f. sp. cubence Race 4 Tropical (FOCR4T), with effective mobilization paths and adaptation to most soils where banana is cultivated in the world (Bermúdez, 2014). Similarly, it has been postulated based on phylogenetic and structural studies that Fusarium can go through divergent-convergent evolution processes in A. tequilana and other crops (Liew et al., 2016; López-Bautista et al., 2020). The SMAP syndrome in adult plants is a clear evidence of the adaptive and parasitic capacity of Fusarium spp., where offsets may have an important implication by moving haplotypes of different species of this fungus, thus favoring dynamic changes of the prevailing prevalence structure at regional level.
Worldwide experience in Fusarium management shows that prevention is the most profitable and biologically viable alternative under an imposing regulatory or voluntary scheme yet general adopted among the productive sectors. Australia was able to contain a regional outbreak of FOCR4T for more than 20 years by taking severe preventive measures that included offsets movement restrictions for planting as their main strategy (M. Dita. 2017. Personal communication). However, since studies conducted in the DOT region of Jalisco suggest that haplotypes of different Fusarium species are widely distributed (Vega-Ramos et al., 2013; Ramírez-Ramírez et al., 2017; López-Bautista et al., 2020), the concept of free area as the basis for establishment of mother plantations and restriction of commercial offset mobilization appear to be unreliable. The alternative would be to optimize the principle of low prevalence with the pathogenic knowledge at the haplotypes level and their regional prevalence levels. This also implies a thorough knowledge of the physico-chemical and biological mechanisms of suppressive soil to operate on the soil health as a mitigation strategy (Fang et al., 2012; Huang et al., 2019). For example, neutral-alkaline pH and organic matter greater than 2.5 do not promote high Fusarium indexes (FI) in soils where agave is grown in Jalisco and are considered low inductive soils (López-Bautista et al., 2020). Although the offset maintains its physiological connection with the mother plant until it is cut with commercial purposes, there also exists an interaction with the soil that must be fully understood. In this study, the offset base-size criterion, which is defined by the size of its “bulbar” base, was intended to demonstrate that the younger the shoot (Lime), the least the exposure to microbial soil and the highest the vigor conferred by maternal factors, and therefore the least presence of Fusarium, thereby justifying its use in new plantations. The results demonstrated that it has no effect on the inoculum load nor on the species composition. The infective or endophytic condition however still needs to be elucidated. On the contrary, the epidemic inductivity of SMAP had an effect on the offset inoculum load. Surprisingly, the region considered as moderate (South, 6.83±4.22) marginally surpassed the most inductive region (Los Altos, 5.58±2.42) in CFU/offset. The less inductive region (Valley, 3.3±3) had 59 and 48% less inoculum load. Nonetheless, Los Altos lowest level of CFU variability suggests that Fusarium spp. have higher prevalence and endemicity. In this region, the establishment of mother plantations from commercial plantations could pose a greater mitigation challenge and soil health recovery.
Considering the importance of the agave industry and the epidemic history of SMAP, official phytosanitary campaigns and regulatory initiatives have been carryout and proposed with preventive purposes (SENASICA-DGSV, 2017). In 2003 it was presented the Norma Oficial Mexicana project NOM-083-FITO-2003 focused on delimiting the phytosanitary requirements for production and mobilization of propagative material of Agave tequilana Weber Blue variety in the DOT region. This norm project has not been published and its approval is uncertain. The objective of NOM-083 is as follow: ‘Establish phytosanitary requirements for the production and mobilization of propagative material of Agave tequilana Weber Blue variety, to protect its phytosanitary condition and prevent the spread of economically important pests affecting it’. In this context, pest has a wide meaning and mainly refers to Cercospora agavicola, Fusarium spp. and Scyphophorus acupunctatus (Coleoptera: Curculionidae). The guiding principle of the normative proposal is the certification of mother plantations, which is defined as ‘The temporary condition of a site where Agave tequilana Blue variety is cultivated within an area with Designation of Origin of Tequila from where offsets can be extracted, or tissue be donated for agave in vitro culture’. This regulatory framework may be a fundamental option for a sustainable phytosanitary management of the DOT, provided that comprehensive understanding of the offset pests risk and the integration of mother plantations into an epidemiological surveillance program to optimize monitoring, prevalence thresholds and phytosanitary actionability criteria are fully incorporated. This study, as well as previous research conducted as part of the regional surveillance network SIVEA (SENASICA-DGSV, 2017), which is currently operated only by the state of Guanajuato, provides biological and epidemiological criteria for identification of risk zones and low prevalence as baseline for establishment of mother plantations (Coria-Contreras et al., 2019; López-Bautista et al., 2020). The emerging global parasitic behavior of Fusarium spp., its taxonomic complexity and adaptive plasticity requires effective exclusion and mitigation strategies based on sound etiological, epidemiological and agroecological criteria, as well as a holistic and systemic vision in the DOT region to ensure its sustainability and resilience.
CONCLUSIONS
This is the first study that demonstrates the association of four Fusarium spp. species complexes with Agave tequilana commercial offsets. The CIFUSAG method was developed and validated to estimate the inoculum load in offsets; this method can be applied to studies on microbiological prevalence, risk management, control, and certification with emphasis on Fusarium spp. The epidemic inductivity to the wilt and dry bud rot syndrome (SMAP) showed a proportional tendency with Fusarium spp. inoculum load, while the offset base-size did not have any differential effect. It is necessary to integrate the study of soil physico-chemical and biological factors to understand the risks associated with the inoculum load in offsets. Selective pathogenicity tests are required to determine the Fusarium specie(s) that represent the highest level of survival, infectivity, and colonization in commercial offsets. This research justifies the scientific, technical, and regulatory analysis of commercial mother plantations and the offsets movement for propagative purposes to effectively manage the dispersion risk, establishment and/or intensity of potential damage to at least four Fusarium species associated with A. tequilana offsets. It is important to implement biological and chemical preventive management strategies that allow effective sanitary management of the Designation of Origin of Tequila (DOT) region.
Acknowledgments
The authors wish to thank CNRF-DGSV for the financial support provided through the SENASICA-COLPOS agreement for conducting this project. To COLPOS and CONACYT for granting the scholarship that allowed the first author to obtain her M.Sc. degree. To Casa Sauza and CESAVEJAL for the logistics and/or infrastructure support. To the CP-LANREF team for their dedicated support during the development of this research.
REFERENCES
Avasthi S, Gautam AK and Bhaduaria R. 2018. Isolation and characterization of Fusarium species causing leaf spot and root rot diseases on Aloe vera. Journal on New Biological Reports 7(1): 1-9. https://www.researchgate.net/publication/323320545 [ Links ]
Bergman BHH and Bekker-Van Der Voort, AMM. 1978. Latent infection in tulip bulbs by Fusarium oxysporum. Netherlands Journal of Plant Pathology 85: 187-195. https://doi.org/10.1007/BF01976820 [ Links ]
Bermúdez CI. 2014. Herramientas biotecnológicas para el combate de la raza 4 tropical de Fusarium oxysporum f. sp. cubense en Musa spp. Biotecnología vegetal 14(4): 195-202. https://revista.ibp.co.cu/index.php/BV/article/view/82 [ Links ]
Coria-Contreras JJ, Mora-Aguilera G, Yañez-Morales MJ, Acevedo-Sánchez G, Santana-Peñaloza B, Mendoza-Ramos C, Jiménez-González L, Martínez-Bustamante VI, García-Martínez DC and Rubio-Cortés R. 2019. Applied regional epidemiology to inductive characterization and forecasting of blue agave gray spot (Cercospora agavicola) in Jalisco, Mexico. Mexican Journal of Phytopathology 37(1): 71-94. http://dx.doi.org/10.18781/R.MEX. FIT.1809-4 [ Links ]
CRT, Consejo Regulador del Tequila. 2020. Geografía de la DOT. https://www.crt.org.mx/index.php/es/ (consulta, enero 2019). [ Links ]
Fang X, You MP and Barbetti MJ. 2012. Reduced severity and impact of Fusarium wilt on strawberry by manipulation of soil pH, soil organic amendments and crop rotation. European Journal of Plant Pathology 134: 619-629. https://doi.org/10.1007/s10658-012-0042-1 [ Links ]
Green MR and Sambrook J. 2012. Molecular Cloning: A Laboratory Manual, 4rd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. 1881p. http://www.cshprotocols.org [ Links ]
Guzmán-Hernández E, Flores-Colorado OE, Acevedo-Sánchez G, Mora-Aguilera G, López-Javier MA y González-Gómez R. 2017. Apps como herramientas de soporte epidemiológico aplicado a sistemas regionales de vigilancia epidemiológica. Revista Mexicana de Fitopatología 35(S): 178. [ Links ]
Halfeld-Vieria BA y Nechet KL. 2005. Morte de plântulas de Acácia mangium por Fusarium solani no Brasil e estudo da sua associação com sementes. Summa Phytopathologica 31: 383-385. https://www.researchgate.net/publication/233391880 [ Links ]
Huang J, Pang Y, Zhang F, Huang Q, Zhang M, Tang S, Fu H and Li P. 2019. Suppression of Fusarium wilt of banana by combining acid soil ameliorant with biofertilizer made from Bacillus velezensis H-6. European Journal of Plant Pathology 154: 585-596. https://doi.org/10.1007/ s10658-019-01683-5 [ Links ]
Jiménez-González LR, Mendoza-Ramos C, Santana-Peñaloza B, Coria-Contreras JJ, Delgado-Mora F, Acevedo-Sánchez G, Guzmán-Hernández E y Mora-Aguilera G. 2017. Escala logarítmica diagramática de severidad para medición de pudrición seca del cogollo, marchitez y mancha gris del agave azul. Revista Mexicana de Fitopatología. 35(S): 177. [ Links ]
Leslie JF and Summerell BA. 2006. The Fusarium Laboratory Manual. Ames, Iowa: Blackwell Publishing. 388p. https://www.researchgate.net/publication/321385629_The_Fusarium_Laboratory_Manual [ Links ]
Liew ECY, Laurence MH, Pearce CA, Shivas RG, Johnson GI, Tan YP, Edwards J, Perry S, Cooke AW and Summerell BA. 2016. Review of Fusarium species in association with mango malformation in Australia. Australasian Plant Pathology 45: 547-559. https://doi.org/10.1007/s13313- 016-0454-z [ Links ]
López-Bautista V, Mora-Aguilera G, Gutiérrez-Espinosa MA, Mendoza-Ramos C, Martínez-Bustamante VI, Coria-Contreras JJ, Acevedo-Sánchez G and Santana-Peñaloza B. 2020. Morphological and molecular characterization of Fusarium spp. associated to the regional occurrence of wilt and dry bud rot in Agave tequilana. Mexican Journal of Phytopathology 38: 79-106. DOI: 10.18781/R.MEX.FIT.1911-4 [ Links ]
NOM-083-FITO-2003. 2003. Proyecto de Norma Oficial Mexicana NOM-083-FITO-2003, Requisitos fitosanitarios para la producción y movilización de material propagativo de Agave tequilana Weber variedad azul. http://187.191.71.192/expedientes/1901 [ Links ]
O´Donnell K, Ward T, Robert V, Crous P, Geiser D and Kang S. 2015. DNA sequence-based identification of Fusarium: Current status and future directions. Phytoparasitica 43: 583-595. https://doi.org/10.1007/s12600-015-0484-z [ Links ]
Ramírez-Ramírez MJ, Mancilla-Margalli NA, Meza-Álvarez L, Turincio-Tadeo R, Guzmán-de Peña D and Ávila-Miranda ME. 2017. Epidemiology of Fusarium agave wilt in Agave tequilana Weber var. azul. Plant Protection Science 53: 144-152. https://doi.org/10.17221/142/2016-PPS [ Links ]
SENASICA-DGSV. 2017. Manual operativo de la campaña contra plagas reglamentadas del agave. Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria, Dirección General de Sanidad Vegetal-Centro Nacional de Referencia Fitosanitaria. 28p. http://www.sivea.org.mx/web/files/vista_principal/ManualOperativoPlagasReglamentadasdelAgave2016septiembreFINAL.pdf [ Links ]
SIAP. 2020. Servicio de Información Agrícola y Pesquera. SAGARPA. https://www.gob.mx/siap (Consultado marzo 2020). [ Links ]
Vega-Ramos KL, Uvalle-Bueno JX and Gómez-Leyva JF. 2013. Molecular variability among isolates of Fusarium oxysporum associated with root rot disease of Agave tequilana. Biochemical Genetics 51: 243-255. https://doi. org/10.1007/s10528-012-9559-4 [ Links ]
Received: June 28, 2020; Accepted: December 10, 2020











 texto en
texto en 


